Replacement of Carboxylate Ligand Substituent on Modulation of Structures and Magnetic Properties in Salen?Type Dinuclear Dy Complexes
LI MinWU Hai?Peng*,ZHANG ShengLIU Yu?FangCHEN Yong?Qiang*,CHEN San?Ping
(1Department of Chemistry and Chemical Engineering,Jinzhong University,Jinzhong,Shanxi 030619,China)
(2College of Chemistry and Materials Science,Northwest University,Xi'an 710127,China)
(3College of Chemistry and Chemical Engineering,Baoji University of Arts and Sciences,Baoji,Shaanxi 721013,China)
Abstract:Three salen?type centrosymmetric dinuclear Dy complexes,[Dy2(Hhms)2(C(CH3)3COO)2(H2O)4](NO3)2(1),[Dy2(Hhms)2(C14H9COO)2(C2H5OH)2(CH3OH)2][ZnCl4](2),and[Dy2(Hhms)2(C6H3(NH2)2COO)2Cl2]·2CH3CN(3)(H2hms=(2?hydroxy?3?methoxybenzylidene)?semicarbazide),were isolated with different substituted carboxylic acid ligand,and were characterized structurally and magnetically.Structural analyses illustrate that the Dyions in complexes 1 and 2 maintain similar monocapped square?antiprism geometries,but the coordination mode of carbox?ylate in 1 is different from that in 2;complexes 2 and 3 possess similar phenoxy oxygen and carboxylate bridged structure whereas the coordination geometries around the Dy ions are different between 3 and 2 due to the differ?ence of coordinated small molecules.Magnetic characterizations reveal that significant single?molecule magnet(SMM)behavior was observed under zero dc field for complex 3,with an effective energy barrier to the reversal of magnetization of 96 K.Conversely,complex 1 only showed fast quantum tunneling relaxation even 2 was SMM?silent.Furthermore,the magneto?structural correlations in these Dy2complexes were discussed.The results indicate that utility of carboxylate ligand substituent can give rise to good modulation in the molecular anisotropy and symme?try,hence the enhanced magnetic relaxation.CCDC:2092500,1;2092502,2;2092503,3.
Keywords:dinuclear complex;dysprosium;magnetic properties;carboxylate ligand;single?molecule magnets
0 Introduction
Single?molecule magnets(SMMs),which exhibits slow relaxation of magnetization at the molecular level,are expected to be the smallest storage units and quan?tum objects,invoking intense research interest in the field of spintronic devices and quantum computing[1?3].Recently,optimizing the crystal field environment of a single Dycenter based on an electrostatic model allows blocking of magnetization reaching unprecedent?ed liquid nitrogen temperatures[4].Whereas,encapsulat?ing two or more spin centers complexes facilitate the generation of more sophisticated architectures and mag?netic behavior,giving the new opportunities to design SMMs with augmented performances[5?7].The exploita?tion of new Dypolymetallic complexes to drive understanding of influence factors that dictate the slow magnetic relaxation is still being established[8?12].
Based on the above considerations,to further effi?ciently control the carboxylic coordination,we selected various carboxylic acid ligands with different steric hin?drance and electron donor ability substituent and com?bined them with polydentate Schiff?base ligand with relatively fixed coordination cavities to construct polymetallic complexes(Scheme 1).Herein,three novel Dy?based complexes,[Dy2(Hhms)2(C(CH3)3COO)2(H2O)4](NO3)2(1), [Dy2(Hhms)2(C14H9COO)2(C2H5OH)2(CH3OH)2][ZnCl4](2),and[Dy2(Hhms)2(C6H3(NH2)2COO)2Cl2]·2CH3CN(3)(H2hms=2?hydroxy?3 ?methoxybenzylidene)?semicarbazide)were isolated in a centrosymmetric binuclear architecture.The structural characterization and magnetic properties of complexes 1?3 were investigated.Through magneto?structural cor?relations analysis,the present work highlights the importance of ligand substitution and molecule symme?try in controlling magnetic relaxation and provides a feasible way to develop high?performance carboxylate?based polymetallic SMMs.
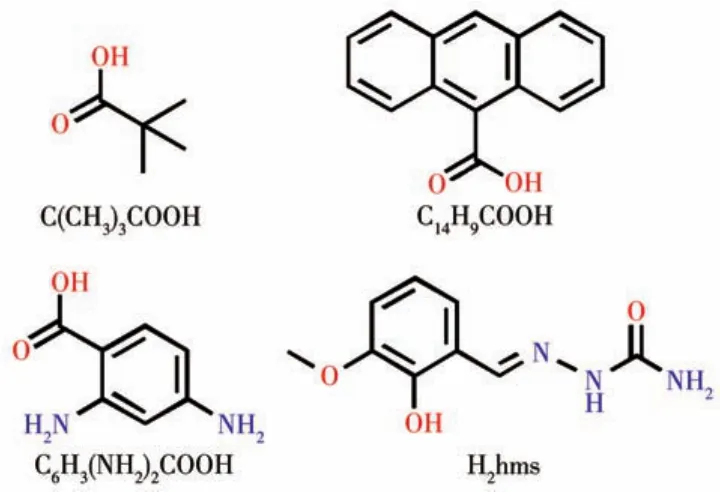
Scheme 1 Structures of carboxylic acid ligands and Schiff?base ligand
1 Experimental
1.1 Materials and measurements
All chemical reagents and solvents were obtained commercially and used in the reactions without further treatment,except for the ligand of H2hms,which was synthesized by an analogous method described in the literature[21].Powder X?ray diffraction measurements were carried out on a Bruker D8 ADVANCE X?ray powder diffractometer at 40 kV and 40 mA,using CuKαradiation(λ=0.154 18 nm)ranging from 5°to 50°(2θ)to verify the phase purity of the polycrystalline samples.The FT?IR spectra were recorded with pressed KBr pellets in a range of 4 000?400 cm-1on a Tensor 27 spectrometer(Bruker Optics,Ettlingen,Germany).Elemental analyses(C,H,N)were collected on an Elementar Vario EL Ⅲ analyzer.Magnetic susceptibility measurements were conducted on a Quantum Design MPMS?XL7 SQUID magnetometer in a temperature range of 2?300 K with 1 000 Oe applied field.Alternating?current(ac)measurements were performed on the same magnetometer at ac frequencies ranging from 1 to 1 000 Hz using a 2.0 Oe oscillating ac field.The measured susceptibilities were corrected for the diamagnetism of the constituent atoms(Pascal's tables).
1.2 Synthesis of [Dy2(Hhms)2(C(CH3)3COO)2(H2O)4](NO3)2(1)
A mixture of Dy(NO3)3·6H2O(0.045 6 g,0.1 mmol),C(CH3)3COOH(0.010 2 g,0.1 mmol)and H2hms(0.021 0 g,0.1 mmol)in methanol(4 mL)and dichloromethane(4 mL)was added sodium azide(0.006 5 g,0.1 mmol).After stirring for 3 h,the resul?tant solution was filtered and allowed to stand undis?turbed at room temperature.The yellowish block crys?tals suitable for single?crystal X?ray diffraction were obtained by slow evaporation of the filtrate after 5 d.Yield:ca.56%(32 mg)based on Dy.Elemental Anal.Calcd.for C28H46Dy2N8O20(%):H,4.07;C,29.51;N,9.83.Found(%):H,4.43;C,29.67;N,9.52.IR(KBr,cm-1):3 340(s),3 043(s),2 584(w),2 420(w),1 667(s),1 568(s),1 451(s),1 415(s),1 385(m),1 366(m),1 357(s),1 225(m),1 089(m),1 018(s),956(m),787(m),741(m),669(m),614(m),493(w).
1.3 Synthesis of [Dy2(Hhms)2(C14H9COO)2(C2H5OH)2(CH3OH)2][ZnCl4](2)
A mixture of DyCl3·6H2O(0.037 7 g,0.1 mmol),ZnCl2(0.027 3 g,0.2 mmol),C14H9COOH(0.133 2 g,0.6 mmol)and,H2hms(0.021 0 g,0.1 mmol)in metha?nol(4 mL)and ethanol(4 mL)was added sodium azide(0.013 0 g,0.2 mmol).After stirring for 3 h,the resul?tant solution was filtered and allowed to stand undis?turbed at room temperature.The yellowish block crys?tals suitable for single?crystal X?ray diffraction were obtained by slow evaporation of the filtrate after one week.Yield:ca.45%(35 mg)based on Dy.Elemen?tal Anal.Calcd.for C54H58Cl4Dy2N6O14Zn(%):H,3.78;C,41.97;N,5.44.Found(%):H,3.52;C,41.69;N,5.41.IR(KBr,cm-1):3 345(s),3 294(s),3 248(s),3 092(m),2 932(m),2 856(m),1 689(s),1 602(m),1 586(s),1 482(s),1 454(s),1 383(s),1 110(s),1 093(m),854(w),823(w),784(w),744(m),669(m),615(m),494(w).
1.4 Synthesis of[Dy2(Hhms)2(C6H3(NH2)2COO)2 Cl2]·2CH3CN(3)
A mixture of H2hms(0.021 0 g,0.1 mmol),C6H3(NH2)2COOH(0.015 2 g,0.1 mmol),and DyCl3·6H2O(0.037 7 g,0.1 mmol)in acetonitrile(4 mL)was placed in a 20 mL Teflon?line autoclave kept for three days at 90℃,and then cooled down to room temperature at a rate of 5 ℃ ·h-1to form yellowish block crystals suit?able for X?ray analysis.Yield:ca.58%(35 mg)based on Dy.Elemental Anal.Calcd.for C36H40Cl2Dy2N12O10(%):H,3.37;C,36.13;N,14.05.Found(%):H,3.55;C,36.49;N,14.02.IR(KBr,cm-1):3 433(s),3 369(s),3 293(s),3 076(m),2 954(s),2 867(w),2 244(w),1 687(s),1 607(s),1 562(s),1 446(s),1 416(s),1 385(s),1 272(m),1 113(s),1 091(s),976(m),857(w),824(w),767(w),732(m),667(m),614(m),494(w).
1.5 X?ray single?crystal diffraction analysis
Single?crystal X?ray data for complexes 1?3 were collected at room temperature on a Bruker Apex Ⅱ CCD diffractometer with graphite monochromated MoKαradiation(λ=0.071 073 nm).Cell determination and data reduction were processed with the SAINT pro?cessing program.The absorption correction based on multiscan was applied in SADABS.By using Olex2[27],the structures were solved by direct methods with SHELXT and refined by full?matrix least?squares tech?niques againstF2using SHELXL?2014 programs[28].All non?hydrogen atoms were refined anisotropically.The acidic hydrogen atoms were found from the elec?tron density map and were refined freely.All other hydrogen atoms were placed in calculated geometrical?ly positions and refined using the riding model.The crystallographic data and structure refinement summa?ry are summarized in Table S1(Supporting informa?tion).Selected bond distances and angles are listed in Table S2.
CCDC:2092500,1;2092502,2;2092503,3.
2 Results and discussion
2.1 Description of crystal structures
Complexes 1?3 were structurally characterized by X?ray crystallography(Table S1).1 and 2 crystallize in the monoclinic space groupC2/c,while 3 crystallizes in space groupP21/n.1?3 are all centrosymmetric binu?clear structures and the asymmetric unit of the com?plex contains one?half of the entire molecule.The Dyions in 1 and 2 have similar monocapped square?antiprism geometries with an {O8N}coordination sphere(Fig.S1 and Table S3).Two antiparallel Hhmsligands bind to the Dyion by two phenoxy oxygen at?oms(O2,O2i),one methoxy group(O1),one aldehyde group(O3),and one Schiff?base nitrogen atom(N1),and the remaining coordination sites are filled by two carboxylic oxygen atoms(O4,O5)and two H2O mole?cules for 1 or a superposition of CH3OH and C2H5OH for 2(O6,O7)(Fig.1).In 1,the C(CH3)3COO-groups act as chelating ligands,with it coordinating to a single Dyion,while in 2,the C14H9COO-groups bridge the two Dycenters in aμ2∶η1∶η1fashion.It is important to note that the different coordination patterns of carboxylate in 1 and 2 do not affect the configurational differences between them.For complex 3,the Dycenters are coordinated by Hhms-ligands and carboxyl?ic oxygen atoms in a manner essentially the same as that in 2 except for two alcohol oxygen atoms substitut?ed by one Cl-ion.Therefore,the Dyion in 3 has a{DyO6NCl}coordination sphere,forming a square?antiprism geometry.The two Dycenters are bridged by two phenoxy oxygen atoms(O2 and O2i)of two Hhms-ligands,besides,the RCOO-groups with distinct terminal substituent bridge two Dyatoms in aμ2∶η1∶η1fashion in 2 and 3,with the Dy…Dy intra?molecular distance of 0.378 1(1),0.365 6(8)and 0.364 8(7)nm respectively,as well as Dy—O—Dy angle of 107.19°,101.81°,and 101.23°respectively.The Dy—O bond lengths are in a range of 0.230 5(3)?0.263 7(2)nm,being the longest related to the methoxy oxygen atom distances.The shortest Dy—O bond lengths in 1?3 involve aldehyde oxygen(for 1)or one of bridged phenoxide oxygen(for 2 and 3)with 0.230 5(3),0.232 7(2),and 0.231 5(5)nm respectively.The Dy—O distances within O,N,O?tridentate pocket of Hhmsligand are shorter than that within O,O?bidentate pock?et of Hhms-ligand.The Dy—Carboxylatebond lengths of the bidentate bridging coordination mode in 2(AVG:0.237 1 nm)and 3(AVG:0.233 2 nm)show shorter distance than that of the chelating coordination mode in 1(AVG:0.244 2 nm),which may be associated with the different terminal substituents of carboxylic acid ligands.The Dy—N(0.247 8(6)?0.256 7(3)nm)and Dy—Cl(0.278 3(1)nm)bonds present quite long distances.Furthermore,the shortest inter?dimer Dy…Dy distance is equal to 0.721 9,1.018 9,and 0.835 6 nm respectively.
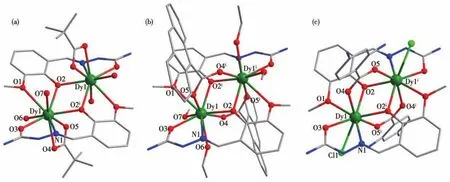
Fig.1 Molecular structures of complexes 1(a),2(b),and 3(c)
2.2 Magnetic properties
The magnetic properties of 1?3 were measured using a SQUID magnetometer.The sample purity was first checked by powder X?ray diffraction experiment comparing experimental diagram to theoretical one ob?tained from the crystal structure(Fig.S2).
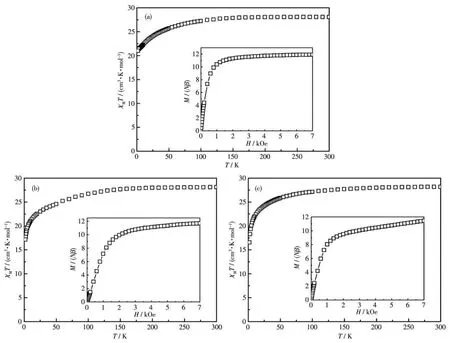
Fig.2 Temperature dependence of χMT for complexes 1?3 under 1 000 Oe field
The variable?temperature magnetic susceptibility for 1?3 was collected between 2 and 300 K under an applied magnetic field ofHdcbeing 1 kOe.At 300 K,χMTproducts were 28.07,28.02,28.16 cm3·K·mol-1for 1?3,respectively(Fig.2),which are slightly lower than the value expected for two ensembles of Dyions in the free ?ion approximation.On cooling,theχMTproducts underwent a slow decline and then decreased rapidly at low temperatures to reach a value of 21.00,20.17,and 16.53 cm3·K·mol-1respectively at 2 K.The reduction ofχMTfor all three complexes is accounted for by the gradual depopulation of the Stark sublevels and/or intramolecular antiferromagnetic interactions.The magnetization data of 1?3 at 2 K are depicted in the inset of Fig.2.The plots ofMvsHshowed a rapid increase below 1 kOe,and the slight increase reaching the maximum magnetizations of 11.9Nβ,11.0Nβ,11.4Nβat 7 kOe,which were much lower than the theo?retical saturation value of 20Nβfor the Dy2dimer.The unsaturated magnetization indicates strong magnetic anisotropy of Dyions[29?30].
The magnetization relaxation dynamics were investigated by studying the temperature (T)and frequency(ν)dependence of ac magnetic susceptibili?ties.Complex 2 displayed no out?of?phase signal in ac susceptibility measurements in zero?field(Fig.S3).As a result of a strong QTM of Dyions,the temperature?dependent out?of?phase signals of 1 only showed a small tail below 4 K(without peak maximum)under zero dc field(Fig.3).

Fig.3 Temperature?dependent out?of?phase χ″ac susceptibility signals(a)and frequency?dependent out?of?phase χ″ac susceptibility signals(b)for 1 under zero dc field
For complex 3,the out?of?phase(χ″)component of the temperature?dependent ac susceptibility exhibited one maximum in the 3.5 K(100 Hz)?8 K(1 000 Hz)range,which indicates the slow relaxation of magnetiza?tion of 3(Fig.4).But a tail of the peak was observed below 3 K,and this observation suggests a temperature?independent QTM regime at lower temperatures,as is often observed for Ln?containing systems.Consis?tently,the frequency dependence of the maximum in association with single relaxation mode appeared on the plot of theχ″vs the frequency between 1 and 1 000 Hz.The Cole?Cole plot ofχ″vsχ'clearly identified sin?gle relaxation process and were fitted using a general?ized Debye model[31]with CCFIT package[32]to extract the relaxation times(τ)(Fig.S4).The fitting gave a rela?tively narrow distribution ofτwith a small coefficientαfrom 0.19 to 0.34.Insight into the dynamic magnetiza?tion under zero dc field of 3 was obtained through plots of lnτvsT-1(Fig.5).An Arrhenius fitting of the high?temperature region gave unphysical parameters ofUeff=68 K andτ0=6.30×10-8s.In the whole temperature range,a combination of multiple relaxation pathways including Orbach,Raman,and QTM processes can be considered here.The data for 3 were fitted using Eq.1:τ-1=τQTM-1+CTn+τ0-1exp[-Ueff/(kBT)],whereUeffis the Orbach parameters,Candnare the Raman parame?ters,andτQTM-1is the QTM rate.The best fit parameters were found to beUeff=96 K,τ0=3.1 ×10-10s,C=0.122 s-1·K-4.77,n=4.77 andτQTM=5.7 ms.To get a better un?derstanding of the magnetic relaxation,we also tried to fit the relaxation time of zero?field data with only QTM and Raman processes,however,no better results were obtained by leaving out the Orbach term(Fig.S5).
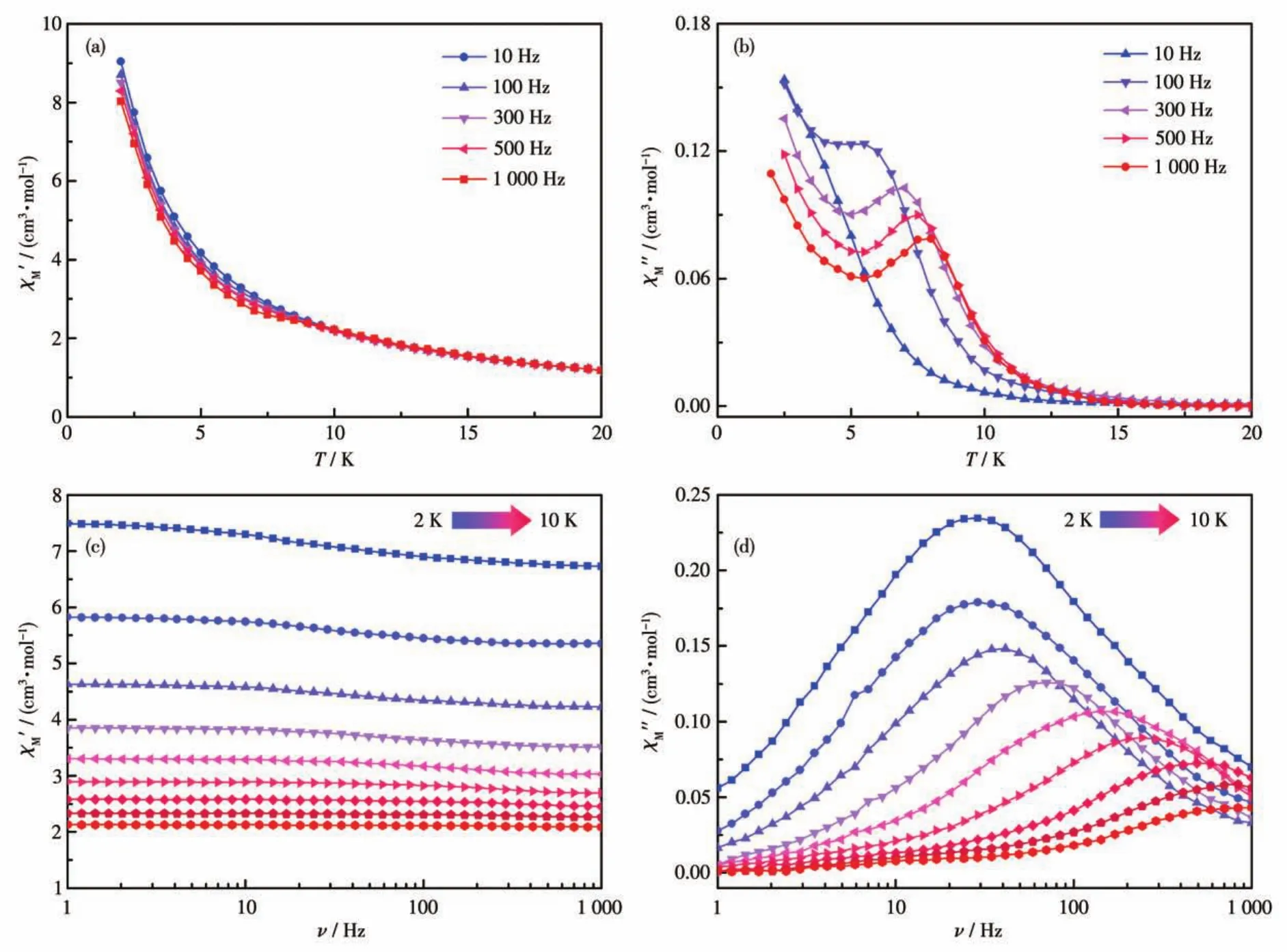
Fig.4 Temperature?dependent in?phase χ'(a)and out?of?phase χ″(b)ac susceptibility signals for 3 under 0 Oe dc field;Frequency?dependent in?phase χ'(c)and out?of?phase χ″(d)ac susceptibility signals for 3 under 0 Oe dc field
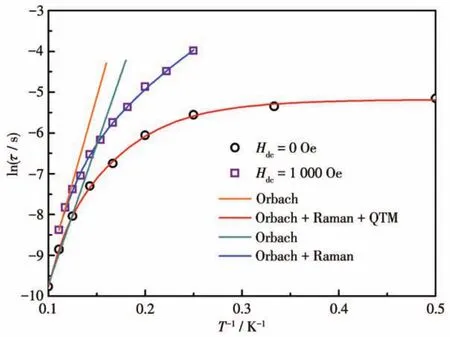
Fig.5 Relaxation data plotted as ln τ vs T-1for 3
The ac magnetic susceptibility was further investi?gated under an applied static field.Applying a dc field of 1 000 Oe induces slower relaxation with shifting peak maxima toward lower frequency(Fig.S6),indicat?ing a significant suppression of QTM.Cole?Cole plots in a range of 4?9 K were obtained(Fig.S7)and fitted using the generalized Debye model[31]to give the relax?ation timeτ(Fig.5)and distribution coefficientα(0.15?0.27).The thermal energy barrier was estimated from the high?temperature data of lnτvsT-1plot to be 83 K withτ0=2.1×10-8s(Fig.5).An additional term of Raman processes was required to fit the relaxation time behavior in the whole temperature range.The obtained satisfied parameters wereUeff=98 K,τ0=7.4×10-9s,C=0.133 s-1·K-4.32,n=4.32.Furthermore,if we only consid?ered the Raman process for τ without the Orbach pro?cess,the fitting line did not fit well the lnτvsT-1plots(Fig.S5),also suggesting the existence of a thermally activated relaxation in 3.

Fig.6 Magnetic anisotropy axes(green lines)predicted by Magellan program for Dy centers in complexes 1?3
From the ac susceptibilities,we can see that com?plex 3 has the highest energy barrier.The structures of 2 and 3 are analogous,with the major difference being in R substituent of carboxylate ligand and the coordi?nated small molecules with four superposed CH3OH/C2H5OH in 2 and two Cl-in 3.It should be noted that there is no change in the coordination modes of Hhmsand RCOO-between them.In addition,complexes 1 and 2 have a similar coordination configuration,with the main distinction only in the bonding mode of car?boxylate which leads to the relative differences in the spatial arrangement of ligands.However,their magnet?ic properties are quite different:1 exhibits a frequency?dependent behavior under zero dc field though without a clear peak,while 2 does not show magnetic relax?ation behavior.
To further better understand the SMM properties of 1?3,Fig.6 depicts the simulated principal magnetic axes of Dyions in each molecule defined by the Magellan program[33].The directions of the calculated anisotropy axis of Dyin 1 are found to be tilted towards the O4 atom belonging to the bidentate—C(CH3)3COO-anion and bridged phenoxy oxygen atoms(O2).Both of them make a small angle of 17.4°and 34°respectively.While the anisotropy axes of com?plex 2 show parallel arrangement through the middle of each C14H9COO-ligands,almost perpendicular to the bridged phenoxy oxygen atoms(O2 and O2i).For dinu?clear complex 3,the anisotropy of the Dyion is near?ly oriented along with the Dy—Ocarboxylate(O4)and Dy—Cl bonds,which deviation angles are in a range of 17.5°?18.9°.These illustrate the fact that the bonding mode of carboxylate and the coordination of chloride anions affect the magnetic anisotropy of the Dyion and the SMM properties.
From the analysis of the anisotropic axis above,the most magnetic axes in the ground states are closer along with the carboxylic oxygen moieties.It is well?known that the magnetic anisotropy of an Lnion is extremely sensitive to its coordination environment[34?39].Even changes in the second coordination sphere ligat?ed atoms could also lead to a significant variation on the single?ion anisotropy of the Lnions,as demon?strated by Sessoli and co ?workers[40?42].In 3,the two strongly electron?donating groups—NH2through inductive effects increase the charge of axially shortest carboxylic oxygen donor thus strengthening the axial component of crystal field relative to 1 and 2.Careful inspection of the bond distance of complexes 1?3 reveals that the different electron?donating effects in?duced by the R substituent are necessarily felt by the car?boxylic oxygen ligands,and consequently has an effect on the strength of the chemical bond to the Dyions.This is indeed suggested by the shortest Dy—Ocarboxylatebond for 3(Dy—O4 0.232 6(4)nm,Dy—O5 0.233 7(5)nm).Whilst simultaneously,coordination of a chloride ion,in place of two solvent molecules(H2O or CH3OH/EtOH),reduces the coordination number,resulting in the Dyions in square antiprismatic environments withD4dsymmetry,which are much different from the monocapped square?antiprism in 1 and 2.Moreover,among complexes 1?3,the shortest intramolecular Dy…Dy distances(0.364 8(7)nm)and the smallest Dy—O—Dy angles(101.23°)are found for 3,which may have a positive influence on the magnetic exchange coupling between two Dyions.In contrast to 3,com?plex 2 did not show magnetic relaxation behavior.It might be associated with the relatively high charge dis?tribution of the Ophenoxoatoms(O2 and O2i)lying on the transverse plane(perpendicular to the magnetic anisot?ropy easy axis)which contributes to transverse ligand field,and thus is disadvantageous to obtain the high magnetic anisotropy of oblate Dyion.In total,the different magnetic properties of 3 and 1?2 mainly result from the differences of ligand substitution by the non?coordinating electron?donating group,coordination geometry,and magnetic interaction between two Dyions.The results emphasize that the utility of the ligand substitution can give rise to good modulation in molecule symmetry,as well as the magnetic anisotropy of Lnion,thus improving SMM performance.
3 Conclusions
In summary,a series of dinuclear Dycomplexes based on different substituted carboxylate ligand and Schiff?base H2hms ligands have been reported.In com?plexes 1 and 2,donor atom sets around the Dyions are the nearly same as a{DyO8N}coordination sphere,whereas,in 3,the Dyions exhibit a{DyO6NCl}coor?dination environment.Complex 3 exhibited zero?field SMM behavior with a high thermal energy barrier for the reversal of the magnetization(96 K),revealing a significant improvement in performance across the series.The largeUeffvalue observed for 3 is a conse?quence of the enhanced magnetic anisotropy and local symmetry promoted by the presence of the significant electron?donating groups—NH2on the carboxylic oxygen atoms and the coordinated chloride ligands reducing the coordination number of the Dyions,resulting in a higher symmetry environment(D4dsym?metry)of the Lnions.The results demonstrate that the non?coordinating ligand substitution and coordina?tion small molecule play essential roles in charge distri?butions of the ligand?field environments and molecular symmetry thus an SMM performance,which should be considered seriously and utilized efficiently during the rational design of new SMMs.
Supporting information is available at http://www.wjhxxb.cn
 無機(jī)化學(xué)學(xué)報(bào)2022年1期
無機(jī)化學(xué)學(xué)報(bào)2022年1期
- 無機(jī)化學(xué)學(xué)報(bào)的其它文章
- 《無機(jī)化學(xué)學(xué)報(bào)》投稿須知(NOTICE TO AUTHORS)
- Synthesis,Structures and Catalytic Activity in Knoevenagel Condensation Reaction of Two Diphenyl Ether Tetracarboxylic Acid?Co Coordination Polymers
- Mn?MOF Derived Mn2O3 Micromotors Applied to Removal of Methyl Blue in Water
- Crystal Structures and Magnetic Properties of LnⅢ2Complexes Based on a Polydentate Schiff Base Ligand
- Synthesis of a Water?Stable Zn?Based Metal?Organic Framework for Luminescence Detecting Fe3+and 2,6?Dichloro?4?nitroaniline
- Constructing and Photocatalytic Performance of Flower?like CeO2/TiO2 Heterostructures
