Synthesis of a Water?Stable Zn?Based Metal?Organic Framework for Luminescence Detecting Fe3+and 2,6?Dichloro?4?nitroaniline
WANG Xiao?Qing MA Xue?Hui FENG Dou?Dou TANG Jing WU Dan
(Department of Chemistry,College of Science,North University of China,Taiyuan 030051,China)
Abstract:A novel thermal?stable and water?stable Zn?based metal?organic framework(MOF),[Zn2(bcpp)2(1,4?bib)2]·1.5H2O(1)was synthesized by solvothermal method based on 3,5?bis(4?carboxylphenoxy)pyridine(H2bcpp)and 1,4?bis(1?imidazoly)benzene(1,4?bib).Complex 1 belongs to the monoclinic system with space group I2/a,which exhibits a 1D tubular structure.The adjacent 1D tubular structures are interpenetrated with each other to obtain a 3D supramolecular structure.Moreover,complex 1 exhibits excellent photoluminescence property,which can be applied as a“turn?off”fluorescence probe to detect Fe3+ion and pesticide 2,6?dichloro?4?nitroaniline in an aqueous solution with high selectivity and sensitivity.CCDC:2095435.
Keywords:metal?organic framework;Zn;luminescence detecting;Fe3+;pesticide
0 Introduction
Currently,the large discharge of hazardous pollut?ants from agriculture,mines,and factories has brought serious damage to the ecological environment and phys?ical health.For example,organochlorine pesticides have been widely applied to kill off insect pests and improve the yield of crops[1].The 2,6?dichloro?4?nitroaniline(DCN)is one of organochlorine pesticides,which is a broad?spectrum insect killer and can be used to prevent cotton rotten bells,wheat powdery mil?dew,and garden stuff rot[2].Whereas the degradation of DCN needs a long time,and the ingestion of DCN resi?dues on food can damage the immune system of humans and cause cancer or other illnesses.Moreover,Fe3+ions exist in living systems that take part in some cellular processes,such as oxygen transport and hemo?globin formation[3].However,the excess of Fe3+ions can lead to some diseases of proteins and nucleic acids.Thus,exploring a rapid and effective detecting method to sense metal ions and organochlorine pesticides is significant.
The fluorescence sensor exhibits rapid response,high sensitivity,selectivity,and simple operation for detecting pollutants.Recently,metal?organic frame?works(MOFs)as novel fluorescence sensors have been used to detect pollutants,including heavy metal cat?ions(Fe3+,Hg2+,Cu2+),inorganic anions(F-,CrO2-,24MnO42-),pesticides(organochlorine pesticides,organo?phosphorus pesticides),nitrobenzene,and so on[4?8],which can be attributed to their high surface areas,diverse chemical and physical properties,excellent flu?orescence properties and abundant action sites.Among them,these MOFs based ond10metal centers(Zn2+,Cd2+)and the conjugated ligands have been potentially used as photoluminescent probe materials to detect pol?lutants due to their excellent fluorescence properties.Nevertheless,the stability of MOFs is still a significant challenge for practical applications as fluorescence sensors in an aqueous solution.
The hard?soft?acid?base(HSAB)theory indicates that the Znis a relatively soft?acid ion,which has stronger coordination bonding with the soft?base N ?donor ligands[9].But the strong coordination bonds could lead to poor crystallinity.Based on the guide?lines,we chose mixed ligands(rigid N?donor ligand and flexible carboxylic ligand)to construct a 1D rhom?bic nanotube structure with high stability and crystal?linity,[Zn2(bcpp)2(1,4?bib)2]·1.5H2O(1)(H2bcpp=3,5?bis(4?carboxylphenoxy)pyridine,1,4?bib=1,4?bis(1?imidazoly)benzene).Based on the high water stability and excellent luminescent property,complex 1 was used as a multi?responsive sensor to detect metal ions and pesticides in aqueous solutions and real samples.
1 Experimental
1.1 Materials and methods
All reagents and materials were commercially available and used directly without further purification.The ligands H2bcpp and 1,4?bib were obtained from Jinan Henhua Sci.&Technol.Co.,Ltd.Powder X?ray diffraction(PXRD)patterns were performed with a Rigaku Dmax 2500 diffractometer(XRD,CuKα,λ=0.154 nm,U=40 kV,I=25 mA,2θ=5°?50°).Infrared spectra were obtained at FTIR?8400S spectrometer and thermogravimetric analysis(TGA)curves were carried out with a ZCT?A analyzer.UV?Vis absorption experi?ments were performed with a Shimadzu UV?2600 spec?trophotometer.Photo?luminescence spectra were taken on a Hitachi F?4600 fluorescence spectrophotometer.
1.2 Synthesis of[Zn2(bcpp)2(1,4?bib)2]·1.5H2O(1)
A mixture of Zn(NO3)2·6H2O(0.016 mmol,4.8 mg),H2bcpp(0.008 0 mmol,2.8 mg),1,4?bib(0.016 mmol,3.4 mg)in EtOH/H2O(4.0 mL,1∶3,V/V)was sealed in a 25 mL reactor,heated to 120℃for 55 h,and then cooled to room temperature.Colorless block crystals were obtained by washed with EtOH,filtration and dried in air.Anal.Calad.for C68H52N8O8Zn2(%):C,65.81;H,4.19;N,9.03.Found(%):C,65.77;H,4.22;N,9.06.IR(KBr,cm-1):3 789(w),3 122(w),2 358(s),1 674(m),1 677(w),1 569(m),1 554(m),1 539(m),1 521(w),1 488(m),1 452(m),1 429(m),1 406(m),1 379(m),1 307(m),1 294(m),1 263(m),1 026(m),999(m),954(w),838(m),729(m),653(m)(Fig.S1,Support?ing information).
1.3 Crystal structure determination
The crystal data of 1 were collected on a Rigaku Oxford Diffraction XtaLAB Synergy?S diffractometer with CuKαradiation(λ=0.154 184 nm)at 150 K.Its structure was solved by the superfilp method in Olex2 program.All non?hydrogen atoms were refined onF2with full?matrix least?squares procedures in SHELXL?2016.Some free disordered solvent molecules were removed with the PLATON/SQUEEZE routine.The SQUEEZE result reveals a residual electron density of 141 electrons/cell(Z=8)which corresponds to the residual electron density of 1.5 H2O molecules.The free 1.5 H2O molecules were also proved by the TGA and elemental analysis.The crystal data and refined parameters are presented in Table 1.Some selected bond distanced and angles are listed in Table S1.

Table 1 Crystal data and refined parameters of complex 1
CCDC:2095435.
2 Results and discussion
2.1 Crystal structure of complex 1
Single?crystal X?ray diffraction analysis presents that complex 1 crystallizes in the monoclinic with space groupI2/a.Fig.1a displays that the asymmetric unit contains two Znions,two bcpp2-ligands,two 1,4?bib ligands,where the free disordered solvent molecules are omitted.The dihedral angles between two benzene rings in two different bcpp2-ligands are 40.941(331)°and 50.180(265)°,respectively(Fig.S2).Both Znions are four?coordinated by two nitrogen atoms from different 1,4?bib ligands and two oxygen atoms from two bcpp2-ligands with a distorted tetrahe?dral geometry.The average bond distances of Zn—N and Zn—O are in the ranges of 0.200 2?0.202 7 nm and 0.187 7?0.202 8 nm,respectively,which are in accordance with the reported values previously[10].
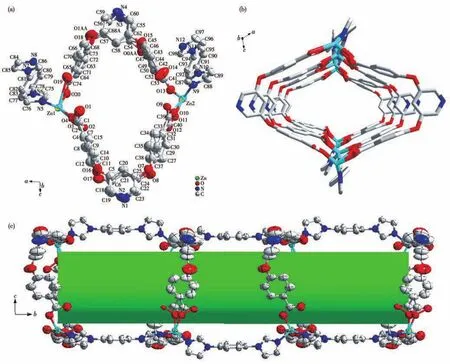
Fig.1 Asymmetric unit of 1 with 50% thermal ellipsoid probability(a);1D tubular structure of complex 1 along b axis(b)and along a axis(c)with 50% thermal ellipsoid probability
In complex 1,H2bcpp ligands are completely deprotonated.Each carboxylate group of bcpp2-bridges one Znion,which exhibits the coordination mode ofη1.Two bcpp2-ligands bridge two Znions to form a ring.The adjacent rings are linked by two 1,4?bib ligands,to form a 1D tubular structure with a rhombic channel(the diagonal length of 1.5 nm×1.9 nm,Fig.1b and 1c).Because of the large rhombic windows in 1,the adjacent 1D tubular structures are interpenetrated with each other.Moreover,the imidazole rings of the adjacent 1,4?bib ligands exist twoπ…πstacking inter?actions with face?to?face fashion,but two imidazole rings are not exactly parallel(Fig.2a).One is theπ?stacking interaction between Cg1 plane and Cg3 plane(Cg1:N5?C75?N6?C77?C76,Cg3:N9?C87?N10?C89?C88,centroid…centroid 0.376 93 nm,dihedral angle 4.7°).The other is theπ?stacking interaction between Cg2 plane and Cg4 plane(Cg2:N7?C85?C84?N8?C86,Cg4:N11?C96?C97?N12?C98,centroid…centroid 0.378 77 nm,dihedral angle 1.4°).The adjacent 1D tubular structures are interpenetrated and linked by weakπ?stacking interactions to form a 3D supramolec?ular structure(Fig.2b).
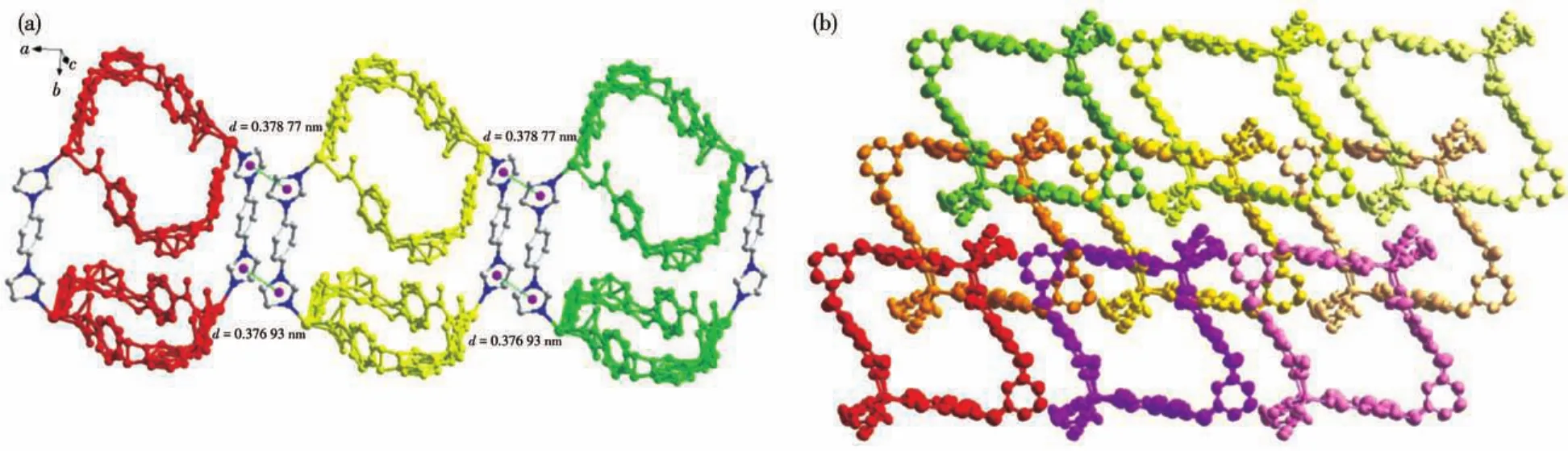
Fig.2 (a)π?stacking interaction in complex 1;(b)3D supramolecular structure of 1 viewed along b axis with 50% thermal ellipsoid probability
2.2 PXRD and TGA
The thermal stability of 1 was studied under an N2atmosphere from 50 to 700℃(Fig.S3).Complex 1 displayed a weight loss of 2.2% between 50 and 113℃because of the loss of one and a half free H2O mole?cules(Calcd.2.1%).There was a platform from 113 to 326℃.Subsequently,there was a rapid weight loss beyond 326℃that indicates the decomposition of 1.Furthermore,the PXRD pattern of 1 can match the simulated one,indicating its phase purity.The PXRD pattern of 1 after being soaked in boiling water almost had no change compared with the simulated one,indi?cating its water stability(Fig.3).
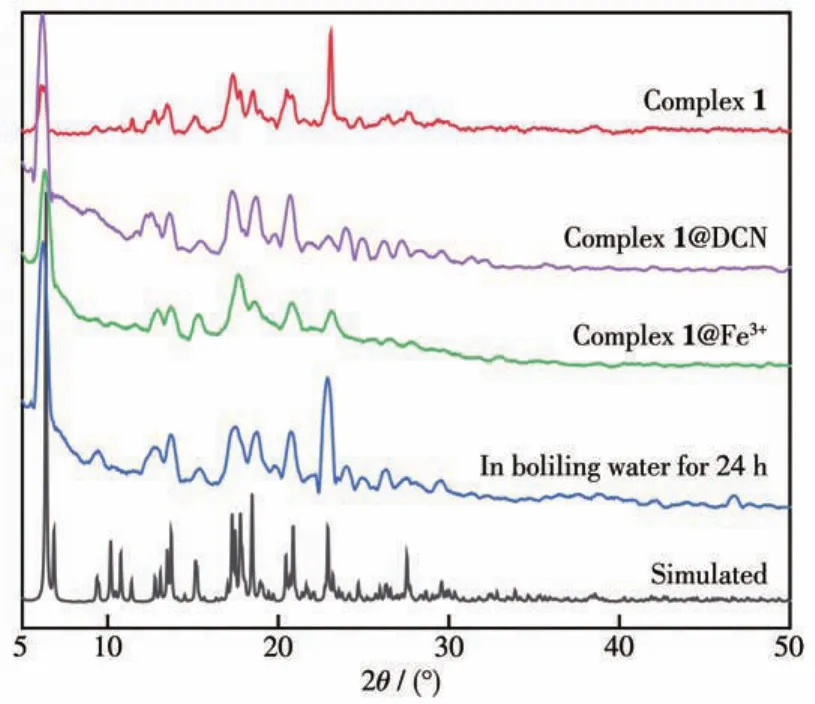
Fig.3 PXRD patterns of complex 1,1 after soaking in boiling water for 24 h,and 1 after detecting various analytes
2.3 Photoluminescence properties
The solid?state luminescence emission spectra of H2bcpp(λex=355 nm),1,4?bib(λex=353 nm),and 1(λex=355 nm)were investigated at room temperature(Fig.4).H2bcpp and 1,4?bib showed the main emission peaks at 463.8 and 475.2 nm,respectively.Their emission peaks may be ascribed toπ* →πorπ* →n[11?12]tran?sitions.Complex 1 exhibited a single intense broad emission band at 461.4 nm,which was close to the emission peak of H2bcpp.Compared with the emission peaks of H2bcpp and 1,4?bib,complex 1 presented a 2.4 nm blue shift and 13.8 nm blue shift in the lumi?nescence emission spectrum,respectively.Considering that Zn2+ions have ad10configuration,the lumines?cence emission spectrum of 1 can be assigned to the metal?to?ligand charge transfer(MLCT)or ligand?to?metal charge transfer(LMCT)[13?18].The slightly blue ?shifted emission bands can be attributed to the coordi?nation effects.Based on the good luminescence property of complex 1,we further studied the luminescence sensing activity of complex 1.
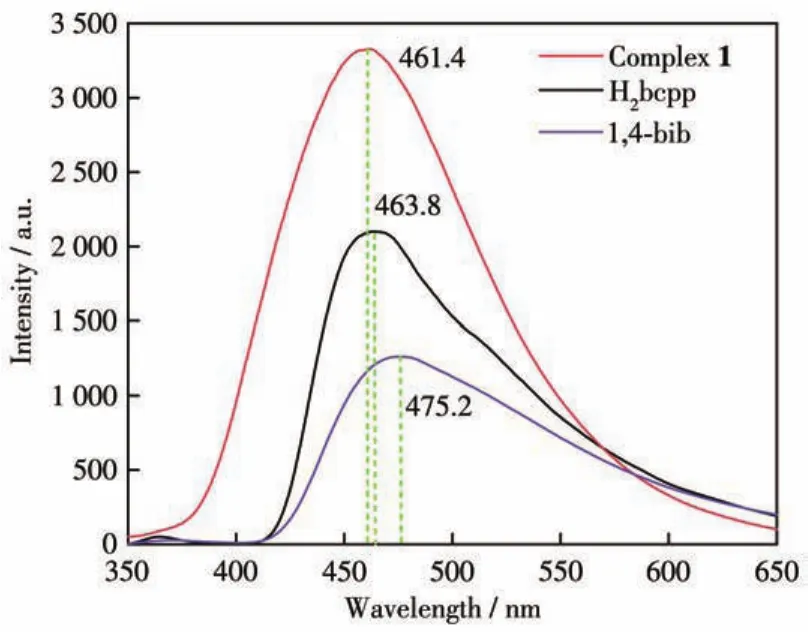
Fig.4 Solid?state luminescence emission spectra of H2bcpp,1,4?bib,and complex 1 at 298 K
2.4 Selective detection of Fe3+ions
The detection ability of complex 1 for Fe3+ions has been explored in an aqueous solution.The powder sample of 1(2.0 mg)was evenly dispersed into 2.0 mL of 10 mmol·L-1M(NO3)naqueous solution(Mn+=Na+,Zn2+,Ni2+,Mg2+,Mn2+,Ca2+,Ba2+,Cu2+,Cd2+,Pb2+,Co2+,Cr3+,Fe2+,Al3+,Fe3+).The fluorescence emission spec?tra of the dispersed suspensions were measured atλex=355 nm.As shown in Fig.5a,the luminescence intensi?ty of 1 has an almost completely quenching with the addition of Fe3+,indicating the excellent sensing ability for Fe3+.The titration experiment was further performed to explore the sensing ability for Fe3+ions(Fig.5b).With adding the Fe3+solution,the fluorescence intensi?ty of 1 dropped gradually with a high fluorescent quenching efficiency of 93.3% in the presence of 1.3 mmol·L-1Fe3+solution.Moreover,the low Fe3+concen?tration and the fluorescence intensity exhibited a good linear relationship(Fig.5c),which can be fitted to a line by Stern?Volmer relationship:I0/I=cFe3+Ksv+1,whereI0andIare the luminescence intensities of 1 without and with the addition of Fe3+,respectively;Ksvis the Stern?Volmer constant.TheKsvwas calculated to be 4.90×103L·mol-1.The limit of detection(LOD)was about 1.8 μmol·L-1calculated by 3σ/Ksv[19].Compared with some reported MOFs?based fluorescence sensors,complex 1 exhibited a comparable or lower LOD for sensing Fe3+in an aqueous solution(Table S2).
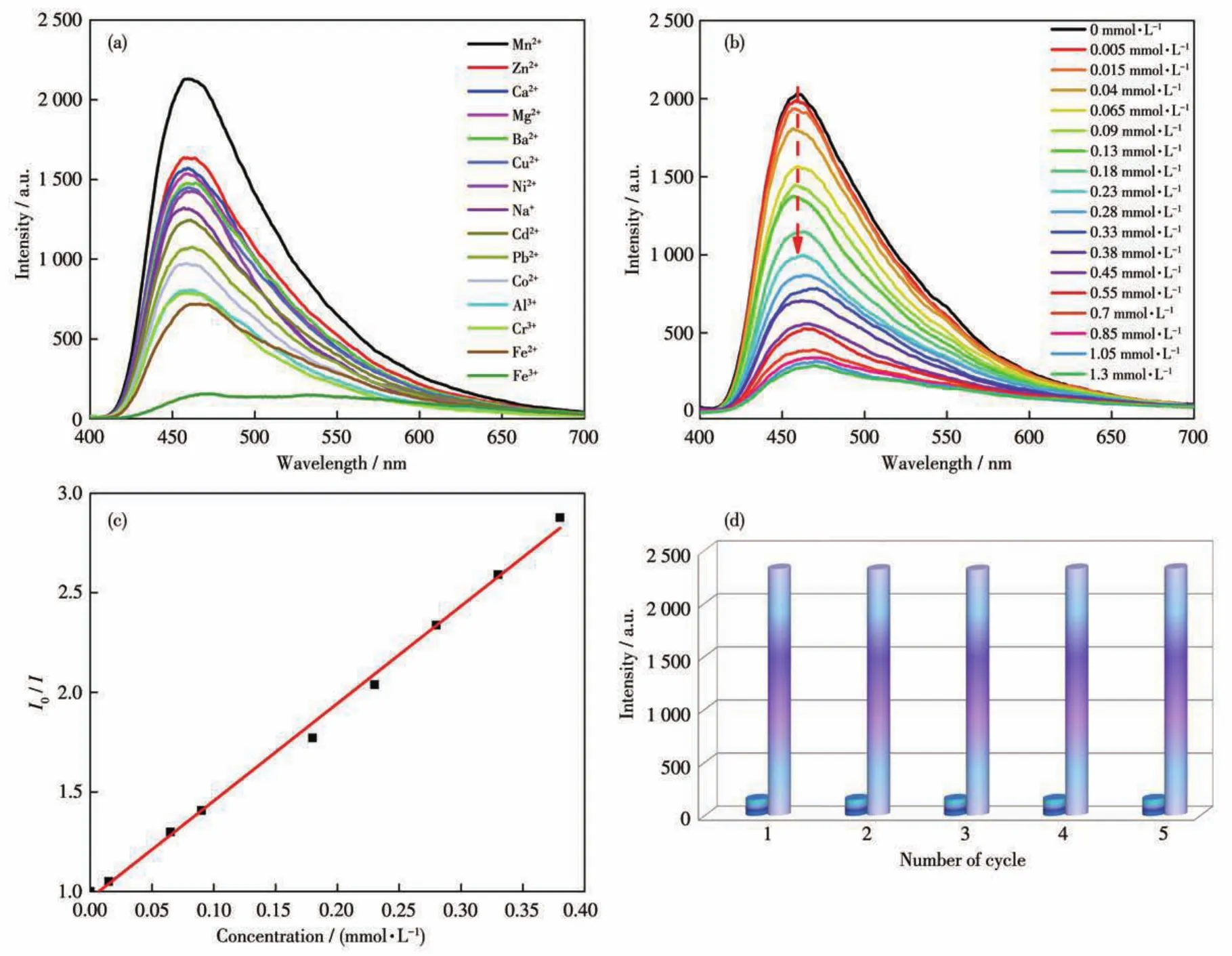
Fig.5 (a)Fluorescence intensity of 1 in aqueous solutions containing various metal cations at 461.4 nm(λex=355 nm);(b)Titration experiment of 1 for detecting Fe3+;(c)Stern?Volmer plot of 1 for sensing Fe3+in aqueous solution;(d)Repeatability experiment of 1 for detecting Fe3+
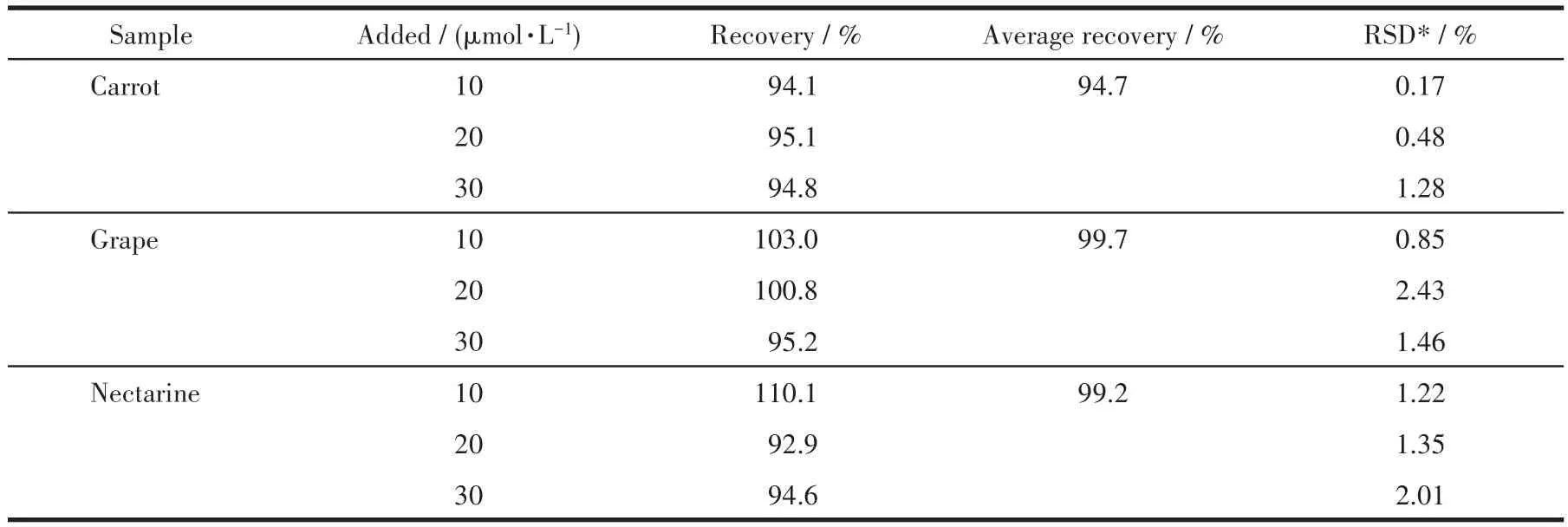
Table 2 Determination of DCN in real samples
Furthermore,selectivity and recyclability are also important for evaluating the detecting ability of 1.Thus,the anti?interference fluorescence detection ex?periments for Fe3+ions were explored by adding Fe3+ions into the suspension of complex 1 with various metal cations.It was found that the Fe3+for the fluores?cence intensity of complex 1 still had an outstanding quenching effect(Fig.S4).The result indicates that complex 1 exhibits good selectivity for Fe3+.In addi?tion,the recyclability of 1 for the detection of Fe3+was verified by the cyclic tests.After repeated for five cycles,the fluorescence sensitivity remained almost unchanged(Fig.5d).The results suggest that complex 1 has high sensitivity,selectivity,and recyclability for sensing Fe3+.In addition,we conducted a time?dependent experiment and found that the fluorescence intensity decreased rapidly in 30 s and remained unchanged in 360 s(Fig.S5a).Herein,the recognition mechanism of 1 for sensing Fe3+has been investigated.The PXRD peaks of complex 1 before and after recog?nizing Fe3+ions almost had no change,indicating the structure of complex 1 keeps perfectly(Fig.3).Com?pared with the UV?Vis spectra of other metal ions,the UV?Vis absorption spectra of Fe3+had the greatest extent overlaps with the excitation spectra of complex 1(Fig.S6a),which suggests that there is an inner filter effect(IFE)between complex 1 and Fe3+.The excita?tion energy of complex 1 is absorbed by Fe3+,which leads to fluorescence quenching[20?21].IR spectra,where 1@Fe3+showed new peaks at 1 745 and 1 411 cm-1compared with complex 1(Fig.S1),further indicate that there is a weak interaction between complex 1 and Fe3+.

Fig.6 (a)Fluorescence intensity of 1 in various organochlorine pesticides at 461.4 nm(λex=355 nm);(b)Titration experiment of 1 for DCN;(c)Stern?Volmer plot of 1 for sensing DCN;(d)Repeatability experiment of 1 for DCN
2.5 Selective detection of DCN
Organochlorine pesticides as a class of widely?used pesticides have gained numerous attention from environmentalists because residual organochlorine pes?ticides are harmful to the environment and human health.Thus,we try to investigate the detection ability of 1(λex=355 nm)for organochlorine pesticides in an aqueous solution.Herein,we selected eight pesticides to explore,including carbaryl,chlorobenzene(CB),atrazine,2,4?dichlorobenzene(2,4?DCP),1,2?dichloro?benzene(1,2?DiCB),1,2,4?trichlorobenzene(1,2,4?TriCB),1,2,4,5?tetrachlorobenzene(1,2,4,5?TetraCB),and DCN.As shown in Fig.6a,DCN shows the highest quenching efficiency for the fluorescence intensity of 1(81.8%).The titration experiments exhibited that the fluorescence intensity of 1 gradually reduced with adding DCN solution(Fig.6b).In addition,the DCN concentration and the fluorescence intensity of complex 1 exhibited a good linear relationship(Fig.6c).TheKsvfor DCN was 2.06×104L·mol-1,and the LOD was about 0.44 μmol·L-1,indicating the excellent sensitivity of 1 for DCN.Moreover,the selective and the anti?interference ability were investigated,which are shown in Fig.6d and Fig.S7.The time?dependence experiment of complex 1 for DCN further proves that complex 1 has high stability(Fig.S5b).The results displayed high selectivity and recyclability,indicating that complex 1 is a potential fluorescent sensor for detecting DCN.Furthermore,1 has higher sensitivity and lower LOD compared with some reported MOF fluorescent probes for detecting DCN(Table S3).
Additionally,the sensing ability of 1 for DCN in real samples,such as the concentrated juices of grapes,nectarines,and carrots,was also investigated(Fig.S8).TheirKsvand LOD values are listed in Table S4.Nota?bly,the LOD values were all lower than the maximum DCN residue reported in China National Food Safety Standards[22].The results indicate the excellent selectiv?ity,sensitivity,and recyclability of 1 for detecting DCN in aqueous solution and real samples.At the same time,complex 1 had a high recovery rate in these real samples(Table 2).Furthermore,the sensing mecha?nism of 1 was investigated.As shown in Fig.S6b,the excitation spectrum of complex 1 has a major overlap with the UV spectrum,indicating that there is an IFE[25?26]between DCN and complex 1.DCN absorbs the excitation energy of complex 1,resulting in fluores?cence quenching.
3 Conclusions
In summary,a novel water?stable Zn?based MOF(1)was constructed with a flexible carboxylic ligand 3,5?bis(4?carboxylphenoxy)pyridine and a rigid N?donor ligand 1,4?bis(1?imidazoly)benzene.Complex 1 exhibits a 1D tubular structure,where the adjacent 1D tubular structures are interpenetrated with each other to obtain a 3D supramolecular structure.Further?more,the photoluminescence property of 1 was investi?gated,which indicates that 1 can be used to detect Fe3+ion and pesticide DCN in an aqueous solution.The LOD values for Fe3+ion and DCN in aqueous solutions are 1.8 and 0.44 μmol·L-1,respectively.The results suggested that complex 1 can be used as a“turn?off”fluorescence probe to trace Fe3+ion and pesticide DCN with high selectivity,sensitivity,and recyclability.
Supporting information is available at http://www.wjhxxb.cn
- 無機化學(xué)學(xué)報的其它文章
- 《無機化學(xué)學(xué)報》投稿須知(NOTICE TO AUTHORS)
- Replacement of Carboxylate Ligand Substituent on Modulation of Structures and Magnetic Properties in Salen?Type Dinuclear Dy Complexes
- Synthesis,Structures and Catalytic Activity in Knoevenagel Condensation Reaction of Two Diphenyl Ether Tetracarboxylic Acid?Co Coordination Polymers
- Mn?MOF Derived Mn2O3 Micromotors Applied to Removal of Methyl Blue in Water
- Crystal Structures and Magnetic Properties of LnⅢ2Complexes Based on a Polydentate Schiff Base Ligand
- Constructing and Photocatalytic Performance of Flower?like CeO2/TiO2 Heterostructures

