Coarse-grained simulations on interactions between spectrins and phase-separated lipid bilayers?
Xuegui Lin(林雪桂) Xiaojie Chen(陳曉潔) and Qing Liang(梁清)
1Center for Statistical and Theoretical Condensed Matter Physics and Department of Physics,Zhejiang Normal University,Jinhua 321004,China
2Department of Industrial Automation,Guangdong Polytechnic College,Zhaoqing 526100,China
Keywords: protein,lipid bilayer,interaction,molecular dynamics simulation
1. Introduction
Erythrocyte is a specific cell without nucleus and most organelles.[1,2]The plasma membrane of erythrocyte is associated with a cytoskeleton composed of erythroid spectrins(referred to herein as spectrins) and some other membrane proteins.[2–4]The interaction between spectrins and erythrocyte membrane is considered as a key factor determining the erythrocyte structural stability and flexibility which are of great importance for the realization of the biological functions of erythrocyte.[5–11]Therefore,it is essential to understand the microscopic mechanism of interactions between spectrins and erythrocyte membrane from the molecular scale.
The single chain of spectrin skeleton consists of two heterodimers, theαandβspectrins, which form antiparallel heterotetramers by laterally interacting with each other.[3,4,12]In the cytoskeleton of the heathy erythrocyte, theαandβspectrins interact with high affinity at the tetramerization site and form a stable network.[13,14]However,if some mutations of amino acids occurs at the tetramerization site, the affinity of theαandβspectrins may be considerably influenced and the stability of the erythrocyte cytoskeleton will be reduced, resulting in the deformation of erythrocyte and some blood diseases such as hereditary elliptocytosis (HE), hereditary pyropoikilocytosis (HPP), and hereditary spherocytosis(HS).[15–22]
The predominant lipids of the plasma membrane of erythrocyte are cholesterol (CHOL) and phospholipids, and the molar ratio of CHOL: phospholipids is about 0.90. The main phospholipids include phosphatidylcholine (PC), phosphatidylethanolamine (PE), sphingomyelin (SM), and phosphatidylserine(PS).In addition,the lipids distribute asymmetrically in the two leaflets of the membrane. PS and PE almost exclusively distribute in the inner leaflet while the outer leaflet of the membranes is mainly composed of PC and SM.[23,24]
The spectrin skeleton anchors to the erythrocyte membrane mainly though connecting with some integral/peripheral membrane proteins or protein complexes.[3,4,17,23,25–35]However,more and more evidences indicated that the direct interactions between spectrins and lipids could also be crucial for the structural stability and function of erythrocyte.[36–40]It has been shown that there are some specific binding sites on spectrins for PC,PE,and PS lipids in the inner leaflet of erythrocyte membrane,and theαandβspectrins interact differently with the same kind of lipids.[41–43]Additionally, CHOL and phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2, referred to as PIP2 in this work)also play important roles in the interactions of spectrins with erythrocyte membrane.[44–47]
Furthermore, spectrins can also influence the nano-sized domain formation in the erythrocyte membrane and exhibit different affinities with the liquid-ordered(Lo)domains(e.g.,lipid rafts)and the liquid-disordered(Ld)domains in the erythrocyte membrane.[48–54]Therefore,the interactions between spectrins and the plasma membrane of erythrocyte are extremely complicated and still remain elusive. More efforts are necessary to reveal the microscopic mechanism of the interactions between spectrins and erythrocyte membrane, in particular,at the molecular scale.
In this work, using coarse-grained molecular dynamics(MD) simulations, we investigate the interactions of spectrins with the phase-separated lipid bilayers composed of saturated lipids (e.g., dipalmitoyl-PC (DPPC), dipalmitoyl-PE (DPPE), or PIP2), unsaturated lipids (e.g., dilinoleoyl-PE (DIPE), dilinoleoyl-PC (DIPC), or palmitoyl-oleoyl-PS(POPS)),and CHOL.We try to reveal the microscopic mechanism of the affinities of spectrins with different lipids and Lo/Ld domains, and the effects of charged lipids and spectrin mutation on the spectrin–membrane interactions at the molecular scale. The simulations provide some new theoretical insights which are helpful for understanding the molecular structure of erythrocyte as well as the molecular mechanism of some relevant blood diseases.
2. Model and methods
2.1. System setup
2.1.1. Lipid bilayers
To capture the main structural and componential properties of erythrocyte membrane and simulate the lipid bilayers with relatively large size for time of microsecond(μs)length,we simplified the components of the plasma membrane of erythrocyte and built some ternary and quaternary lipid bilayers composed of the main components of erythrocyte membrane,PC, PE, PS, PIP2, and CHOL, with the coarse-grained Martini model.[55–57]The headgroups of PC and PE are zwitterionic while those of PS and PIP2 are anionic. PS carries one negative charge on the headgroup. PIP2 carries five negative charges in the unprotonated state since salt was added in our systems,[58]and its molecular structure was adopted from Ref. [59]. The coarse-grained molecular structures of the lipids used in our simulations are presented in Fig. 1(a)and the compositions of all the lipid bilayers are summarized in Table 1. Here, the tails of DPPC, DPPE,or PIP2 are fully saturated while those of DIPC, DIPE, or POPS are unsaturated. For all the lipid bilayers,the leaflet directly interacting with the spectrins is the inner leaflet and the other leaflet is the outer leaflet. The previous work has shown that the membrane composed of saturated lipids, unsaturated lipids, and cholesterol can spontaneously separate into Lo and Ld domains at 305 K.[60]In this work, the initial distribution of the lipids is homogeneous and the phase separation of the Lo and Ld domains spontaneously occurs in all the systems within several microseconds.
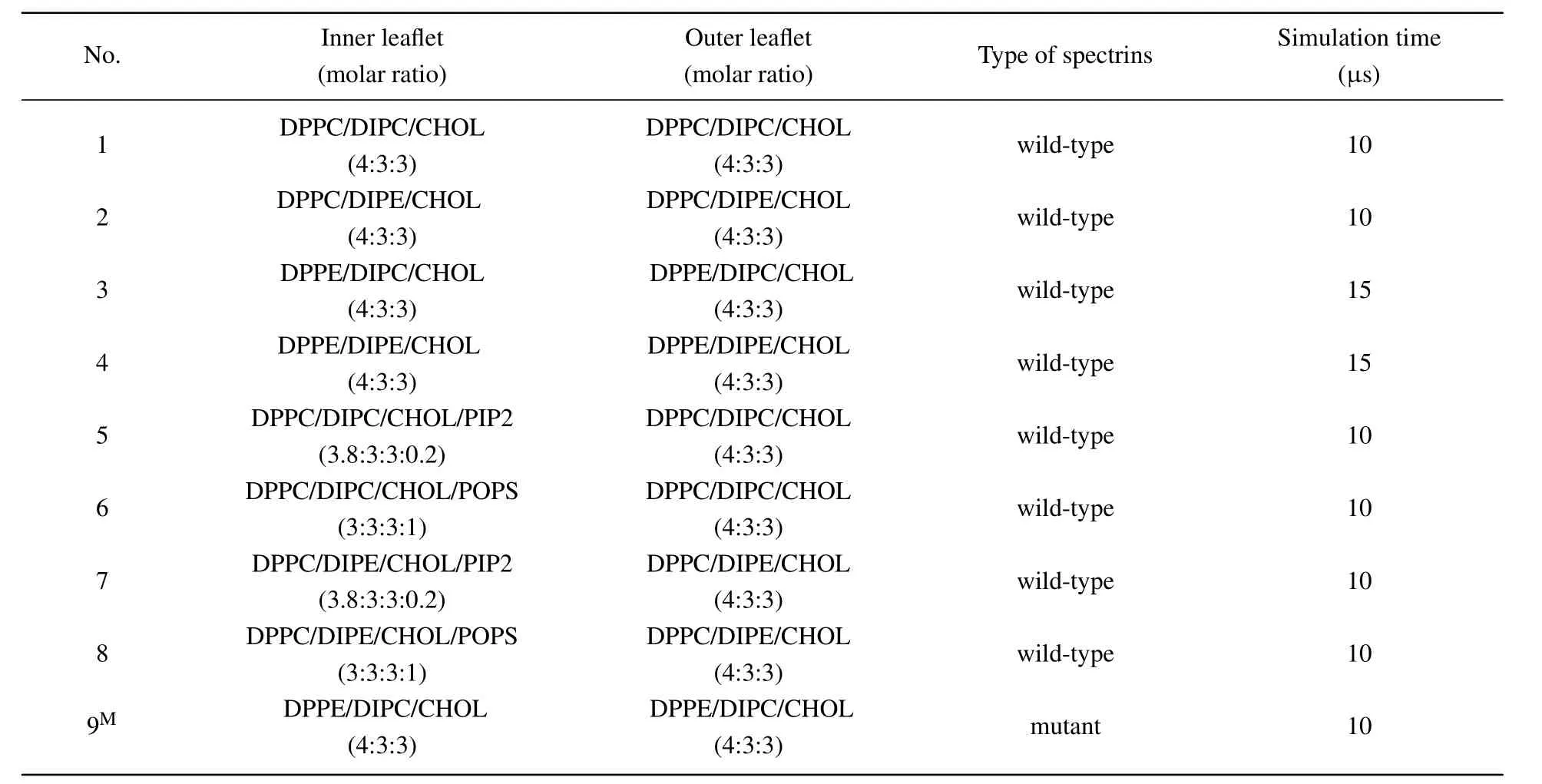
Table 1. Overviews of the lipid compositions in the two leaflets of the lipid bilayers, the spectrin type in the corresponding system, and the simulation time. The spectrins are mutant with the mutation sites of W263R and W300R on the β17 spectrin in the last system marked with‘M’.
2.1.2. Spectrin
The structure of spectrin domains(PDB ID:3LBX)used in this work is shown in Fig. 1(b). The spectrins contain four homologous repeats: the repeats 0 and 1 of N-terminus of theαspectrins (α0 andα1, residues 19–158) and the repeats 16 and 17 of C-terminus of theβspectrins (β16 andβ17, residues 1900–1931 and 1934–2083 ).[12]The spectrin repeats interact head-to-head betweenα0 andβ17 through non-bonded interactions. The atomistic structure of the spectrins was coarse-grained with Martini model and the residues of the coarse-grained spectrins are renumbered from 1 to 322.Correspondingly,the residues 1–140 belong to theαspectrins while the residues 141–322 belong to theβspectrins in the coarse-grained model. Besides the wild-type spectrins, we also examined the interactions of the mutant spectrins with the lipid bilayer composed of DPPE/DIPC/CHOL.Here,we considered the mutant spectrins with the mutation sites of W263R and W300R (W2024R and W2061R in the original atomistic structure)on theβ17 spectrin as shown in Fig.1(c). Namely,the amino acids of tryptophan(W)at the positions of 263 and 300(2024 and 2061 in the original atomistic structure)in the wild-typeβ17 spectrin are replaced by arginine (R), respectively. This type of mutation is considered one of the causes of the blood disease of HE.[22]

Fig. 1. (a) Coarse-grained (CG) molecular structures of the lipids used in this work. The lipids are shown in colors as follows: DPPC: red; DPPE:magenta;DIPC:pink;DIPE:blue;PIP2: yellow;POPS:green;CHOL:gray.This coloring scheme is used throughout the paper. (b)Atomistic and coarsegrained structures of the α (white)and β (cyan)spectrins. (c)Illustration of the mutation sites of W263R(yellow)and W300R(red)on the β17 spectrin.
All the lipid bilayers with the spectrins were built using the script of INSANE provided by the Martini developers.[61]To save the simulation time for the adsorption of the spectrins onto the surface of the lipid bilayers,we put the spectrins near the surface of inner leaflets of the bilayers and the distances between them are about 1.4 nm–2.5 nm. The initial box size of the simulations is about 20 nm×20 nm×30 nm and periodic boundary conditions were used in all three directions. The size of the simulation box inzdirection was set relatively large to ensure the free adjustment of the spectrins.In all ternary lipid bilayers, the molar ratio of the saturated lipids, the unsaturated lipids, and CHOL molecules is 4:3:3.To examine the effect of charged lipids on the interactions between spectrins and the lipid bilayers,some saturated lipids in the inner leaflet of the ternary bilayers were substituted by the anionic lipids POPS or PIP2. For the convenience of comparison,we kept the charge number of the anionic lipids the same in the charged bilayers,i.e., the fractions of PIP2 and POPS are 2 mol% and 10 mol%, respectively, since the number of charged beads in the headgroup of PIP2 is five times larger than that of POPS.Each system contains about 8×104coarsegrained water beads to solvate the lipid bilayer. Furthermore,0.15-M salt was added into each system to mimic the physiological conditions and additional sodium ions were introduced to maintain electroneutrality of the systems containing anionic lipids(PIP2 or POPS).
2.2. Coarse-grained MD simulations
All the systems were simulated using Martini coarsegrained force field. Although ignoring the hydrogen-bonding interactions, some molecular details of lipids, and some secondary structural details of proteins,the Martini model,which used a four-to-one mapping scheme, is still one of the most successful models for predicting theμs-long dynamical properties of complex lipid bilayers and its interactions with proteins or nanoparticles.[56,62–65]In many cases, Martini model got good agreement with the atomistic models or experiments due to its good optimization on the parametrization of the lipid–lipid and lipid–protein interactions.[56,62,66]In the present simulations, the non-bonded interaction parameters were set as follows: the Coulomb interaction was shifted to zero between 0 nm and 1.2 nm and the Lennard–Jones interaction was shifted to zero between 0.9 nm and 1.2 nm. The NPT ensemble was used for the simulations. The temperature of all the simulations was maintained at 300 K using velocityrescaling coupling scheme with a relaxation time of 1 ps.[67]The bilayer(xy)plane and the normal(z)direction were separately coupled to a 1-bar pressure bath with a relaxation time of 2 ps by using the semi-isotropic Parrinello–Rahman pressure coupling scheme.[68,69]The compressibility of systems was set as 3×10?4bar?1(1 bar=105Pa)in both lateral and normal directions of the bilayer.After energy minimization and a 1-ns equilibrating simulation,except for the systems 3 and 4 which were simulated for 15μs,the other systems were simulated for 10μs. To ensure the reproducibility of the results, each simulation was run for 2–3 replicas with the same initial states of the lipid bilayers and proteins but different distances between them. The GROMACS package(version 2019.3)was used to perform the simulations with an integration time-step of 20 fs.
2.3. Data analysis
We used Visual Molecular Dynamics (VMD) to visualize the structures of the lipid bilayers and the spectrins.[70]To characterize the molecular details of the interactions between the spectrins and lipid bilayers and the structural stability of the spectrins,some tools from the GROMACS package were used to analyze the results. The tool ofgmx mindistwas adopted to calculate the numbers of contacts between the spectrins (or amino acids) and the headgroups of DPPC, DIPE,DPPE, DIPC, and CHOL molecules with a cutoff length of 0.6 nm as a function of simulation time. The tool ofgmx rmswas used to calculate the root-mean-square deviation(RMSD)of the spectrins. The RMSD is the most commonly-used function for characterizing the variation of the protein configuration at certain time(t)compared with the reference configuration of the protein at the initial time(t=0)and defined as
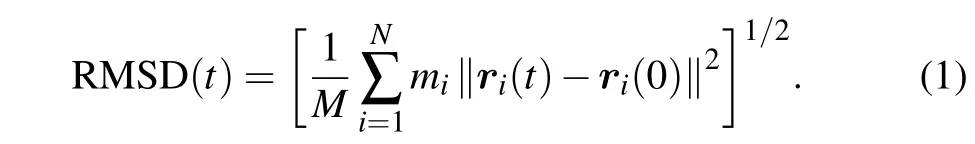
Here,miis the mass of thei-th atom on the protein backbone,andri(t)is the position of thei-th atom at timet;Nis the total number of the atoms on the protein backbone;Mis the total mass of the atoms on the protein backbone andM=∑Ni=1mi.
Additionally, to examine the effect of the spectrin binding on the structure of the lipid bilayer, we also analyzed the curvature of the lipid bilayers by projecting the coordinates of the lipid headgroups of the inner leaflet to a two-dimensional plane and fitting with a curve and the maximal curvature of the curve was used to estimate the curvature of the lipids.[71]
3. Results and discussion
3.1. Interactions between spectrins and lipid domains of the neutral lipid bilayers
We first examine the interactions of the spectrins with the ternary bilayers only containing neutral lipids. We considered four lipid bilayers composed of DPPC/DIPC/CHOL, DPPC/DIPE/CHOL,DPPE/DIPC/CHOL, and DPPE/DIPE/CHOL, respectively,with the molar ratio of 4:4:3. Figure 2 shows the final structures of the 10-μs or 15-μs simulations on these lipid bilayers with the spectrins and the contact numbers of theαandβspectrins with the lipids during the simulations. The results show that the spectrins exhibit different interactions not only with PE and PC lipids but also with saturated and unsaturated lipids.
For the ternary lipid bilayer composed of DPPC, DIPC,and CHOL (Fig. 2(a)), both theαandβspectrins contact weakly with the bilayer only near the end of the simulation time while they do not tend to contact with the bilayer during most of the simulation time, which indicates that the interaction between the spectrins and PC lipids is very weak. For the bilayers containing PE lipids (Figs. 2(b)–2(d)), the spectrins are steadily adsorbed on the surface of the bilayers. However,the spectrins interact with saturated and unsaturated PE lipids differently in the different lipid bilayers. In the lipid bilayer composed of DPPC/DIPE/CHOL, the spectrins are adsorbed on the surface of Ld domain enriched in unsaturated DIPE.In the bilayer composed of DPPE/DIPC/CHOL,the spectrins prefer to contact with the Lo domains enriched in the saturated DPPE. In the bilayer composed of DPPE/DIPE/CHOL,in most time of the simulation, the spectrins show no obvious preference with either DPPE or DIPE, and the contacts between the spectrins with DPPE and with DIPE are comparable. Only in the last 2μs,the number of contacts between the spectrins and DIPE becomes slightly larger than the number of contacts between the spectrins and DPPE, which implies that the spectrins tend to contact with the DIPE-enriched Ld domain.
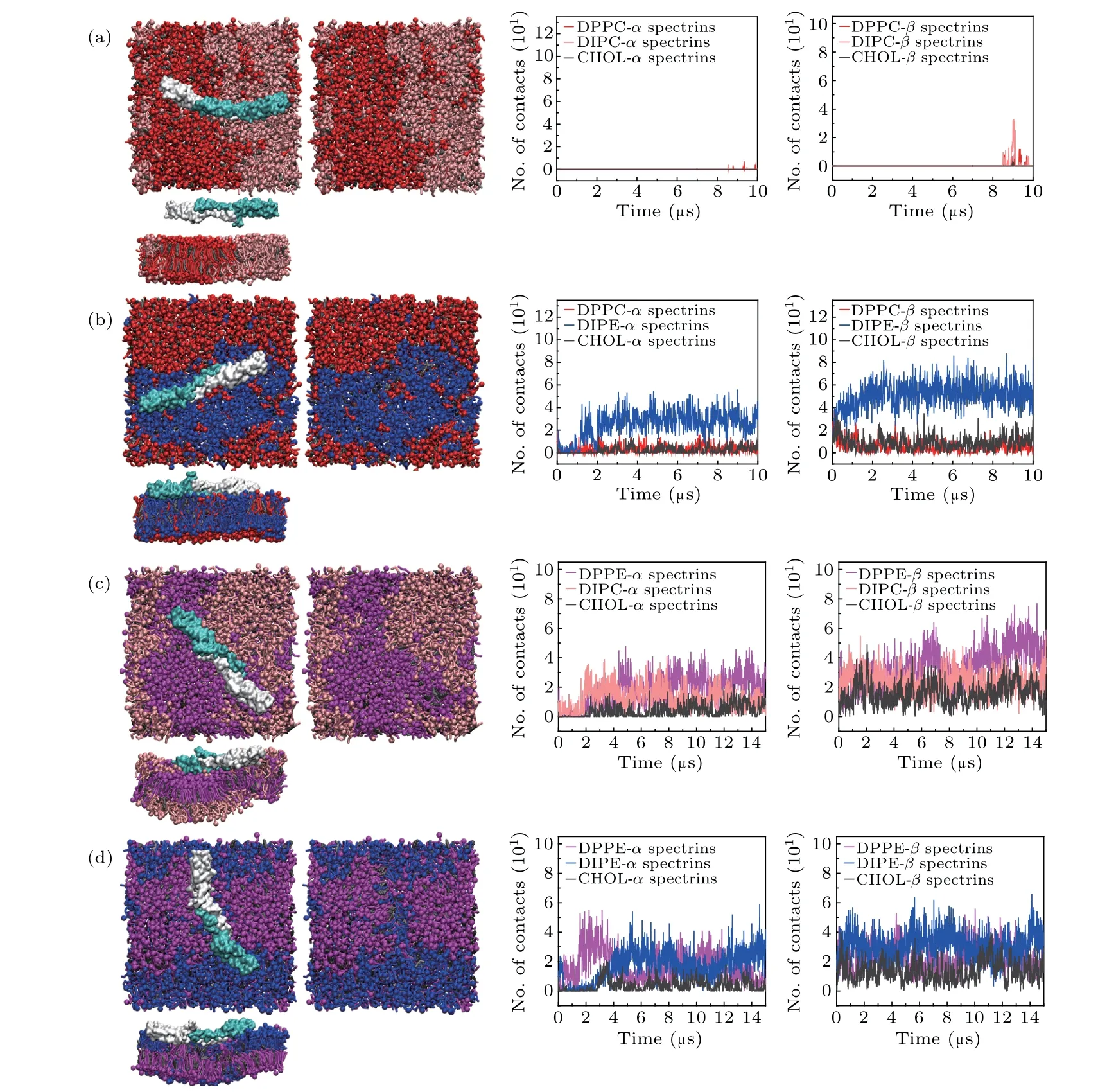
Fig. 2. Top views and lateral views of the final structures of the lipid bilayers composed of (a) DPPC/DIPC/CHOL, (b) DPPC/DIPE/CHOL,(c) DPPE/DIPC/CHOL, and (d) DPPE/DIPE/CHOL, with the spectrins, and the numbers (No.) of contacts between the α (β) spectrins and the lipid headgroups. For clarity,the structures of the lipid bilayers with the spectrins displayed and not displayed are both presented,and water and ions are not shown.
The results of these four systems show that the spectrins exhibit a stronger interaction with PE lipids than with PC lipids,indicating that there are some specific binding sites for PE on the surface of the spectrins. This result agrees well with some experimental results.[41,42]Additionally, the affinity of the spectrins with the Lo and Ld domains is determined by the compositions of the lipid bilayers. For the bilayer of DPPE/DIPC/CHOL(Fig.2(c)),because of the stronger attraction between the spectrins and DPPE, the spectrins prefer to contact with the DPPE-enriched Lo domain. For the bilayers with DIPE-enriched Ld domains(Figs.2(b)and 2(d)),the spectrins tends to contact with the Ld domains. This is possibly because that the disordered packing of DIPE in the Ld domains facilitates the binding or insertion of the spectrins onto/into the lipid domains.[35]
Furthermore, comparing the contact numbers of theαand theβspectrins with the lipids shown in Fig. 2, we find that the numbers of the contacts of theβspectrins with the lipids are obviously larger than those of theαspectrins with the lipids. Therefore, the binding ofβspectrins dominates the interactions between the spectrins and lipid bilayers,which also agrees well with the experimental result.[43]
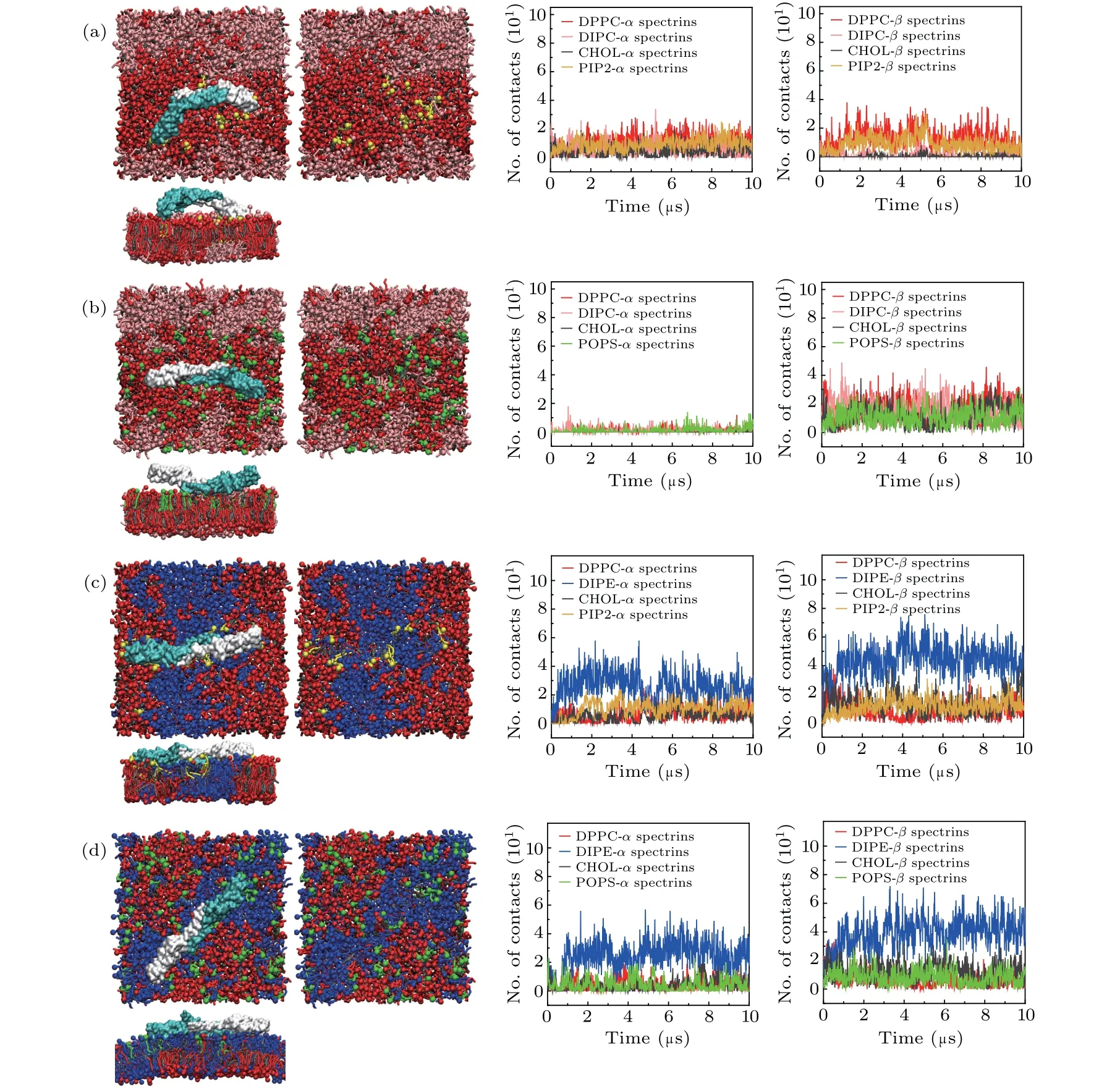
Fig. 3. Top views and lateral views of the final structures of the spectrin-bound lipid bilayers composed of (a) DPPC/DIPC/CHOL/PIP2,(b)DPPC/DIPC/CHOL/POPS,(c)DPPC/DIPE/CHOL/PIP2,and(d)DPPC/DIPE/CHOL/POPS,with the spectrins,and the numbers(No.) of contacts between the spectrins and the lipid headgroups.
3.2. Effects of anionic lipids on the interactions between spectrins and lipid domains
The anionic lipids such as PS and PI are important components of the inner leaflet of erythrocyte membrane. To reveal the effect of anionic lipids on the interactions between the spectrins and erythrocyte membrane, we substituted some of the saturated lipids with the anionic lipids PIP2 or POPS in the inner leaflet of the ternary lipid bilayers of DPPC/DIPC/CHOL and DPPC/DIPE/CHOL, but kept the compositions of the outer leaflets unchanged. We examined the interactions between the spectrins and the lipid bilayers with the inner leaflets composed of DPPC/DIPC/CHOL/PIP2,DPPC/DIPC/CHOL/POPS, DPPC/DIPE/CHOL/PIP2, and DPPC/DIPE/CHOL/POPS, respectively, as presented in Table 1 and the simulation results are shown in Fig.3.
In the lipid bilayers with the inner leaflets of DPPC/DIPC/CHOL/PIP2 and of DPPC/DIPC/CHOL/POPS,after introducing the anionic lipids which mainly distribute in the Lo domains,the spectrins are attracted by the anionic lipids and adsorbed onto the surface of the PIP2-or POPS-containing Lo domains as shown in Figs. 3(a) and 3(b). We also calculated the numbers of contacts between the headgroups of the anionic lipids and the amino acids on the spectrin surface as shown in Fig.4. We find that the main binding sites of the anionic lipids on the spectrin surface are the positively charged(Lys, Arg) or polar (Thr, Ser) amino acids. Therefore, the binding of the spectrins on the surface of the Lo domains in Figs. 3(a) and 3(b) is dominated by the electrostatic interactions between the anionic lipids(PIP2 or POPS)and the positively charged or polar amino acids on the spectrin surface since the interactions between the spectrins and PC lipids is very weak as shown in Fig. 2(a). Additionally, as shown in Fig.4,PIP2 and POPS bind to the spectrins at different sites,indicating they show specific interactions with the spectrins,respectively.
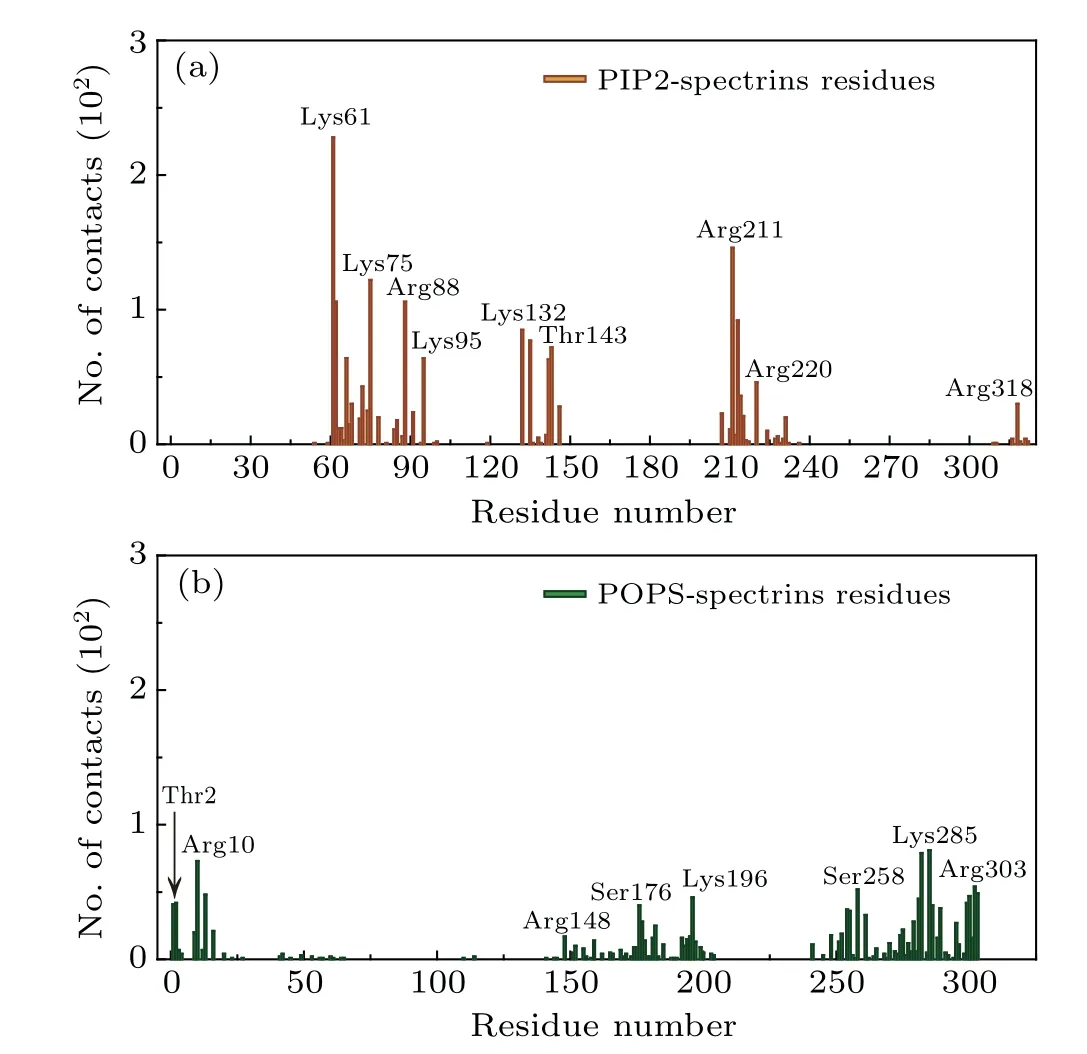
Fig. 4. The numbers of contacts between the headgroups of the anionic lipids (PIP2 and POPS) and the amino acids on the surface of the spectrins which are adsorbed on the surface of the lipid bilayers composed of DPPC/DIPC/CHOL/PIP2 and DPPC/DIPC/CHOL/POPS, respectively.Here,the last 1-μs trajectories of the MD simulations are used for analysis.
However,in the bilayers with inner leaflets composed of DPPC, DIPE, CHOL, and PIP2 (or POPS) (Figs. 3(c) and 3(d)),the interaction between the spectrins and DIPE is strong and the molar ratio of DIPE is much larger than that of PIP2 or POPS, the interaction between the spectrins and the lipid bilayers is dominated by the attraction between the spectrins and DIPE.Therefore,the spectrins are still adsorbed on the surface of Ld domain just as in the neutral DPPC/DIPE/CHOL bilayer.Furthermore, because of the electrostatic interaction between the anionic lipids and the positively charged or polar amino acids on the spectrin surface, the distribution of the anionic lipids is disturbed by the spectrins. PIP2 lipids with a lower molar fraction mainly distribute in the Ld domain while POPS lipids with a higher molar fraction mostly distribute in the Lo domain and are partially partitioned into the Ld domain by the attraction of the spectrins.
3.3. Effect of mutation on the interactions of spectrins with lipid domains
It has been shown that the mutation of the spectrins may result in the erythrocyte deformation which associates with some blood diseases in previous work.[15–22]Here, we also considered the effect of mutation on the interactions between spectrins and erythrocyte membrane. We considered the mutations of W263R and W300R in theβ17 spectrin(Fig.1(c)),which can cause the blood disease of HE.[22]We examined the interactions of the mutant spectrins with the lipid bilayers composed of DPPE/DIPC/CHOL and the simulation results are shown in Fig.5. We find that, compared with the case of the wild-type spectrins(Fig.2(c)),the contacts between theαspectrins and the Lo domains significantly increases.Most importantly, the mutant spectrins become more strongly curved and the lipid bilayer is strongly bent by the spectrins correspondingly.
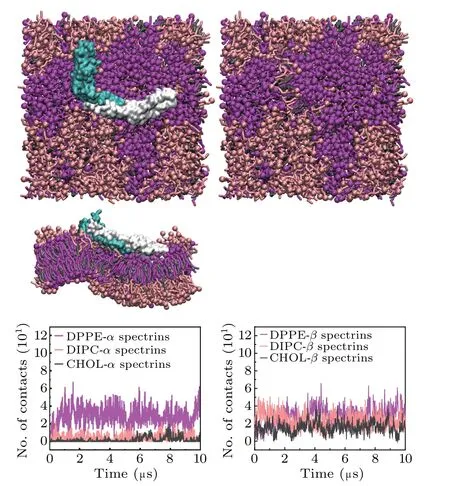
Fig.5.Top views and lateral view of the final structure of the mutant spectrinbound lipid bilayer composed of DPPE/DIPC/CHOL,with the spectrins,and the numbers of contacts between the spectrins and the lipid headgroups.Here,the mutation sites are W263R and W300R on the β17 spectrin.
We compared the RMSDs of the wild-type and the mutant spectrins(Fig.6). The results show that the RMSD of the mutant spectrins is larger than that of the wild-type spectrins,especially in the later stage of the simulation, indicating that the mutation may lead to the decrease of the structural stability of the spectrins. Because tryptophan is hydrophobic while arginine is positively-charged at physiological conditions,they have completely different properties and interact differently with the other amino acids in the spectrins. Thus, when the mutations of W263R and W300R occurs, the interaction between theαandβspectrins will be greatly influenced and the spectrin tetramer becomes more unstable compared to the wild-type spectrins. In the relevant experiment,[22]it has also found that these two mutations can lead to the decrease of the affinity of theαandβspectrins. Therefore, our simulation results agree very well with the experimental results.
Furthermore,we also compared the curvature of the lipid bilayers interacting with the wild-type and the mutant spectrins in Fig.7. Due to its unstable structure,the mutant spectrins can fluctuate strongly on the surface of the lipid bilayer and result in the stronger curvature of the bilayer. Therefore,the maximal curvature of the bilayer bound with the mutant spectrins is obviously larger than that of the bilayer bound with the wild-type spectrins as shown in Fig. 7. This result demonstrates that, in the blood diseases such as HE or HPP,the deformation of erythrocyte is possibly induced by the mutant spectrins with an unstable structure.[22]
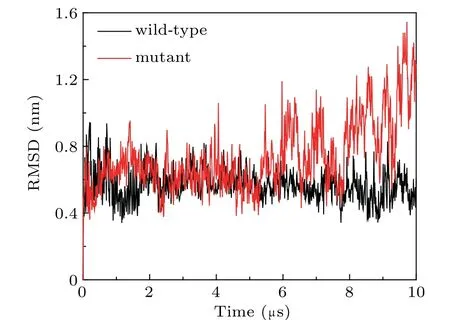
Fig.6. The RMSDs of the wild-type spectrins and the mutant spectrins with the mutation sites of W263R and W300R adsorbed on the surface of the lipid bilayer composed of DPPE/DIPC/CHOL.
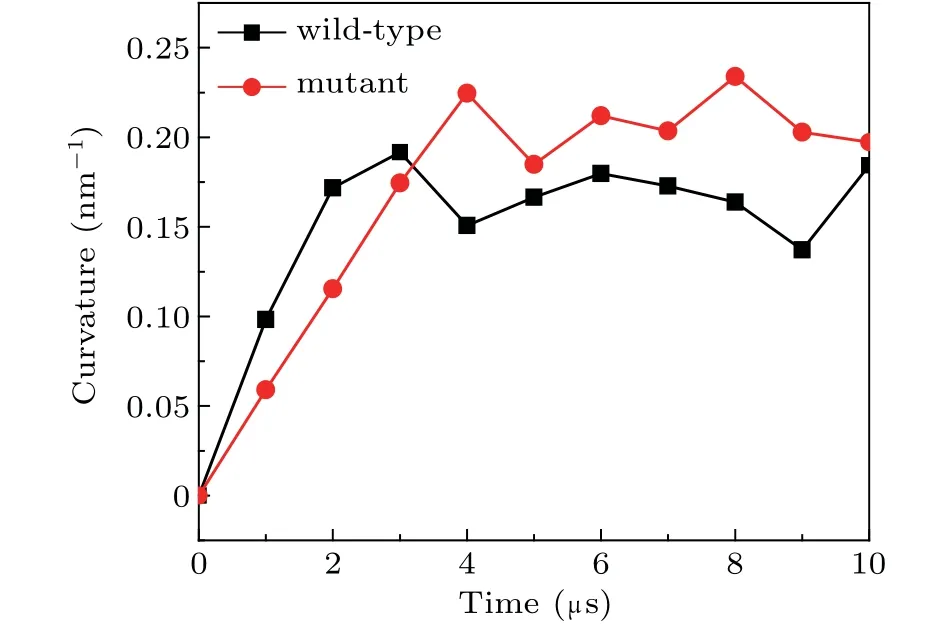
Fig. 7. The maximal curvatures of the DPPE/DIPC/CHOL lipid bilayer bound by the wild-type and the mutant spectrins.
4. Conclusion
In this work,using coarse-grained MD simulation,we examined the interactions between the wild-type or mutant spectrins and lipid nanodomains in the multicomponent lipid bilayers. The results show that the spectrins exhibit a stronger interaction with PE lipids than with PC lipids, which agrees well with the experimental results. Additionally, the spectrins prefer to contact with the Ld domains in the ternary neutral lipid bilayers containing unsaturated DIPE. The anionic lipids can enhance the interactions between the spectrins and the lipid bilayers through the electrostatic interaction between the positively charged or polar amino acids on the surface of the protein and the anionic headgroups of the lipids, and the binding of the spectrins obviously influences the distribution of the anionic lipids in the lipid bilayers. Furthermore, we also considered the effect of mutation on the interactions between the spectrins and the lipid bilayer. It is found that the spectrin mutation reduces the structural stability of the spectrins and further leads to the strong deformation of the lipid bilayer,which could be attributed to the causes of some blood diseases. Our results may yield some theoretical insights into understanding the erythrocyte structure and the treatment of some spectrin-associated blood diseases.
- Chinese Physics B的其它文章
- Constraints on the kinetic energy of type-Ic supernova explosion from young PSR J1906+0746 in a double neutron star candidate?
- Computational model investigating the effect of magnetic field on neural–astrocyte microcircuit?
- Gas sensor using gold doped copper oxide nanostructured thin films as modified cladding fiber
- Exact explicit solitary wave and periodic wave solutions and their dynamical behaviors for the Schamel–Korteweg–de Vries equation?
- Suppression of ferroresonance using passive memristor emulator
- Suppression of ice nucleation in supercooled water under temperature gradients

