Synthesis and Characterization of Palladium Nanoparticles with High Proportion of Exposed(111)Facet for Hydrogenation Performance
LU Zhang-Yin HONG Yun-Yang DAI Yu-Yu LI Xiao-Qing YAN Xin-Huan
(State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology,Zhejiang University of Technology,Hangzhou 310014,China)
Abstract:Pd nanoparticles with different(111)facet proportions were prepared at by a liquid phase hydrogen reduction method,then preparing corresponding Pd/C catalysts.The results of transmission electron microscopy(TEM),fast Fourie transition(FFT),and X-ray diffraction(XRD)revealed that the proportion of the(111)facets on the Pd surface was higher at lower temperatures.Hydrogen oxygen pulse titration(H2-O2)and H2-temperature programmed desorption(H2-TPD)showed that the hydrogen adsorption volume of Pd/C catalysts was correlated linearly with the Pd(111)facet proportions.All Pd/C catalysts had an average particle size of 4.3 nm with narrow particle size distribution,which could eliminated the effect of particle size.The similar pore parameters and Pd loading of all catalysts allowed the reasonable comparison for Pd(111)facet proportions influenced the hydrogenation performance in three typical reactions.Moreover,linear correlations were found between the H2 consumption rate with Pd(111)facet proportions in each of styrene,cyclohexene,and p-nitrotoluene hydrogenation.The good catalytic performance of high Pd(111)facet proportion catalyst for hydrogenations could be attributed to the H2 molecule prior to absorbed the Pd(111)facet promoting the formation of dissociated hydrogen atoms.These results above indicated that Pd-based catalysts with high(111)facet proportion facilitated hydrogenation performance.
Keywords:palladium;single crystal;Pd(111)facet;hydrogen dissociation;hydrogenation
0 Introduction
Nanoparticles(NPs)surface faceting has profound effect for chemical transformations,such as heterogeneous catalysis[1-3],hydrogen storage[4-5],and fuel cells[6].Recently,a growing number of reports from academia and industry demonstrate that noble metals′facet plays crucial roles in hydrogen dissociation for catalytic hydrogenation[7-11].For example,in alkyne hydrogenation,which is a selective reaction used in the food industry,palladium(111)-octahedra have higher catalytic activity than(100)-cubes[12].Similarly,surface faceting has been reported to control hydrogen sensors.For example,in TiO2nanocrystal,H2tend to be adsorbed and dissociated on the(002)and(101)surface,leading to high sensitivity and short response time[13].
The nature of hydrogen dissociation on Pd surface faceting for the reaction is a longstanding scientific question.Up to now,it remains a major challenge to discover the consensus of dominant catalytic facet for the hydrogenation by Pd nanocatalysts.The previous study of the Kim group reported that the Pd(100)has easier decomposition of hydrogen than the Pd(111)contributed to high performance for selective hydrogenation of acetylene[14].On the other hand,Yarulin et al.thought that the Pd(111)is more active than the Pd(100)[15].Moreover,DFT(density functional theory)calculations suggested that the styrene hydrogenation activity of the clean Pd(111),Pd(100),and Pd(110)surfaces decrease in the order of Pd(111)>Pd(100)>Pd(110)[16].Yang et al.revealed that performance for selective hydrogenation of acetylene to ethylene on several Pd surfaces is Pd(211)>Pd(111)> Pd(100)[17].In addition,to our best knowledge,the discrimination of the hydrogen dissociation for each Pd surface is rarely studied from experimental observations.
Most studies comparing particle morphology are performed over an ensemble of NPs with varied size and shape[18-19].While NPs synthesis have different shape with facet distributions,the bigger sizes lead to lower atom efficiency[20-22].On the other hand,single crystal particle studies can identify facet-specific activity and give better insight in the role of hydrogen dissociation on facets.
In this work,we use the hydrogenation of single crystal Pd NPs to investigate the hydrogen dissociation on three low-index facets.Single crystal Pd NPs with different proportions of Pd(111),Pd(100),and Pd(110)facets were prepared at temperatures of 10,15,25,30,and 35℃.The Pd NPs were then loaded onto activated carbon and labeled Pd/C-x,wherexdenoted the temperature value at which the Pd NPs were prepared.The Pd/C-xcatalysts were characterized by performing transmission electron microscopy(TEM),X-ray diffraction(XRD),N2adsorption-desorption,inductively coupled plasma-optical emission spectroscopy(ICP-OES),hydrogen oxygen pulse titration(H2-O2),and H2temperature programmed desorption(H2-TPD)analysis.Finally,we evaluated these catalysts for their styrene,cyclohexene,andp-nitrotoluene hydrogenation activities.We confirmed that Pd(111)facet proportion was linear with the hydrogenation activity of these Pd/C-xcatalysts.These call for better understanding on improvement of hydrogenation activity by increasing the Pd(111)facet proportion,aiming to guide the rational design and facet optimization of the Pd-based catalyst.
1 Experimental
1.1 Materials
Tris-(dibenzylideneacetone) dipalladium(0)(Pd2(dba)3,AR)was purchased from Sigma-Aldrich Co.,Ltd.Propylene carbonate(PC,AR)was purchased from Dongguan Youte environmental protection materials Co.,Ltd.Active carbon(AR)was brought from Shanghai Lvqiang New Material Co.,Ltd.Styrene(C8H8,AR),cyclohexene (C6H12,AR),andp-nitrotoluene(C7H7O2N,AR)were purchased from Shanghai Aladdin Reagent Co.,Ltd.
1.2 Preparation of Pd nanoparticles
Pd nanoparticles with different Pd(111)proportion were synthesized by the methods in different temperatures.Specifically,a measured amount of Pd2(dba)3as a precursor and 100 mL PC were added into a 250 mL stainless steel stirred reactor.The reactor was initially purged with H2for 6 times,then slowly heated until the desired reaction temperature of 10,15,25,30,and 35℃.After pressurized to 4.0 MPa with H2,the reaction was started with a stirring rate of 500 r·min-1for 3 h.Then the prepared black Pd reactant was adsorbed by quantitative activated carbon for 24 h until the solution was colorless and transparent after filtration.The samples were washed by ethanol and acetone,then natural dried for 24 h.All these materials were defined as Pd/C-10,Pd/C-15,Pd/C-25,Pd/C-30,and Pd/C-35,respectively.
1.3 Catalysts characterization
TEM was taken on a JEOL JEM-1200EX with an accelerating voltage of 200 kV.Before being transferred into the TEM chamber,the samples dispersed in ethanol were deposited onto holey carbon films supported on Cu grids.
Fast Fourier transformation(FFT)was performed on Digital-Micrograph software.The selected area of the high resolution TEM(HRTEM)images was treated by FFT,thus the reciprocal lattices corresponding to the reciprocal space were obtained.Then the distance from different lattices to the origin of reciprocity was measured,and the countdown of the distance was the actual interplanar distance.Referring to PDF card data,the specific crystal plane of corresponding substance was gained.
The XRD patterns of the Pd/C-xwere performed on a Rigaku D/Max-2500 X-ray diffractometer,which used a CuKαradiation(λ=0.154 nm)in the 2θscan range(40 kV and 100 mA)from 10°to 80°with a step of 0.05°.
The Pd content of the prepared Pd/C-xcatalysts was determined by ICP-OES.The experiments were done by Aglient 720ES.
N2adsorption at-196℃was measured using a Micromeritics ASAP 2010 system,the samples were degassed at 200℃for 6 h under high vacuum.The surface area was calculated by using the Brunauer-Emmett-Teller(BET)method.The total pore volume was determined by nitrogen adsorption at a relative pressure of 0.99,and the pore size distributions were calculated from the nitrogen adsorption isotherms by the Barrett-Joyner-Hallenda(BJH)method.
The H2-O2and H2-TPD experiments were done by Micromeritics Autochem 2920 with a TCD detector.The principle of H2-O2was as follows,the routine of“pre-reduction(adsorption of hydrogen)→titrated oxygen→titrated hydrogen→titrated oxygen→titrated hydrogen”was measured sequentially.As shown in Eq.1~3,titrating a single palladium atom requires three hydrogen atoms.Specifically,loop ring(a quantitative loop,the volume was 0.5 mL)titration was performed with 5% H2/Ar by injection,until the peak height remained constant,indicating that hydrogen adsorption on the Pt surface had reached saturation,hydrogen titration operation was completed.The adsorbed hydrogen volume on the Pd/C-xwas calculated by Formula 4,whereAH2,Vr,Vm,andmrepresent quantity of adsorbed H2,H2titration volume,molar volume of gas(22.4 L·mol-1),and quality of sample,respectively.
1.4 Catalytic test
In each experiment,the autoclaves were purged 6 times with H2to remove air.After a fixed reaction time,the autoclaves were cooled down to room temperature and H2pressure was carefully released.In the hydrogenation process,stirring speed was kept at 1 200 r·min-1to avoid mass transfer limitations.The H2pressure changes of the 250 mL gas tank was recorded automatically with a pressure sensor,which connected to the autoclaves.
The hydrogenation reaction rates were computed based on calculated H2consumption per unit time(r)using the equation given by Formula 5.Thet2-t1represents the time period when hydrogenation reaction is stable.Then2-n1represents variable quantity in amount of substance of H2.The amount of substance of H2were calculated by Redlich-Kwong Eq.6~8,whereP,V,T,R,Pc,andTcrepresent the H2pressure in storage tank,H2molar volume,H2temperature,thermodynamic constant(8.314 J·mol-1·K-1),critical condition pressure and temperature,respectively.
2 Results and discussion
2.1 Characterization results
We investigated the Pd NPs of Pd/C-10,Pd/C-15,Pd/C-25,Pd/C-30,and Pd/C-35 by TEM,and the results are shown in Fig.1.Spherical Pd nanocrystals were observed in each image.The size of the Pd NPs was approximately 4.3 nm.As shown in Fig.2,three typical Pd NPs were magnified by HRTEM,which were characterized by eight triangular(111)facet,six square(100)facet,and dodecahedron(110)facet,respectively.For each sample,50 Pd crystals chosen randomly from several HRTEM images were examined and classified into three categories:Pd crystals exposed only(111),(100)and(110)facets(Fig.S1~S5).Based on statistical analysis,the proportion of Pd(111)facet in the Pd/C-10,Pd/C-15,Pd/C-25,Pd/C-30,and Pd/C-35 catalyst were 84%,75%,63%,55%,and 43%,respectively.This suggests that the reaction temperature influences the formation of Pd crystals exposed by only the(111)facet.

Fig.1 TEM images and derived particle size distributions of Pd/C-x samples:(a)Pd/C-10,(b)Pd/C-15,(c)Pd/C-25,(d)Pd/C-30 and(e)Pd/C-35
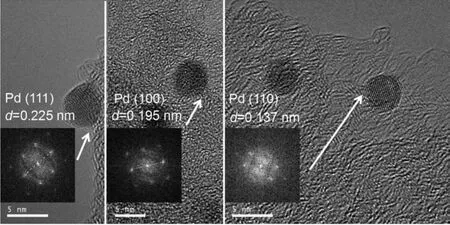
Fig.2 HRTEM and FFT images of single Pd NPs
Fig.3 shows the XRD patterns of Pd/C-xcatalysts.In each XRD pattern,three diffraction peaks were observed at 2θ=40.1°,46.7°,and 68.1°,which are assigned to(111),(200)and(220)facet of face-centered cubic Pd,respectively;this suggests the formation of metallic Pd.The ratios of peak intensity of the(111)facet to that of the(220)facet for Pd/C-10,Pd/C-15,Pd/C-25,Pd/C-30,and Pd/C-35 were 16.7,11.2,9.7,6.5,and 4.3,respectively.This suggested that Pd NPs had a higher proportion of Pd(111)facet synthesized in lower temperature.Meanwhile,the particle size of Pd NPs of different catalysts,which calculated from FWHM of diffraction peaks according to Scherrer equation[23],are listed in Table 1,which is consistent with the TEM results.There is little difference in crystallinity between catalysts.Above results eliminated the possibility of a particle size effect and difference of crystallinity,allow us to directly compare their catalytic performance[24].
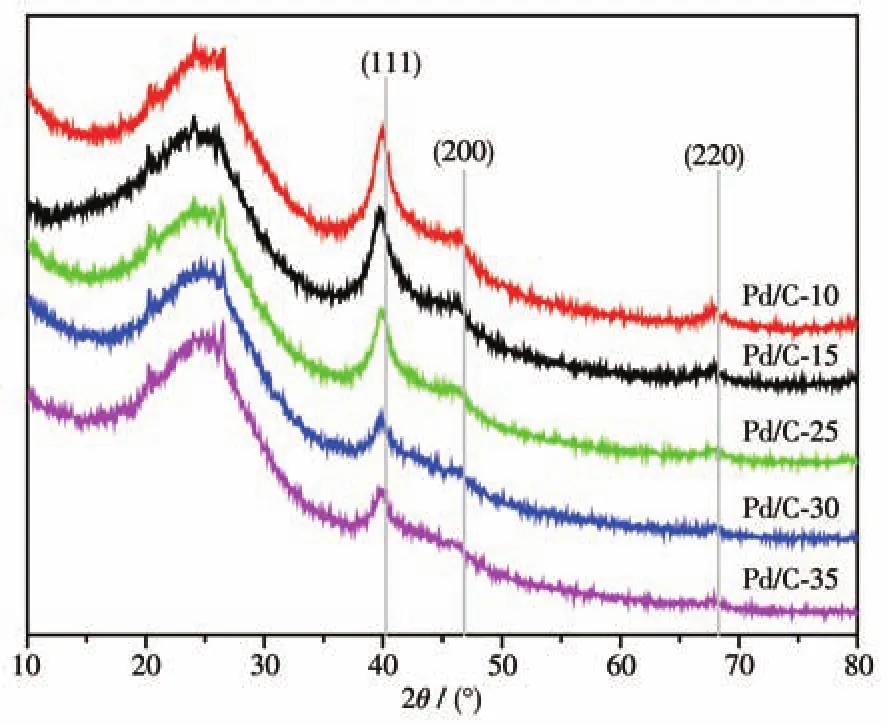
Fig.3 XRD patterns of Pd/C-x catalysts
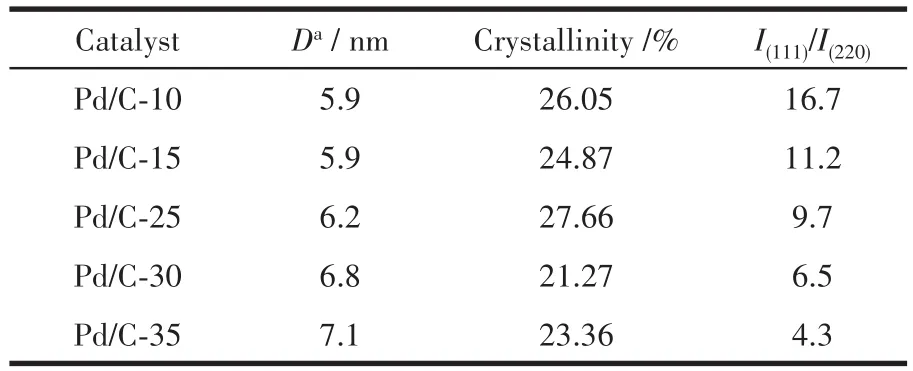
Table 1 XRD analysis of the catalysts
It is known that the surface of crystals can be easily controlled via adjusting supersaturation of crystal growth units during the crystal growth process[25].Xie et al.extensively proposed that the faster reduction rate results in the higher surface energy of crystallites[26].On the other hand,the surface energy on Pd single crystals has been reported to increase in the order of Pd(111)<Pd(100)<Pd(110)[27].The change in the temperature can exponentially influence reduction rate of metal precursor which explains the slower reduction rate lead to higher Pd(111)proportion in lower reaction temperature.
The texture properties of different samples are measured by N2adsorption and desorption experiment and the results are summarized in Table 2.Compared to carbon,the mesoporous volume and mesporous area of different catalysts slightly decrease,which is attributed to Pd NPs clogged the pores of active support carbon during catalyst preparation process[28].However,the external surface area of all catalysts substantially remains unchanged.Based on the total amount of Pd in the impregnation solution,the theoretical Pd loading(mass fraction)was 1.00% of that in Pd/C-xcatalysts.The Pd loading of all catalysts varied from 0.90% to 0.95%(within the range of test errors).
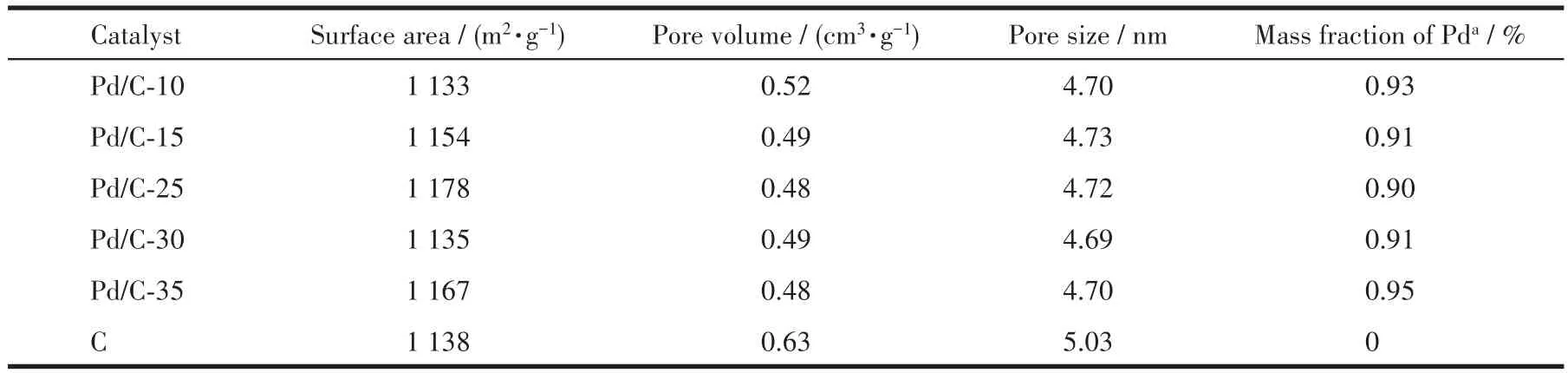
Table 2 Properties of the catalysts
To explore effect of(111)facet proportion on H2adsorption capacity,H2-O2titration was performed for the Pd/C-xcatalysts.The adsorbed hydrogen volume of the Pd/C-xwas calculated by integral quantity of stable peak area(Fig.4a).Fig.4b shows that the quantity of adsorbed H2was plotted against the Pd(111)proportion(%).The amount of adsorbed H2on Pd/C-10 was 39.46 μmol·g-1,which was nearly 2.13 times greater than the amount of H2on Pd/C-35(18.54 μmol g-1).The Pd(111)proportion of Pd/C-10 was 1.95 times than that of Pd/C-35.The amounts of adsorbed H2on Pd/C-15,Pd/C-25,and Pd/C-30 were 34.92,25.47,and 22.84 μmol·g-1,respectively.It is clear that a linear relationship between the quantity of adsorbed H2and the Pd(111)proportion of each Pd/C-xcatalyst.The linear curve in Fig.4b had a high correlation coefficient(R2)of 0.98.It should be pointed out that the line through the origin point,indicating nonoccurrence H2dissociation with absence of Pd(111)facet.On the other hand,the volume of adsorbed H2was not positively correlated with the proportions of the Pd(100)and Pd(110)facets(Fig.S6).The results confirms that Pd(111)facet plays a central role in hydrogen dissociation.

Fig.4 Results of H2-O2 titration of absorbed hydrogen for Pd/C-x catalysts:(a)H2-O2 titration peak map;(b)relationship between Pd(111)proportion and the quantity of adsorbed H2
H2-TPD was used to detect the metal properties of the catalysts with Pd NPs of different(111)facet proportions,which is shown in Fig.5.Generally,the hydro-gen adsorbed on the Pd surface can be assigned to two kinds of hydrogen species,including the surface hydrogen adsorbed on the Pd surface and subsurface hydrogen adsorbed on the subsurface or the bulk of Pd[29].As shown in Fig.4,the desorption peak centered at 65℃can be assigned to the desorption of H2molecules from Pd surface[30-31],while the peak centred at 380℃can be assigned to the desorption of H2molecules from the active support carbon[32].The dissociation adsorption capacity of Pd for H2of Pd/C-35 was too weak,whereas that of Pd/C-10 was too strong,which suggests that the high Pd(111)proportion has stronger ability to activate H2.
2.2 Catalyticactivity of Pd/C-x catalysts for hydrogenation
Generally,the facet of Pd NPs may affect product conversion and selectivity using defined experiments and DFT simulations[33].Therefore,it is imperative to study the(111)facet proportion of Pd influence the hydrogenation activity.The performance of styrene,cyclohexene,andp-nitrotoluene hydrogenation were evaluated for the different catalysts prepared with Pd NPs of different(111)facet proportion.Fig.S7 presents the lines of hydrogen consumption curves for three hydrogenation reactions,suggests the first order reaction for styrene,cyclohexene,andp-nitrotoluene hydrogenation reactions[34-36].The curves in the initial time was not linear,due to the instability of system when the reaction started[37].As the Pd(111)proportion increased the hydrogen consumption gradually increased for all catalysts due to hydrogenation active sites on Pd(111)facet.Moreover,it can be found that the hydrogen consumption rate over different catalysts follows the Pd/C-10>Pd/C-15>Pd/C-25>Pd/C-30>Pd/C-35,in consistent with the results of H2-O2.
Table 3 shows the hydrogen consumption rate for three reactions in Pd/C-xcatalysts with different Pd(111)proportions.All Pd/C-10 catalyst exhibited higher hydrogenation activity than other catalyst in every hydrogenation reaction.At styrene hydrogenation,the hydrogen consumption rate in Pd/C-xcatalysts were 9.17,8.11,7.30,5.68,and 4.59mmolH2·min-1for Pd(111)proportion of 84%,75%,63%,55%,and 43%,respectively.The hydrogenation activity of Pd/C-10 catalyst was 2.00 times that of the Pd/C-35 catalyst,in consistent with the 1.95 times of that Pd(111)ratios.The H2consumption rate in Pd/C-xcatalysts for cyclohexene hydrogenation were 0.59,0.54,0.47,0.40,and 0.34mmolH2·min-1for Pd(111)proportion of 84%,75%,63%,55%,and 43%,respectively,whereas 2.00,1.79,1.60,1.38,and 1.17mmolH2·min-1of that forpnitrotoluene hydrogenation.The data shown in Fig.6 clearly showed a linear relationship between the proportion of Pd(111)facet in Pd/C-xcatalysts and their H2consumption rate in every hydrogenation.Interestingly,each curve passed through the original point,and had a highR2of 0.99.This suggests that no hydrogenation occurred in the absence of Pd(111)under ideal conditions.In contrast,the proportion of Pd(100)and Pd(110)facets in Pd/C-xand their H2consumption rates were not positively correlated(Fig.S8).For the linear correlation between H2consumption rate and Pd(111)proportion,the dissociation adsorption capacity of Pd(111)for H2were further proved from hydrogenation aspects,suggesting that the hydrogenation active site originated from Pd(111)facet.
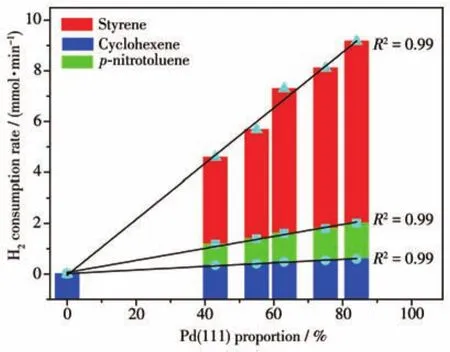
Fig.6 Relationship between the Pd(111)proportion of the catalysts and H2 consumption rates in three hydrogenation reactions
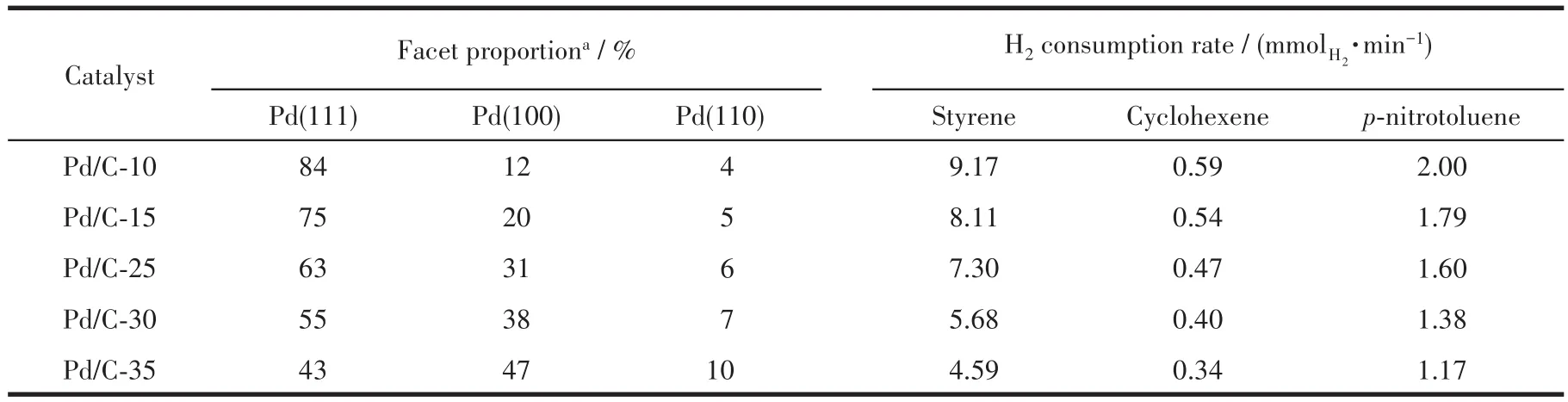
Table 3 Catalytic hydrogenation performance over different catalysts
The reusability study was conducted with Pd/C-10 catalyst for thep-nitrotoluene hydrogenation.As shown in H2consumption curves(Fig.S9),the test was performed up to 10 successive cycles for the reactions.The catalyst stayed active and showed consistent performance(Fig.7).Interestingly,the catalyst was able to retain the activity after successive reuse.
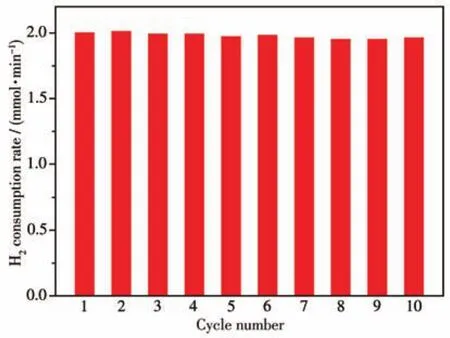
Fig.7 Recyclability test of Pd/C-10 catalyst for p-nitrotoluene hydrogenation
Furthermore,XRD patterns of both fresh and recycled catalysts for thep-nitrotoluene hydrogenation indicated that there was no change in phase purity and the crystalline structure remained stable after ten recycles(Fig.8).In addition,FFT measurement of HRTEM images for recycled catalysts was performed(Fig.S10).The result indicated that the Pd(111)facet proportion could be substantially unchanged.About 82% of Pd(111)facet proportion in recycled Pd/C-10 was consistent with the result of 84% of that in fresh Pd/C-10.
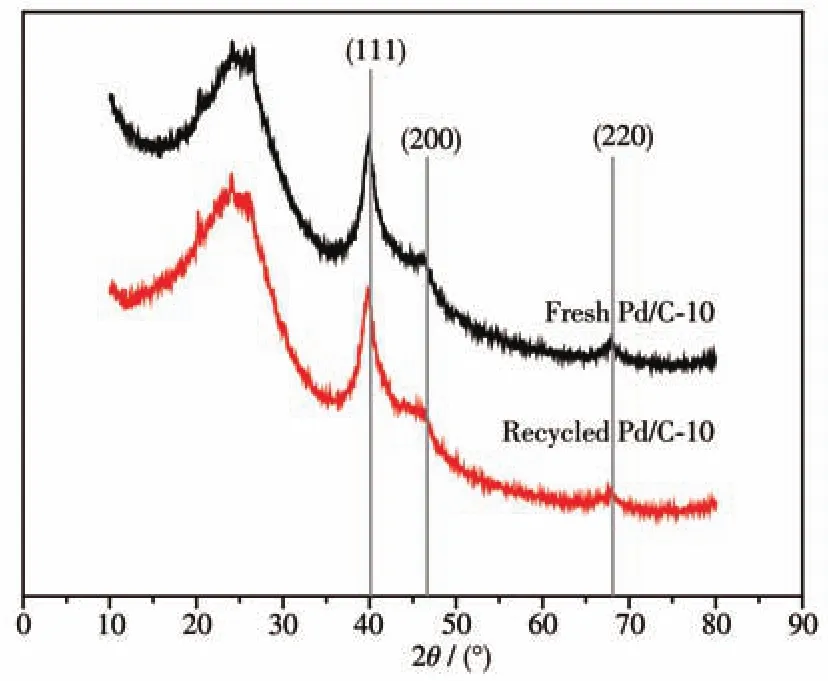
Fig.8 XRD patterns of fresh and recycled Pd/C-10 catalyst
3 Conclusions
In summary,we have described a method for the synthesis of different(111)facet proportions of Pd loaded active carbon catalysts with small size in well dispersion.Through systematic results of H2-O2,H2-TPD and three typical hydrogenation reactions,Pd NPs with high Pd(111)proportion were found to be remarkably active for catalyzing hydrogen.Therefore,we propose that H2molecules prior to adsorb on the Pd(111)facet and dissociate into individual H atoms,which then participate in hydrogenation reactions.This concept of hydrogenation active sites on Pd(111)unlocks the possibility for future nanocrystal catalyst design where the critical facet role can be optimized for a given catalytic reaction.
Supporting information is available at http://www.wjhxxb.cn
Acknowledgements:The authors gratefully acknowledge the National Key Research and D&P of China(Grant No.2017YFC0210900).
- 無機化學學報的其它文章
- Syntheses,Crystal Structures,Luminescence and Catalytic Activity of Manganese(Ⅱ)and Cadmium(Ⅱ)Coordination Polymers Based on 2,3-Dihydroxy-terephthalic Acid
- Scale-Up Strategy to Develop Highly-Effective Co-N-C@KB Composites as Sulfur Host for Lithium-Sulfur Battery
- Self-Assembled Zn2+,Co2+ and Ni2+ Complexes Based on Coumarin Schiff Base Ligands:Synthesis,Crystal Structure and Spectral Properties
- Synthesis,Characterization,and X-ray Crystal Structure Analysis of Cu(Ⅰ)/Cu(Ⅱ)Complexes of Phenanthridine and Triphenylphosphine
- 添加叔丁醇鉀對Mg(NH2)2-2LiH體系儲氫性能的影響
- 熒光猝滅型硼氟二吡咯類Cu2+探針的合成及其生物細胞成像的應用

