Synthesis,Characterization,and X-ray Crystal Structure Analysis of Cu(Ⅰ)/Cu(Ⅱ)Complexes of Phenanthridine and Triphenylphosphine
Hikmat Ali Mohamad Karwan Omer Ali Eric Hosten Thomas Gerber
(1Department of Chemistry,College of Education,University of Salahaddin,Erbil,Iraq)
(2Department of Physics,College of Science,University of Halabja,Halabja,Iraq)
(3Department of Chemistry,Faculty of Science,Nelson Mandela Metropolitan University,Port Elizabeth,South Africa)
Abstract:Two new complexes of copper(Ⅰ) and copper(Ⅱ) with phenanthridine(Phend)of the following composition:[Cu(κ1-Phend)2Cl2](1)and[Cu2(κ1-Phend)2(κ1-PPh3)2(μ-Cl)2](2)have been prepared in the presence of triphenylphosphine(PPh3)as a co-ligand.The structures of these complexes have been investigated by elemental analysis,molar conductivity,FT-IR,UV-Vis,and single-crystal X-ray diffraction.X-ray diffraction analysis of typical complex 1 reveals the distorted square planar geometry around copper(Ⅱ)whereas the binuclear complex 2 was irregular tetrahedral geometry around Cu(Ⅰ)center containing bridge Cl- ion.The FT-IR spectra,elemental analysis as well as UV-Vis spectra confirmed their components,geometries,and ligand interactions.The structures of both complexes have been optimized by density-functional theory(DFT)calculations to explain the electronic spectral properties.CCDC:1983822,1;1983821,2.
Keywords:copper(Ⅰ);copper(Ⅱ);heteroleptic complex;phenanthridine;X-ray crystallography;density-functional theory computation
0 Introduction
The monovalent and divalent copper complexes have been widely investigated essentially due to their applications in photovoltaics for the conversion of solar energy into electricity,light-emitting diodes,luminescent probes,and anticancer photoluminescent[1-3].The Cu(Ⅰ)/Cu(Ⅱ) complexes with tetrahedral and irregular square planar coordination geometry are in perpetual interest due to their low toxicity property,more abundant resource,and relatively low cost related to thirdrow transition metal complexes such as rhenium(Ⅰ)[4],iridium(Ⅲ)[5],platinum(Ⅳ)[6],and gold(Ⅰ)[7].The coordinated ligands around Cu(Ⅰ)/Cu(Ⅱ) center play an important role in modifying the physical and chemical characters of the synthesized complexes and hence accurate choice of the ligand is of significant importance that will modify the properties of the resulting complex[8-9].Monodentate ligands have shown effective in decreasing steric interactions in the ligand itself that inhibit the metal coordination environment[10].The most common N-heterocyclic compound in drug chemistry is phenanthridine(Phend)due to its antitumor and antiviral activities[11].The pharmacological properties of Phend and its derivatives come-back to the planarity and conjugateπ-system of the compound[12].Triphenylphosphine(PPh3),generally known as ancillary ligand that can be used as co-ligands with most transition metal complexes and possibly important since it can stabilize metal complexes[13].Research on Phend is commonly limited in coordination chemistry[12].As N-donor ligand Phend can be generated a complex ofcis-[Pt(NH3)2(C13H9N)Cl]NO3,that illustrates a new antitumor property[14].[Au(Phend)Cl3]and [Au(Phend)Br3]complexes have been prepared and exhibit antitumor activity[15].Cu(Ⅰ)/Cu(Ⅱ) ion complexes are potentially effective in the synthesis of a variety of polymeric compounds due to their emissive property[16].In this article,we report our results in the synthesis of two new Cu(Ⅰ)/Cu(Ⅱ) complexes containing PPh3and N-heterocycle,Phend ligands with general composition:[Cu(κ1-Phend)2Cl2](1)and[Cu2(κ1-Phend)2(κ1-PPh3)2(μ-Cl)2](2).The synthesized Cu(Ⅰ)/Cu(Ⅱ) complexes were characterized by elemental analysis,UV-Vis,FT-IR,molar conductivity,and X-ray crystallography analysis.In addition,density functional theory(DFT)computation technique was used to explain the electronic spectral properties of molecules.
1 Experimental
1.1 Materials and instruments
The copper halides(CuCl2·2H2O and CuCl),Phend,PPh3,methanol(99.8%),dimethyl sulfoxide(DMSO,99.9%),and dimethyl formamide(DMF,99.8%)were procured from Sigma-Aldrich and used without further purification.
FT-IR spectra of the samples in a range of 4 000~400 cm-1or 600~200 cm-1were recorded on SHIMADZU FTIR-8400S as KBr or CsI discs,respectively.The electronic spectra were measured on a Shimadzu double-beam AE-UV1609 spectrophotometer after preparing the corresponding sample in DMSO and after 24 h standing at room temperature in a wavelength range of 800~200 nm.C,H,and N microanalysis were performed on a EURO EA 300 CHNS instrument.Molar conductivities were measured from 1 mmol·L-1solutions in DMF at 25℃using a model CON 700 Benchtop conductivity meter.Melting points were recorded on a Stuart SMP3 melting point apparatus.
1.2 Synthesis of[Cu(κ1-Phend)2Cl2](1)
To a solution of CuCl2·2H2O(0.042 g,0.25 mmol)in CH3OH(25 mL),a solution of Phend(0.089 g,0.5 mmol)in CH3OH(25 mL)was added dropwise under stirring.Thereafter,the mixture was stirred for 4 h at room temperature with a formation of a green precipitate,which was then filtered,and the filtrate was evaporated slowly at room temperature to yield green crystalline products within 4 d.Yield:0.122 g(93%).m.p.291~291.4 ℃.Anal.Calcd.for C26H18Cl2CuN2(%):C 63.36,H 3.68,N 5.69;Found(%):C 63.25,H 3.71,N 5.93.Molar conductivity:5.3 S·cm2·mol-1.IR(KBr,cm-1):3 061mν(=C—H),1 608mν(HC=N),1 591 w,1 492mν(C=C),746sν(C—H,oop).IR(CsI,cm-1):349wν(Cu—N),285mν(Cu—Cl).UV-Vis(DMSO,λmax/nm(?/(dm3·mol-1·cm-1))):403(24 813),344(29 069),330(30 303),308(32 467),292(34 246).
1.3 Synthesis of[Cu2(κ1-Phend)2(κ1-PPh3)2(μ-Cl)2](2)
A mixture of Phend(0.089 g,0.5 mmol)and PPh3(0.131 g,0.5 mmol)in 25 mL MeOH was dissolved by mechanical stirring at 25℃for 10 min.The reaction mixture was further treated with cuprous chloride(0.049 g,0.5 mmol)in 25 mL of MeOH solution.The solution mixture was stirred for 3 h at 25℃with a formation of a yellow-green precipitate.The resultant mixture was filtered and the filtrate was kept to evaporate slowly at room temperature,yellow-colored crystals appeared after 10 d.Yield:0.253 g(94%).m.p.135~135.3℃.Anal.Calcd.for C62H48Cl2Cu2N2P2(%):C 68.89,H 4.47,N 2.60;Found(%):C 68.74,H 4.53,N 2.27.Molar conductivity:3.20 S·cm2·mol-1.IR(KBr,cm-1):3 055mν(=C—H),1 612mν(HC=N),1 544w,1 481mν(C=C),746sν(C—H,oop),1 435vsν(P—CAr,PPh3),692vsν(P—C,PPh3).IR(CsI,cm-1):368wν(Cu—N),229mν(Cu—Cl),343mν(Cu—P).UV-Vis(DMSO,λmax/nm(?/(dm3·mol-1·cm-1))):438(22 831),346(28 901),330(30 303),308(32 467),294(34 013),278(35 971).
1.4 X-ray crystal structure determination
Green and green-yellow single crystals with a suitable quality of 1 and 2 were grown by slow evaporation of the methanol solution.X-ray diffraction studies of 1 and 2 were performed at 296 K on a Burker Kappa Apex Ⅱ diffractometer with graphite monochromatic MoKαradiation(λ=0.071 073 nm).The SHELXS-2014 was used for the solution of the complex structure[17]and refined by least-squares procedures using SHELXL-2014 with SHELXE as a graphical interface[18].Non-hydrogen atoms were refined anisotropically.The positions of hydrogen atoms on carbon were placed geometrically with C—H hydrogen and refined isotropically[17].APEX-Ⅱand SAINT were used for data collection,data reduction and cell refinement[19].Crystallographic data and structure refinement details of 1 and 2 are summarized in Table 1.
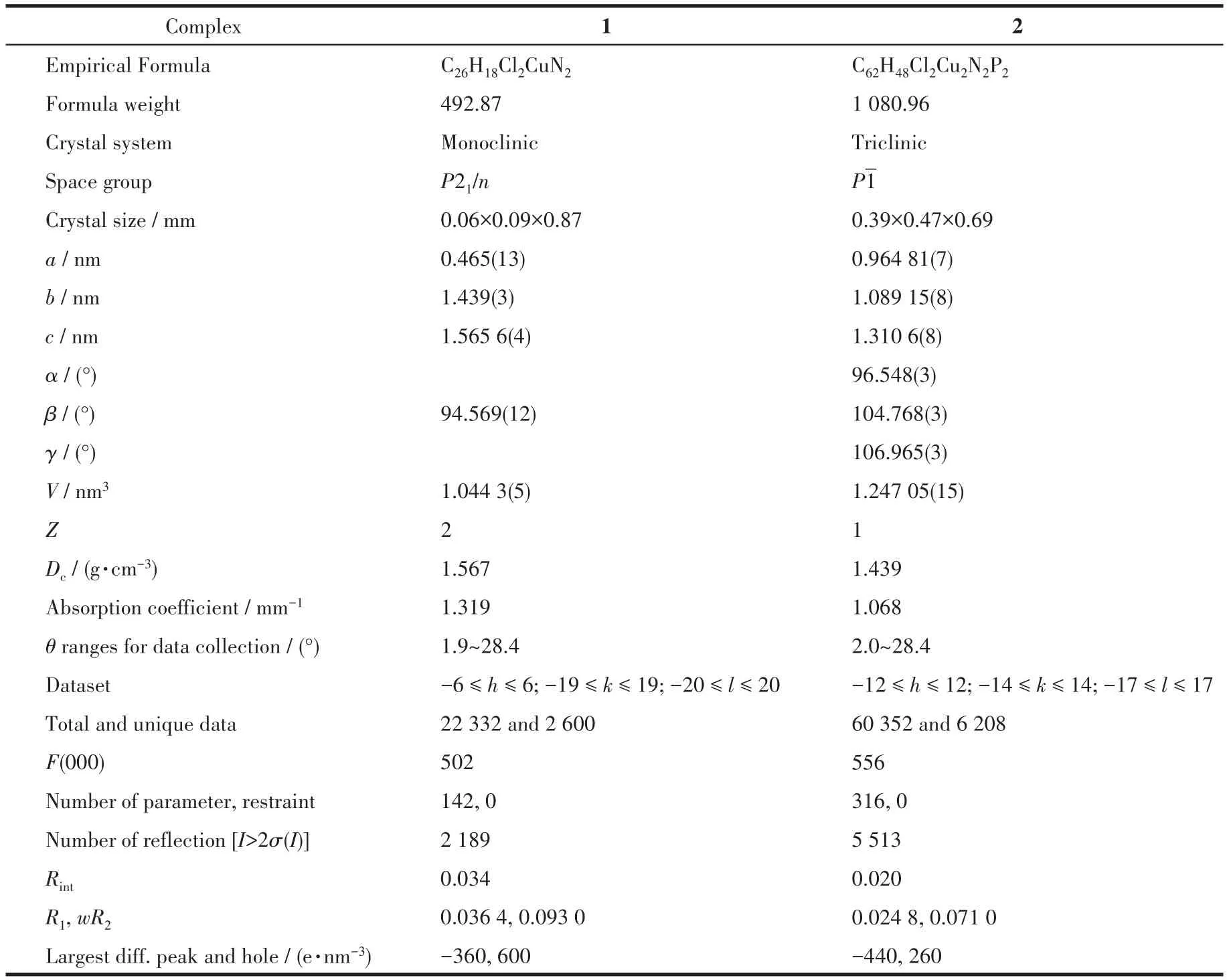
Table 1 Crystal data and structure refinement details of complexes 1 and 2
CCDC:1983822,1;1983821,2.
1.5 DFT studies
The molecular geometries were optimized using the density functional theory(DFT)method at the B3LYP level for the Phend ligand and both complexes with the Gaussian 09 program package.The 6-311G++basis set was for C,H,N,and P,while the LANL2DZ basis set was employed for the heavy metals Cu(Ⅰ)/Cu(Ⅱ)[20].
2 Results and discussion
2.1 Synthesis of complexes 1 and 2
PPh3and aromatic N-heterocycle,Phend were used as ligands for the complexation of Cu(Ⅱ) and Cu(Ⅰ)ions(Scheme 1).The reaction of equimolar amounts of CuCl2·2H2O salt with Phend in methanol as solvent at 25℃obtained complex 1 as a final product.But,in the reaction of CuCl salt with a mixture of Phend and PPh3under similar experimental conditions,complex 2 was achieved as a pure product.The composition and identity of each complex have been deduced from agreeable elemental analysis,UV-Vis,FT-IR,molar conductivity,and single-crystal X-ray crystallography study.
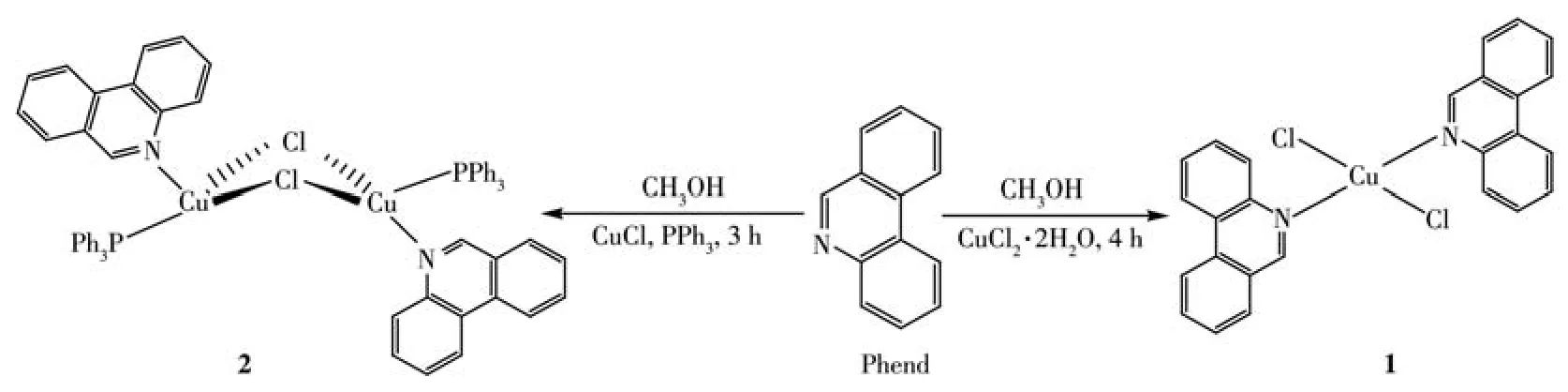
Scheme 1 Synthesis of complexes 1 and 2
2.2 X-ray crystal structures description
The crystals of 1 and 2 were grown by slow evaporation of methanol solution and their structure were determined by single-crystal X-ray crystallography.The crystal structure of complexes 1 and 2 along with the atom numbering scheme is demonstrated in Fig.1 and 2,respectively,and selected bond angles and bond distances are collected in Table 2.In complex 1 the Cu(Ⅱ)center is tetra-coordinated and coordinated to two chlorine atoms and two nitrogen atoms of the ligand Phend,leading to the distorted square planar geometry.The Cu1—N1 bond length,0.201 8(2),is shorter than Cu1—Cl1 bond length,0.224 39(9)nm,which means that the attraction between Cu(Ⅱ)center and Cl atom is stronger than that between Cu(Ⅱ)center and N(Phend)[21].The deviation from 90°for the ideal square planar geometry is observed for the angles about the Cu(Ⅱ)center;Cl1—Cu1—N1,Cl1—Cu1—N1i,Cl1i—Cu1—N1 and Cl1i—Cu1—N1iare 89.66(6)°,90.34(6)°,90.34(6)°,and 89.66(6)°,respectively[22].

Fig.1 Molecular structure of complex 1
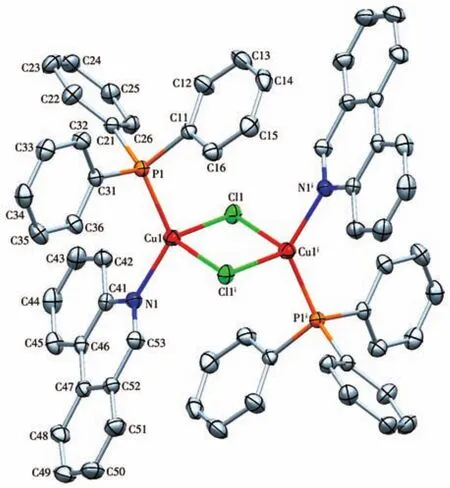
Fig.2 Molecular structure of complex 2
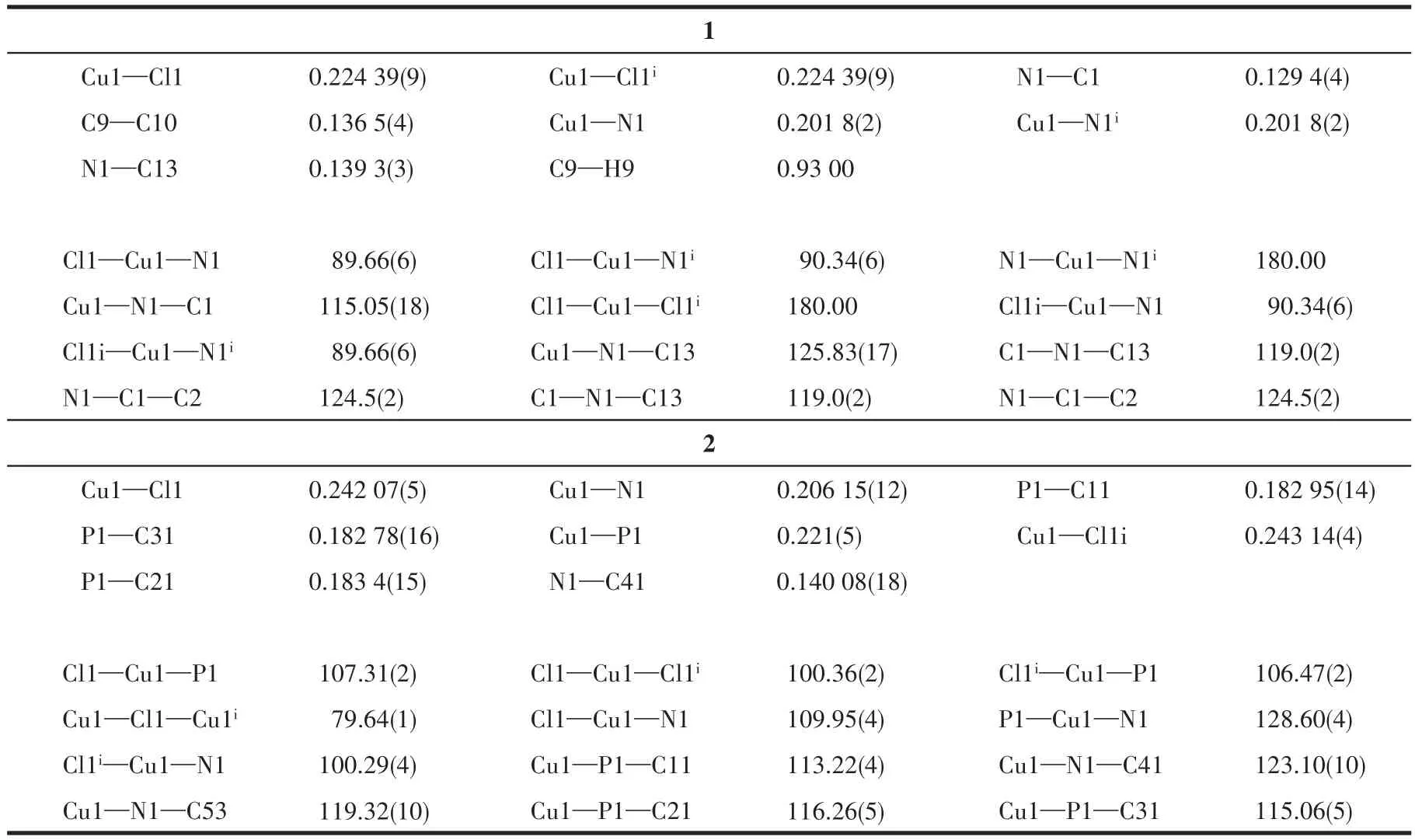
Table 2 Selected bond lengths(nm)and bond angles(°)for 1 and 2
X-ray data reveals that complex 2 crystallize in triclinic space group and exhibits a distorted tetrahedral geometry around each Cu(Ⅰ)with[PNCuCl2]coordination.The coordination geometry of Cu(Ⅰ)center is completed by one N atom of the ligand Phend,P atom of the ligand PPh3and two bridge chlorine atoms.The significant deviation from the ideal tetrahedral geometry arises from the steric effect of the bulky ligands.The bond angle of Cl1—Cu1—P1 is less than 109.5°,being 107.31(2)°[23].However,theP1—Cu1—N1is128.60(4)°.The average Cu1—Cl1,Cu1—P1,Cu1—N1,and Cu1—Cl1ibond lengths are 0.242 07(5),0.221(5),0.206 15(12),and 0.243 14(4)nm,respectively[24].
2.3 Theoretical investigations
B3LYP correlation function by the Gaussian 09 package using density functional theory(DFT)computation technique has been performed to better understand the electronic structure of ligand Phend and both complexes.The optimized structure of ligand Phend and complex 1 are displayed in Fig.3;while that of the complex 2 is shown in Fig.4.The HOMO energy levels of Phend and 1 were-6.792 and-6.019 eV while the LUMO energy levels were-1.560 and-2.225 eV,respectively.The HOMO-LUMO energy gap of Phend and 1 were 5.232 and 3.794 eV,respectively.The HOMO energy level of 2 was-4.158 eV while the LUMO energy level of 2 was-3.218 eV.The energy gap between HOMO-LUMO of 2 was 0.943 eV.The calculated HOMO and LUMO band gap energy(3.794 eV)for the copper complex 1 was found to be lower than that of free ligand Phend(5.232 eV),which justifies the coordinating of Phend ligand to the Cu(Ⅱ)center in a tetracoordinate fashion[25].The surface plot of the highest occupied molecular orbitals(HOMOs)of 2 reveals that the HOMOs are delocalized and reside mainly on the orbitals of Cu+ion[26].
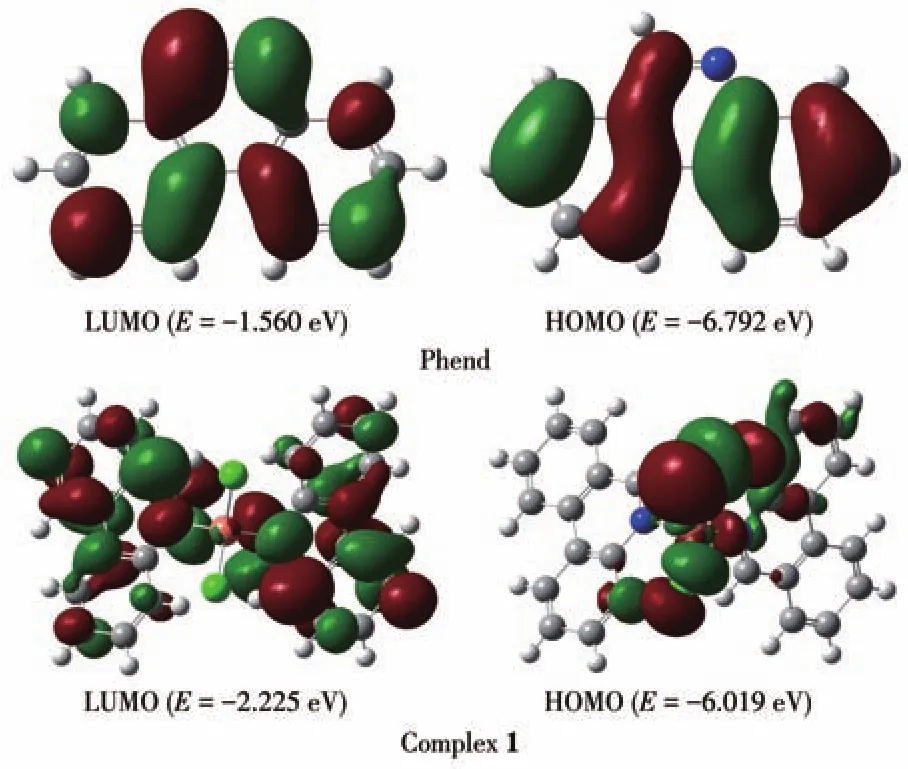
Fig.3 Surface plots of HOMO and LUMO of ligand Phend and complex 1
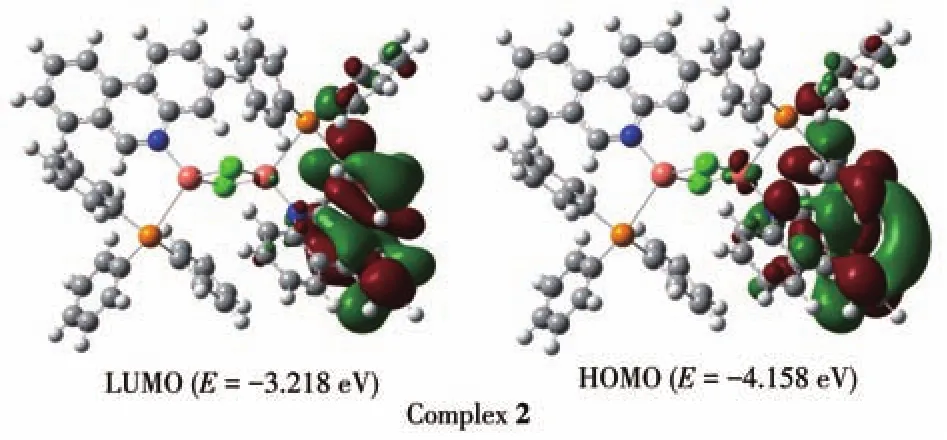
Fig.4 Surface plots of HOMO and LUMO of complex 2
2.4 UV-Vis spectral and conductivity study
The electronic absorption spectra of the ligands and the corresponding complexes 1 and 2 were recorded in DMSO solution(1 mmol·L-1)at room temperature.In the solution spectra of free ligand Phend,bands were observed at 274,290 nm and 334,342 nm,which may be attributed toπ-π*andn-π*transition respectively[27-28].In the absorption spectra of complexes 1 and 2 these bands were observed at 292,308,330,344 nm and 278,294,330,346 nm,respectively(Fig.5).Free PPh3exhibited a broad band at 274 nm,which may be assigned to theπ-π*transition[29].Complex 1 exhibited a low energy band at 403 nm(24 813 cm-1),which corresponds to charge transfer transition from the HOMO of nitrogen(pπ-orbital)to the LUMO of Cu(Ⅱ) (dπ-orbital)[30].
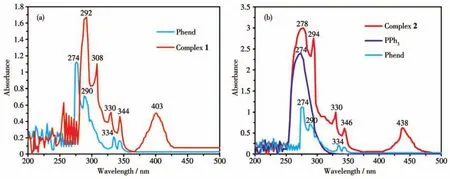
Fig.5 UV-Vis absorption spectra of the ligands and complexes 1 and 2
A broad band was observed for complex 2 at 438 nm(22 831 cm-1),which is related to metal-to-ligand charge transferd10Cu(Ⅰ) (HOMO)to(LUMO)π*orbital of ligand Phend(MLCT)transition[31].The molar conductivity values of complexes 1 and 2 were found to be very low suggesting that they are non-ionic in nature[32].
2.5 IR study
FT-IR spectra of the studied compounds are in good agreement with the X-ray crystallography data with respect to the monodentate nitrogen and phosphorus donating character of the ligands investigated.In the FT-IR spectra of complexes 1 and 2,significant peaks were observed above 3 000 cm-1and in the region of 1 400~1 600 cm-1,indicating the aromatic C—H and C=C stretching vibrations respectively[33-34].The infrared spectra of complexes 1 and 2 revealed medium intense bands at 1 608 and 1 612 cm-1,which are attributed toν(C=N),respectively.These two bands were shifted to a lower-frequency than that in the free Phend which appeared at 1 618 cm-1.This low frequency shift ofν(C=N)demonstrates that ligand Phend is coordinated to the metal ion through the nitrogen atom[35].The IR spectrum of Cu(Ⅰ)complex exhibited an expected band due to the PPh3ligand at 1 456,1 435,692,and 540 cm-1[36].In a range of 400~200 cm-1the weak-intense bands at 349 and 368 cm-1are assigned to Cu—N stretching in 1 and 2,respectively.In this IR region,the bands at 285 and 343 cm-1are attributed toν(Cu—Cl)in 1 and 2,respectively[37].In addition,the IR spectrum of complex 2 displayed a strong-intense band at 692 cm-1corresponding toν(Cu—P)[38].
3 Conclusions
In the current study,we have reported two new homoleptic and heteroleptic Cu(Ⅰ)/Cu(Ⅱ) halide complexes[Cu(κ1-Phend)2Cl2](1)and[Cu2(κ1-Phend)2(κ1-PPh3)2(μ-Cl)2](2)bearing a monodentate Phend ligand and PPh3co-ligand.From the single-crystal X-ray analysis,it has been exhibited that the complex 1 is distorted square planar arrangement around the Cu(Ⅱ)center while the complex 2 is distorted tetrahedral arrangement around Cu(Ⅰ)ion.The UV-Vis spectra of 1 and 2 displayed bands at 403 nm(24 813 cm-1)and 438 nm(22 831 cm-1),respectively,which are corresponding to LMCT and MLCT transitions respectively.The energy of molecular levels is supported by DFT computations.
Acknowledgments:We wish to thank the university of Salahaddin and university of Halabja for limited support of this work.We acknowledge Mr.Mzgin Ayoob and Ms.Aveen Jalal for help in the FT-IR and UV-Vis measurements.
Supporting information is available at http://www.wjhxxb.cn
 無機(jī)化學(xué)學(xué)報(bào)2021年6期
無機(jī)化學(xué)學(xué)報(bào)2021年6期
- 無機(jī)化學(xué)學(xué)報(bào)的其它文章
- Synthesis and Characterization of Palladium Nanoparticles with High Proportion of Exposed(111)Facet for Hydrogenation Performance
- Syntheses,Crystal Structures,Luminescence and Catalytic Activity of Manganese(Ⅱ)and Cadmium(Ⅱ)Coordination Polymers Based on 2,3-Dihydroxy-terephthalic Acid
- Scale-Up Strategy to Develop Highly-Effective Co-N-C@KB Composites as Sulfur Host for Lithium-Sulfur Battery
- Self-Assembled Zn2+,Co2+ and Ni2+ Complexes Based on Coumarin Schiff Base Ligands:Synthesis,Crystal Structure and Spectral Properties
- 添加叔丁醇鉀對Mg(NH2)2-2LiH體系儲(chǔ)氫性能的影響
- 熒光猝滅型硼氟二吡咯類Cu2+探針的合成及其生物細(xì)胞成像的應(yīng)用
