The effects of the identity of shrub species on the distribution and diversity of ground arthropods in a sandy desert ecosystem of northwestern China
JiLiang Liu, WenZhi Zhao, FengRui Li
Linze Inland River Basin Research Station, Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences, Lanzhou, Gansu 730000, China
The effects of the identity of shrub species on the distribution and diversity of ground arthropods in a sandy desert ecosystem of northwestern China
JiLiang Liu*, WenZhi Zhao, FengRui Li
Linze Inland River Basin Research Station, Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences, Lanzhou, Gansu 730000, China
Shrub is an important factor on structuring ground arthropod communities in desert ecosystems. In this study, in order to determine how shrubs and their species influence ground arthropod distribution patterns in a sandy desert scrubland dominated by two different shrub species,Calligonum mongolicumandNitraria sphaerocarpa, the ground arthropods were sampled with pitfall traps during spring, summer and autumn. At the community level, total arthropod abundance was shown to be significantly higher under shrubs than in intershrub bare areas in spring; similar patterns occurred in terms of the richness of arthropod groups in the spring and over three seasons, suggesting season-specific shrub presence effects on arthropod activity. In addition, more arthropods were found underN. sphaerocarpashrubs than underC. mongolicumshrubs in autumn, suggesting season-specific effects of shrub species of arthropod activity, whereas more arthropods taxa were captured underC. mongolicumthanN. sphaerocarpa. At the trophic group level, the abundances of predator and herbivore arthropods were significantly greater under shrubs than in intershrub bare habitats, whereas herbivore arthropods were more abundant underN. sphaerocarpathanC. mongolicum, and an opposite rule was detected for predator arthropods. At the family level, the mean abundances of Carabidae, Curculionidae, Gnaphosidae and Lycosidae were significantly higher in the shrub microhabitats than in the intershrub bare habitat, there was no significant difference between habitats on the mean abundances of Formicidae and Tenebrionidae. The study results suggested that shrub presence and shrub species variation are important determinants of ground arthropod assemblages in this desert ecosystem, but the responses of arthropods differed among trophic and taxonomic groups.
Heihe River Basin; sandy desert ecosystem; ground arthropods; shrub; shrub species
1 Introduction
Shrubland is a widely distributed vegetation type in arid and semiarid ecosystems, found both in China and other parts of the world (Polis, 1991). In shrubland ecosystems, shrubs, serving as the primary producers and providers of resources, as well as important modulators of environmental conditions (e.g., microclimate, soil nutrients and water availability), are of vital importance in the distribution and composition of the above- and below-ground arthropod communities (Stapp, 1997; Bezemeret al., 2010). Therefore, understanding the relationships between the shrubs and arthropods above and below ground isa fundamental issue in ecological research (Sylvain and Wall, 2011; Zhao and Liu, 2013). This study may support the development of a suitable strategy for biodiversity conservation management in shrubland ecosystems.
The role of shrubs as a source of spatial variation in abiotic environmental conditions in arid and semiarid ecosystems has received considerable attention in the past few decades (Charley and West, 1975; Barth and Klemmedson, 1978; Rostagnoet al., 1991; Gutiérrezet al., 1993; López-Pintoret al., 2006). Similarly, shrubs are a source of spatial variation in seed deposition and seedling recruitment of herbaceous species (Callaway, 1992; Vetaas, 1992; Pugnaire and Lázaro, 2000; Shumway, 2000; Facelli and Temby, 2002; Li, 2008). However, the role of shrubs as a source of spatial variation in ground arthropod community composition and structure in arid and semiarid ecosystems has received relatively less attention (Fallaciet al., 1997; Stapp, 1997; Petersonet al., 2001; Sanchez and Parmenter, 2002). The presence of shrubs may significantly affect soil fauna communities through their effects on microhabitat conditions (e.g., temperature and water availability, food resources, oviposition sites, shelters or refuges against predators) (Fallaciet al., 1997; Stapp, 1997; Antvogel and Bonn, 2001; Sanchez and Parmenter, 2002; Boteset al., 2007; Pétillonet al., 2008; Sackmann and Flores, 2009; Liuet al., 2011; Zhao and Liu, 2013).
As of yet, relatively few researchers have addressed the combined effects of shrub presence and shrub species on the assemblage structure of ground arthropods with greater dispersal ability, compared with soil arthropods in arid and semiarid ecosystems (e.g., Rogerset al., 1988; Stapp, 1997; Mazíaet al., 2006; Yinet al., 2010; Zhao and Liu, 2013). Therefore, little is known about the functional roles of these major factors as a source of spatial variation in ground arthropod communities (Liuet al., 2010). In order to obtain a better understanding of the functional roles of shrub presence and shrub species in structuring ground arthropod communities, a field study was conducted in a sandy desert ecosystem of northwestern China. A natural shrub community dominated by two native shrub species,i.e.,Calligonum mongolicumandNtraria sphaerocarpa, was studied to address the following issues: (1) whether or not the presence and species of shrub influences the abundance and richness of ground arthropods; and (2) whether or not these effects vary among taxa, as well as seasons. It was hypothesized that shrub presence and species identity would affect the spatial distribution and diversity of the arthropod community by altering the spatial patterns of habitat use of ground arthropods, but their effects would vary among different trophic groups and taxa, as well as across seasons.
2 Materials and methods
2.1 Study area
This study was conducted in a typical sandy desert ecosystem in the middle reaches of the Heihe River (39°21′N, 100°09′E; 1,380 m a.s.l.), the second largest inland river basin in the arid regions of northwestern China. The site has a temperate continental climate. The average annual temperatures is 7.6 °C, with a mean minimum temperature of ?10.7 °C in January and mean maximum temperature of 23.8 °C in July. The average annual precipitation over the past 30 years is 117 mm, with 75% of rainfall falling between June and September (Liuet al., 2010). The soil is classified as aeolian sandy soil according to the Chinese classification system, with 19.89%±3.41% (mean ± SD) coarse sand content (>0.25 mm), 78.98%±3.42% medium and fine sand content (0.05-0.25 mm) and 1.13%±0.07% silt and clay content (<0.05mm) (Liuet al., 2010). The soil bulk density is 1.56±0.01 g/cm3, and pH is 9.24±0.08. Soil organic carbon, total nitrogen and total phosphorus concentrations are 0.69±0.02 g/kg, 0.011±0.001 g/kg and 0.25±0.02 g/kg, respectively (Liuet al., 2010).
Vegetation at the sandy shrubland site is dominated by two xerophytic shrub species,i.e.,Calligonum mongolicumTurcz. andNitraria sphaerocarpaMaxim., along with herbaceous species such asAgriophyllum squarrosum(L.) Moq.,Bassia dasyphyllaKuntze,Halogeton arachnoideusMoq.,Echinops gmeliniTurcz., andPugionium calcaratumKom. Vegetation cover in the sandy shrubland is 39.11%±3.27%. The shrub density is 1,222±480 plants/km2forC. mongolicumand 1,556±720 plants/km2forN. sphaerocarpa. The density of herbaceous vegetation is 3.28±0.35 plants/m2. The amounts of plant litter are 11.1±1.5 g/shrub underC. mongolicumand 5.1±1.2 g/shrub underN. sphaerocarpa.
2.2 Sampling of ground arthropods
To determine the influence of shrubs and species identity on the distribution and diversity of ground beetles, three sampling plots (200m×200m) at least 500 m apart (Ziesche and Roth, 2008) were established in a sandy shrubland that has been used as a permanent vegetation monitoring site by the Linze Inland River Basin Research Station, Chinese Ecosystem Research Network. In each sampling plot, four replicate individualC. mongolicumshrubs of similar sizes (76.6±7.3 cm in plant height and 94.4±9.5 cm in canopy diameter) and four replicate individualN. sphaerocarpashrubs (50.4±3.1 cm in plant height and 72.8±13.6 cm in canopy diameter), as well as four replicate bare soil sites between shrubs were selected in each plot. The distance between the target shrubsthat were sampled within a plot was >6 m (Mazíaet al., 2006). Pitfall traps (8 cm in diameter, 10 cm in depth) filled with approximately 70 mL of 70% ethanol solution were buried flush with the ground surface under canopies of both shrubs, as well as in the intershrub bare areas (the distance to the nearest shrub sampled was >5 m). Beetles were sampled with pitfall traps positioned in three different microhabitats (i.e., under canopies ofC. mongolicumandN. sphaerocarpashrubs, and intershrub bare ground) in late spring, mid-summer, and early autumn corresponding to the main activity period of ground beetles in the study area (Liuet al., 2010). Each of the three sampling periods consisted of 15 consecutive days (day and night). All traps were checked every three days during the 15-day sampling period. We added ethanol solution throughout the 15-day sampling period when the solution evaporated completely. Captured ground arthropod (preserved in 75% ethyl alcohol) were counted and identified to the family level following the keys presented by Zheng and Gui (1999). Tenebrionidae were identified to the species level according to the method described by Ren and Yu (1999). We also categorized beetles into predators, detritivores, omnivores and herbivores based on their diet and feeding habits (Zheng and Gui, 1999; Zhanget al., 2002).
2.3 Data analysis
A total of 13,001 adult arthropods were captured from the three microhabitat types during the three seasons, which belonged to 13 taxonomic families in two orders (namely Arachnida and Insecta). The arachnid assemblage included six families and the insect assemblage included seven (Appendix 1). In order to determine the community, trophic group and taxa-level responses of the ground arthropods to the presence and species of the shrubs, all 12 families and 7 species of Tenebrionidae were classified into one of three trophic groups based on feeding habitat, as follows: predators, herbivores, and decomposers (including detritivores and omnivores). The following parameters were then calculated at the community level: total abundance (total number of ground arthropods captured per pitfall trap, averaged over the four traps per plot) and total taxonomic richness (total number of families found in the four traps per plot), using a mean of the four traps per plot. At the trophic group level, the abundance (total number of ground arthropods captured per trap, averaged over the four traps of each plot) of each of the three trophic groups, such as predators, herbivores and decomposers, was calculated. At the family level, the abundance (total number of ground arthropods captured per trap, averaged over the four traps per plot) of the top six dominant families (Carabidae, Curculionidae, Formicidae, Gnaphosidae, Lycosidae and Tenebrionidae) was calculated. One-way ANOVA and Tukey’s (HSD) post-hoc tests were performed using SPSS 16.0 for Windows (SPSS Inc., Chicago, Illinois), to examine the effects of shrub presence and species identity on the distribution of ground arthropods at the different taxonomic levels by quantifying the differences in the response variables among the microhabitats. Due to the seasonal variation in the arthropod community, the data were analyzed separately for each season to determine whether the influence of shrub presence and species identity was consistent across the seasons. In these analyses, all data were lg(χ+1)-transformed to improve assumptions of normality and homogeneity of variances.
Two different methods were applied to explore seasonal changes in the arthropod community composition among the microhabitats. First, principal components analysis (PCA) was performed to visually identify the habitat preferences of ground arthropods, and compare the spatial and temporal patterns of the arthropod community. Second, seasonal variation in the arthropod community composition was compared among the microhabitats using the S?rensen index, which is a measure of proportional similarity, ranging from 0 (no similarity) to 1 (identical).
3 Results
3.1 Community-level responses to the presence and species of shrubs
After being averaged over the three seasons, no significant differences in terms of ground arthropod abundance were observed among the three microhabitats (Table 1). However, total arthropod family richness was higher in theC. mongolicumshrub microhabitats than in theN. sphaerocarpashrub microhabitats or in the intershrub bare ground (Table 1). The data pooled from the three seasons indicate that shrub species did not affect arthropod abundance, but a significant difference between the two shrub species was observed in the total arthropod family richness. A greater number of arthropod families were captured under theC. mongolicumshrub than under theN. sphaerocarpashrub, but this effect was only detected over the three seasons (Table 1). Separate analyses for each season showed clear seasonal variation in terms of the influence of shrub presence on arthropod abundance and richness. The mean total number of arthropods trapped in theC. mongolicumshrub microhabitats was lower than in theN. sphaerocarpashrub microhabitats or in the intershrub bare ground in autumn (Table 1). Similarly, a higher arthropod family richness was found in theC. mongolicumshrub microhabitats than the intershrub bare ground in spring (Table 1).
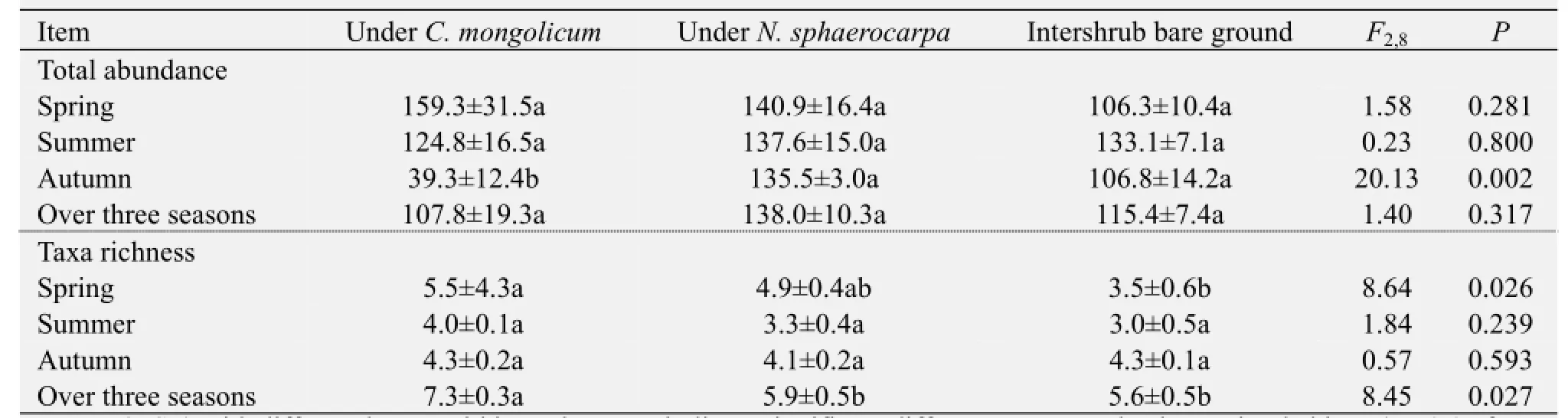
Table 1 Abundance and taxa richness of ground arthropod communities in the three microhabitat types (under the canopies ofCalligonum mongolicum,Nitraria sphaerocarpa, and in the intershrub bare ground microhabitat)
3.2 Trophic-level responses to the presence and species of shrubs
The abundance of the three trophic groups was affected differently by the presence of shrubs. The data pooled from the three sampling periods indicated that the mean abundance of predator arthropods was significantly higher in theC. mongolicumshrub microhabitats than in theN. sphaerocarpashrub microhabitats or in the intershrub bare ground (Table 2). An opposite pattern was observed in the abundance of herbivorous arthropods (Table 2). However, mean abundance of decomposer arthropods did not differ significantly among the three microhabitats (Table 2). Separate analyses for each season of each trophic group showed that shrub presence effects on the abundance of predator and herbivorous arthropods varied among the sampling periods. For example, the influence of shrub presence on predator arthropod abundance was significant in the spring and summer sampling periods, but that on herbivorous arthropods was significant in all three sampling periods (Table 2).
Similarly, the abundance of the three trophic groups differently responded to the identity of the shrub species. Based on their average over the three sampling periods, the difference between the two shrub species in terms of herbivore and predator arthropods was observed to be significant, but was not significant between the two shrub species in terms of decomposer arthropods (Table 2). In these cases, the predator arthropods were more frequently captured in the traps underC. mongolicumthanN. sphaerocarpa, whereas the opposite pattern was observed for the herbivore arthropods. Unlike the herbivore and decomposer arthropods, however, decomposer arthropod abundance did not show a significant difference between the two shrub species (Table 2). Separate analyses for each season and of each trophic group showed that the shrub species effects on the abundance of predator and herbivore arthropods were highly season-dependent.
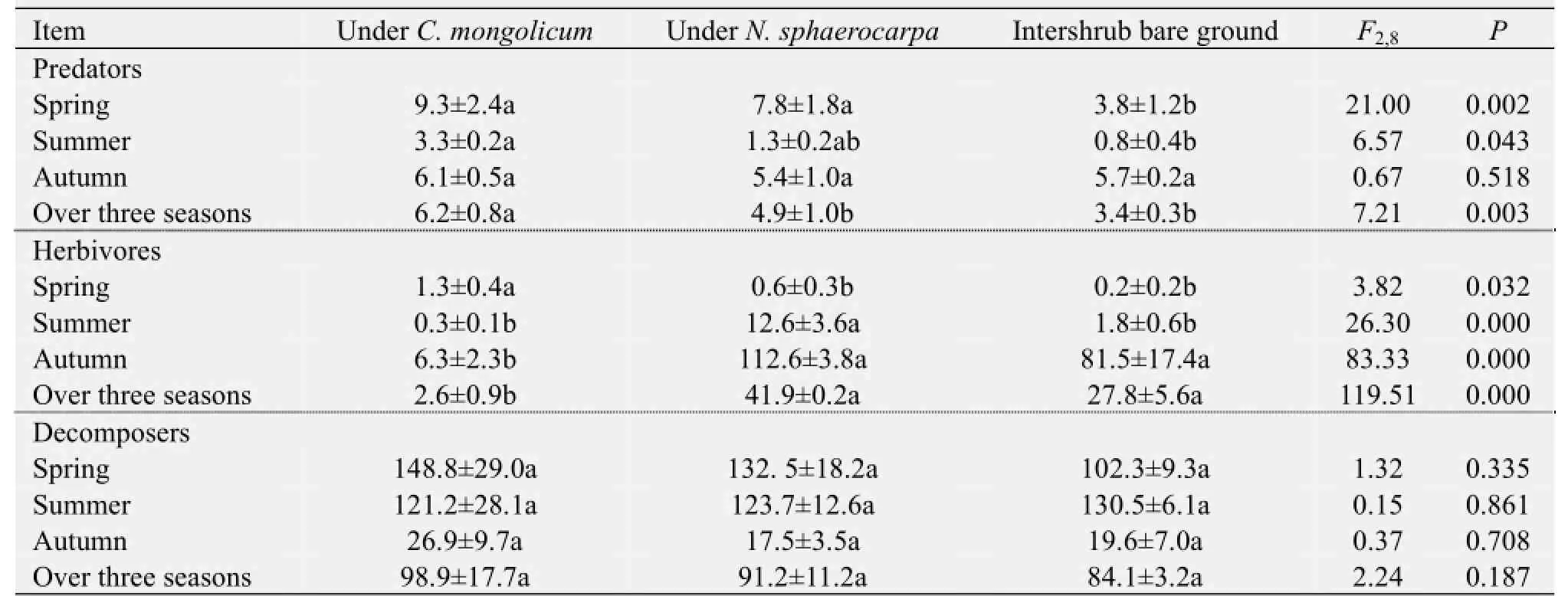
Table 2 Abundance of three trophic groups of ground-dwelling arthropods under the canopies ofCalligonum mongolicum,Nitraria sphaerocarpa, and in the intershrub bare ground microhabitat
3.3 Taxon-level responses to the presence and species of shrubs
Four response groups were identified based on the responses of the six dominant families to the presence and species of shrub (Table 3). The first group included Carabidae, the abundance of which did not differ significantly among the three microhabitats when all three sampling periods were pooled. The second group included Gnaphosidae and Lycosidae, which were significantly more abundant in theC. mongolicumshrub microhabitats than in the intershrub bare habitat. This indicates that the presence of shrubs strongly affected the pattern of habitat use of these two families. The third group included Curculionidae, the abundance of which was strongly affected by the species identity of the plant. The mean abundance of Curculionidae was significantly higher underN. sphaerocarpathanC. mongolicum. The fourth group included Formicidae and Tenebrionidae, which did not significantly differ among the three microhabitats.
Separate analyses for each season and of each family showed that the family-specific shrub presence and species effects were also season-dependent. For example, the positive effects of shrub presence on Gnaphosidae abundance was detected in all three seasons, whereas that on Carabidae abundance was only observed in spring (Table 3). Similarly, the effects of shrub species on the abundance of Curculionidae were significant in all three seasons, but Gnaphosidae abundance was strongly affected by shrub species only in summer and autumn (Table 3).
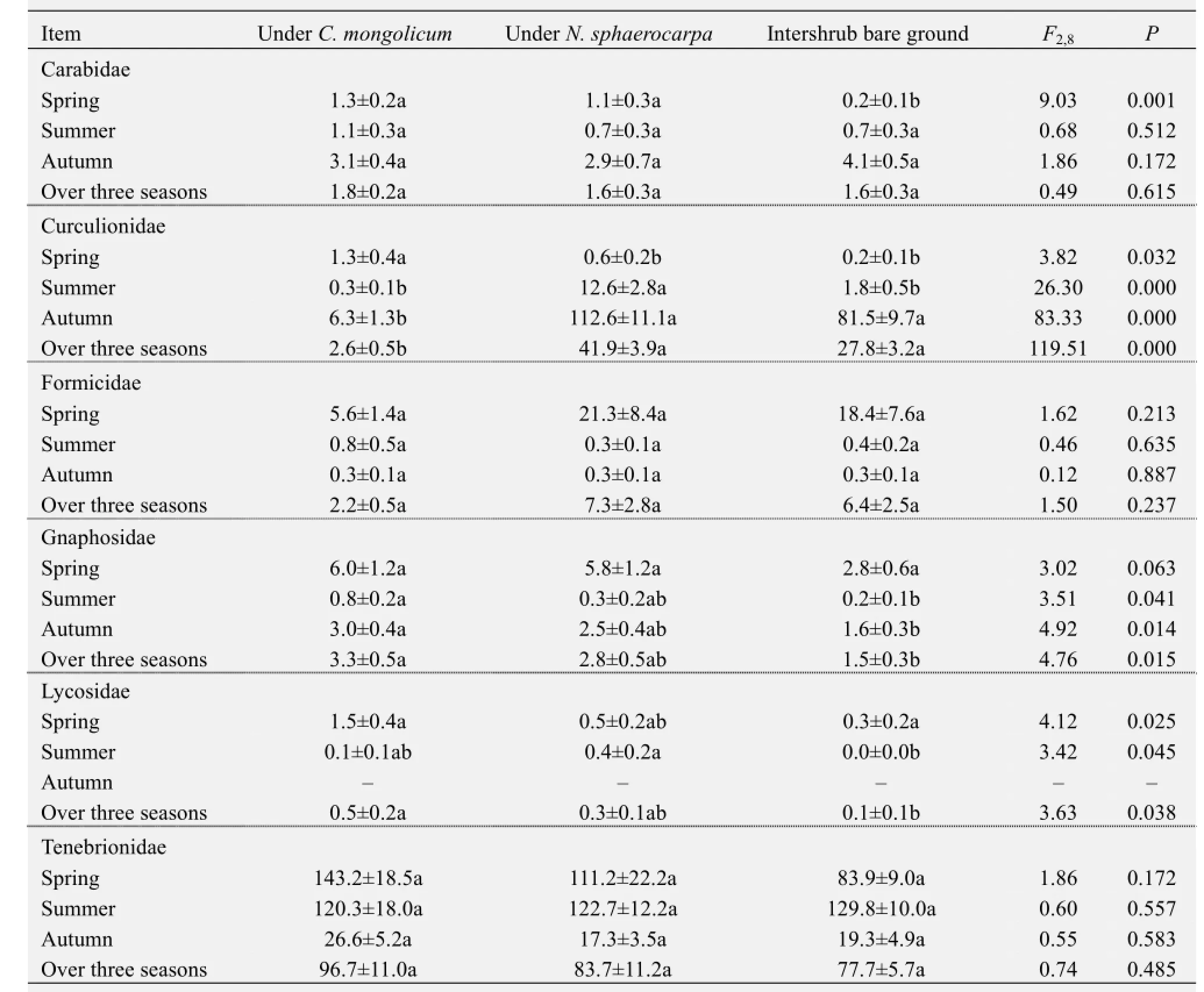
Table 3 Abundances of the six dominant families in the ground arthropod communities under the canopies ofCalligonum mongolicum,Nitraria sphaerocarpa, and in the intershrub bare ground microhabitat
3.4 Assemblage composition
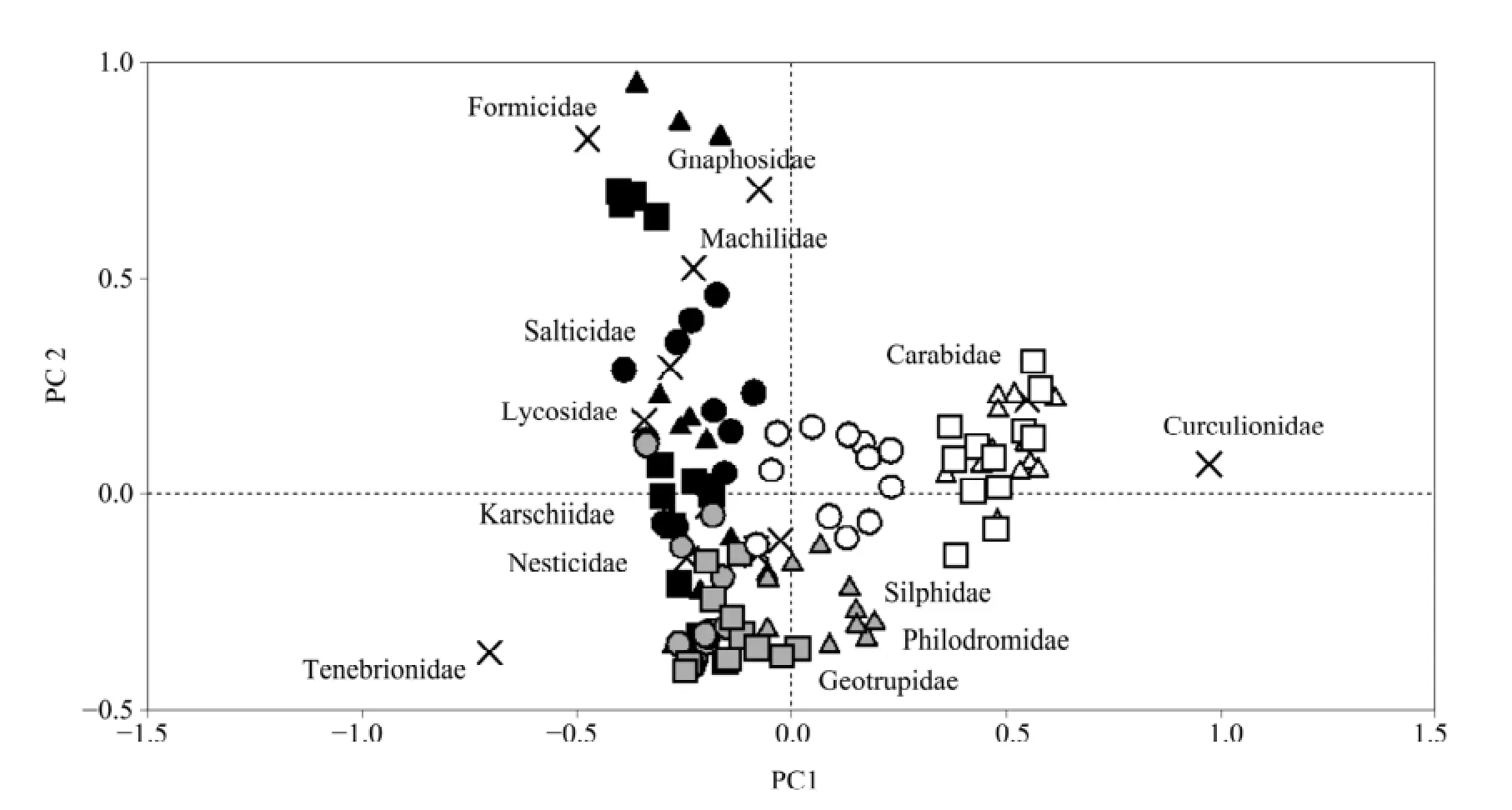
Figure 1 PCA ordination of ground arthropod assemblage composition for the different microhabitats during the three sampling periods. In the biplot, the arthropod families are represented by "×". The three microhabitat types are represented by circles (underCalligonum mongolicumcanopies), triangles (underNitraria sphaerocarpacanopies), and squares (intershrub bare ground). The three sampling periods are distinguished by the colors of the symbol,i.e., black (spring), gray (summer), and white (autumn)
The PCA ordination of ground arthropod assemblages, considering both microhabitats and sampling periods, showed that the assemblage composition in the different microhabitats is strongly affected by seasonal variation. The PCA plot showed a rather distinct group consisting of arthropod assemblages in autumn, whereas the assemblages in the spring and summer differed less significantly (Figure 1). These results are further supported by the S?rensen similarity analysis, which shows strong seasonal variation in the similarity between arthropod assemblages across the three microhabitats. Overall, the assemblage composition underC. mongolicumwas more similar to that underN. sphaerocarpain all three seasons (with the respective S?rensen index values for comparing community composition between the two shrub habitats being 0.82, 0.78 and 1.00 in spring, summer and autumn). However, the communities in the shrub microhabitats differed markedly from those in the intershrub bare habitat in summer (with the S?rensen index values comparing the community composition between the intershrub bare habitat and the shrub microhabitat being 0.59 forC. mongolicumand 0.77 forN. sphaerocarpa). However, the similarity in the assemblage composition between the shrub microhabitats and the intershrub bare habitat increased markedly in autumn (with the S?rensen index values comparing the community composition between the intershrub bare habitat and shrub microhabitat being 1.00 forC. mongolicumand 1.00 forN. sphaerocarpa).
4 Discussion
This study provides the first investigation regarding the influence of shrub presence and species identity on the distribution and diversity of ground-dwelling arthropod communities in a sandy desert of northwestern China. The study demonstrates that the presence of shrubs may have a beneficial influence on the assembly of local ground arthropod communities, by altering patterns of the habitat use of ground arthropods in sandy shrubland desert ecosystems. This finding is consistent with those of studies involving other shrubland ecosystems, which demonstrates that shrub cover has strong effect on the abundance and diversity of soil macroarthropod communities (Petersonet al., 2001; Pen-Mouratovet al., 2004; Doblas-Mirandaet al., 2009; Liuet al., 2011; Zhao and Liu, 2013), as well as ground beetle communities (Rogerset al., 1988; Stapp, 1997; Mazíaet al., 2006; Liuet al., 2012). The observed positive effects of shrubs on arthropod richness may be attributed to the ecological role which individual shrubs play in modifying their nearby biotic and abiotic environmental conditions, which in turn determine the distribution and recruitment of ground arthropod communities (Antvogel and Bonn, 2001; Barber and Marquis, 2011; Liuet al., 2012; Liet al., 2013). However, no significantly positive effects of shrubs on arthropod abundance in sandy desert shrub-lands were found. In addition, PCA ordination and S?rensen similarity analyses show considerably similar patterns in the arthropod community composition between the shrub microhabitats and intershrub bare habitat. This suggests that the presence of shrubs exerted only a weak influence on the composition of ground arthropod communities. The current findings show that in the sandy desert shrubland ecosystem, with its rugged conditions and loose soil texture (greater than 99% sand content), shrubs have a weak effect on ground arthropods. Furthermore, no significant difference of soil texture (percentage content of sand;F2,24=2.70,P=0.087) was found between shrub microhabitat and intershrub bare microhabitat. The results of some previous studies have demonstrated that soil texture have strong effects on the distribution of ground-dwelling arthropod in desert ecosystems (Stapp, 1997; Liet al., 2011; Liuet al., 2012). However, shrubs have no effects on soil texture in the sandy desert ecosystem, and in turn weak effects on the assemblage structure of the ground-dwelling arthropod community at the small spatial scales. Therefore, one can conclude that the ameliorated microclimatic conditions and greater food resources under shrubs have been shown to strongly affect the distribution of ground arthropods in a sandy desert ecosystem (Crawford, 1988; Stapp, 1997; Mazíaet al., 2006; Shelef and Groner, 2011; Liuet al., 2012; Liet al., 2013).
Unexpectedly, however, a significant difference in arthropod abundance was observed between the two shrub species in autumn, as was observed in arthropod richness over three seasons, indicating that shrub species had effects on both arthropod abundance and richness. Based on the data pooled from all three seasons, the mean number of families trapped underC. mongolicumwas significantly higher than that underN. sphaerocarpa, which suggests that a greater number of ground arthropods preferC. mongolicumtoN. sphaerocarpa, thus highlighting the importance of this shrub species to the persistence of ground arthropod assemblages in our system. At the same time, the mean number of ground arthropods trapped underN. sphaerocarpawas also found to be higher than underC. mongolicum, which suggests that some ground arthropod taxes preferN. sphaerocarpatoC. mongolicum. These results are only partially consistent with the findings of Petersonet al. (2001), Doblas-Mirandaet al. (2009) and Liuet al. (2012). In a study regarding the influence of a single shrub on earthworms and soil macroarthropods in a southern California chaparral ecosystem, Petersonet al. (2001) observed significant differences in earthworm abundance among the four dominant shrub species, but the four shrub species did not differ in terms of the litter-active macrofaunal abundance. Similarly, in a study regarding the effects of three shrub species (Ar-temisia herba-albaAsso,Retama sphaerocarpaL., andSalsola oppositifoliaDesf) on the structure of soil macroinvertebrate communities in an arid Mediterranean ecosystem, Doblas-Mirandaet al. (2009) reported significant differences in terms of soil macrofaunal diversity, abundance, and biomass among shrub species. Additionally, in a study on the effects of two shrubs (i.e.,Nitraria sphaerocarpaandReaumuria soongorica) on the structure of darkling beetle (Tenebrionidae) communities in a Gobi desert ecosystem, Liuet al. (2012) reported significant differences on darkling beetle abundance and richness between shrub species. The above results suggest that various soil and ground-dwelling faunal groups or communities respond differently to the identity of shrub species. The observed differences in the response of ground arthropod abundance to the two shrub species may be explained by the differences in morphological, chemical, and phenological plant traits between the two shrub species (Whithamet al., 1999), as well as by differences in resource quantity and quality which both shrub species provide (Wilderet al., 2011). In the system of the present study, the two shrub species differ in the following aspects: first, more resources (litter production) under adultC. mongolicumplants were observed than under adultN. sphaerocarpaplants; and second, leaves ofN. sphaerocarpa(22.2 g/kg) have much higher nitrogen content than those ofC. mongolicum(9.2 g/kg). Therefore, the high leaf production ofC. mongolicumand high leaf-nitrogen content ofN. sphaerocarpamay explain why in the system used in this study most families of arthropods preferredC. mongolicumand only some arthropods preferredN. sphaerocarpa.
Furthermore, evidence was found that the influence of shrub presence and species identity differed among the trophic level of ground arthropods; in particular, such trophic group-specific effects were also found to be season-dependent (Table 2). The emergence of such season-specific shrub presence and species effects most likely reflects the growth pattern of the shrubs with regard to the seasonal dynamics of their leaf production. At the same time, the seasonal effects may be also related to the activity pattern of ground arthropods which are controlled by the staggered characteristics of their growth and development (L?vei and Sunderland, 1996; Weeks and Holtzer, 2000; Zhanget al., 2002; Zotov and Alpatov, 2005). For the three trophic groups covered in this study, the predator and herbivore arthropods generally had significantly higher abundance in the shrub microhabitats than in the intershrub bare habitat (Table 2). This finding suggests that shrub presence is associated with increased species abundance of predator and herbivore arthropods, but does not greatly affect decomposer arthropod abundance. This observation also suggests apositive relationship between shrubs and predator and herbivore arthropods. However, no evidence of a positive response between shrubs and decomposer arthropods was found, as decomposer arthropod abundances underN. sphaerocarpaandC. mongolicumwere similar to those in the intershrub bare habitat. In addition, a significant difference was also found between the two shrub species in terms of predator and herbivore arthropod abundance. More predator arthropods were captured underC. mongolicumthanN. sphaerocarpaover the three seasons; however, an opposite rule was observed for herbivore arthropods. The above results suggest that different trophic groups respond differently to the identity of the shrub species. It was also found that the fact that predator arthropods commonly preferredC. mongolicumwas affected by litter production, and that herbivore arthropods commonly preferredN. sphaerocarpawas affected by leaf quantity.
For the six dominant arthropod families, the abundances of Carabidae, Curculionidae, Gnaphosidae and Lycosidae were consistently much greater under shrubs than in the intershrub bare habitats, both in any given season or over the three seasons. This suggests a consistently positive response of these four taxa to shrub cover, and highlights the importance of shrubs in terms of the population persistence of these five taxa. In contrast, there were no differences observed in the abundances of Formicidae and Tenebrionidae between the shrub microhabitats and the intershrub bare habitats, indicating that these two arthropod taxa are relatively insensitive to the identity of the microhabitat. A similar finding was reported by Petersonet al. (2001), who described that the abundance of ants (Formicidae) under shrubs was similar to that in shrub-free bare area. Furthermore, we also found more the nesting of ants was observed in the intershrub than under shrubs in studied region (personally observation). Therefore, the pattern of preference for shrubs on Formicidae was not observed in the sandy desert ecosystem. An opposite finding was reported by Liuet al. (2012), who observed that the abundance of darkling beetles (Tenebrionidae) under shrubs was significantly higher than that in shrub-free bare areas, which may be determined by habitat conditions (vegetation and soil texture). The two shrub species also differed in terms of their influence on the six dominant arthropod families. Evidence that more arthropods of Curculionidae were captured underN. sphaerocarpathan underC. mongolicumsuggests that Curculionidae belong to herbervore arthropods, which preferN. sphaerocarpa. In addition, we also observed some Curculionidae feeding on grass between shrubs; furthermore, more Curculionidae were captured in the intershrub bare habitat than in theC. mongolicumshrub microhabitats due to the primary production of the grasses. This result was consistent with that reported by Liuet al. (2012). However, no differences were found between the two shrub species in terms of the abundances of Carabidae, Formicidae, Gnaphosidae, Lycosidae and Tenebrionidae, suggesting that these five taxa were not significantly affected by the identity of the shrub species. The considerable difference in the extent of the response to shrub species identity between the three trophic groups and six dominant families may be explained by the differences in their ecology and biology (L?vei and Sunderland, 1996; Colemanet al., 2004; Nielsenet al., 2010).
5 Conclusions
The purpose of this paper is to analyze the impact of shrub presence and shrub species identity on the distribution of the ground arthropod community in a sandy desert ecosystem of northwestern China. The results suggest that shrub presence and shrub species variation are important determinants of ground arthropod assemblages in this arid desert ecosystem, but the response of arthropods differed among trophic or taxonomic groups. The results also suggest that in the sandy shrubland, shrub presence and shrub species effects on the distribution of ground arthropods were season-specific, and overall effects varied considerably across arthropods due to their significant differences in species identity and biological traits (feeding types). However, in addition to shrubs, it was also found that the distribution of ground-dwelling arthropods was shaped by habitat conditions (e.g., soil texture and vegetation texture). Over the past several decades, prolonged overgrazing disturbances have caused extensive degradation in the desert ecosystems of northwestern China, resulting in significant reductions in the cover, biomass and species richness of the shrub communities. This degradation is likely to have a strong impact on ground arthropods. The findings of this study provide valuable information supporting the development of a regional conservation plan. Our understanding of the season- and species-specific effects of shrub presence and species on the assembly of ground arthropod communities highlights the importance of protecting shrub vegetation, as well as its species diversity, for conserving species pools of ground arthropod assemblages in sandy ecosystems of northwestern China.
Acknowledgments:
The authors are very thankful to two reviewers for proposing good suggestions. This research was funded by one of National Basic Research Program of China (No. 2013CB429903) and National Natural Science Foundation of China (Grant Nos. 41201248 and 31170496).
Antvogel H, Bonn A, 2001. Environmental parameters and microspatial distribution of insects: a case study of carabids in an alluvial forest. Ecography, 24(4): 470?482. DOI: 10.1111/j.1600-0587.2001.tb00482.x.
Barber NA, Marquis RJ, 2011. Leaf quality, predators, and stochastic processes in the assembly of a diverse herbivore community. Ecology, 92(3): 699?708.
Barth RC, Klemmedson JO, 1978. Shrub-induced spatial patterns of dry matter, nitrogen, and organic carbon. Soil Science Society of America Journal, 42(5): 804?809.
Bezemer TM, Fountain MT, Barea JM,et al., 2010. Divergent composition but similar function of soil food webs of individual plants: plant species and community effects. Ecology, 91(10): 3027?3036. DOI: http://dx.doi.org/10.1890/09-2198.1.
Botes A, McGeoch MA, Chown SL, 2007. Ground-dwelling beetle assemblages in the Northern Cape Floristic Region: Patterns, correlates and implications. Austral Ecology, 32(2): 210?224. DOI: 10.1111/j.1442-9993.2007.01681.x.
Callaway RM, 1992. Effect of shrubs on recruitment of Quercus douglasii and Quercus lobata in California. Ecology, 73(6): 2118?2128. DOI: http://dx.doi.org/10.2307/1941460.
Charley JL, West NE, 1975. Plant-induced soil chemical patterns in some shrub-dominated semi-desert ecosystems of Utah. The Journal of Ecology, 63: 945?963.
Coleman DC, Crossley Jr DA, Hendrix PF, 2004. Fundamentals of Soil Ecology. 2nd edition. Academic Press, San Diego.
Crawford CS, 1988. Surface-active arthropods in a desert landscape: influences of microclimate, vegetation, and soil texture on assemblage structure. Pedobiologia, 32(5-6): 373-385.
Doblas-Miranda E, Sánchez-Pi?ero F, González-Megías A, 2009. Different microhabitats affect soil macroinvertebrate assemblages in a Mediterranean arid ecosystem. Applied Soil Ecology, 41(3): 329?335. DOI: 10.1016/j.apsoil.2008.12.008.
Facelli JM, Temby AM, 2002. Multiple effects of shrubs on annual plant communities in arid lands of South Australia. Austral Ecology, 27(4): 422?432. DOI: 10.1046/j.1442-9993. 2002.01196.x.
Fallaci JM, Colombini I, Palesse L,et al., 1997. Spatial and temporal strategies in relation to environmental constraints of four tenebrionids inhabiting a Mediterranean coastal dune system. Journal of Arid Environments, 37(1): 45?64. DOI: 10.1006/ jare.1997.0258.
Gutiérrez JR, Meserve PL, Contreas LC,et al., 1993. Spatial distribution of soil nutrients and ephemeral plants underneath and outside the canopy of Porlieria chilensis shrubs (Zygophyllaceae) in arid coastal Chile. Oecologia, 95(3): 347?352. DOI: 10.1007/BF00320987.
Li FR, 2008. Presence of shrubs influences the spatial pattern of soil seed banks in desert herbaceous vegetation. Journal of Vegetation Science, 19(4): 537?548. DOI: 10.3170/ 2008-8-18404.
Li FR, Liu JL, Liu CA,et al., 2013. Shrubs and species identity effects on the distribution and diversity of ground-dwelling arthropods in a Gobi desert. Journal of Insect Conservation, 17(2): 319?331. DOI: 10.1007/s10841-012-9512-1.
Li XR, Jia RL, Chen YW,et al., 2011. Association of ant nests with successional stages of biological soil crusts in the Tengger Desert, Northern China. Applied Soil Ecology, 47(1): 59?66. DOI: 10.1016/j.apsoil.2010.10.010.
Liu JL, Li FR, Liu CA,et al., 2012. Influences of shrub vegetation on distribution and diversity of a ground beetle community in a Gobi desert ecsystem. Biodiversity and Conservation, 21: 2601-2619. DOI: 10.1007/s10531-012-0320-4.
Liu JL, Li FR, Liu QJ,et al., 2010. Seasonal variation in soil fauna community composition and diversity in an arid desert ecosystem of the Heihe Basin. Journal of Desert Research, 30: 342-349.
Liu RT, Zhao HL, Zhao XY,et al., 2011. Facilitative effects of shrubs in shifting sand on soil macro-faunal community in Horqin Sand Land of Inner Mongolia, Northern China. European Journal of Soil Biology, 47: 316-321. DOI: 10.1016/j.ejsobi.2011.07.006.
López-Pintor A, Gómez Sal A, Benayas JMR, 2006. Shrubs as a source of spatial heterogeneity: the case of Retama sphaerocarpa in Mediterranean pasture of central Spain. Acta Oecologica, 29: 247-255. DOI: 10.1016/j.actao.2005.11.001.
L?vei GL, Sunderland KD, 1996. Ecology and behavior of ground beetles (Coleoptera: Carabidae). Annual Review of Entomology, 41: 231-256. DOI: 10.1146/annurev.en.41.010196.001311.
Mazía CN, Chaneton EJ, Kitzberger T, 2006. Small-scale habitat use and assemblage structure of ground-dwelling beetles in a Patagonian shrub steppe. Journal of Arid Environments, 67(2): 177-194. DOI: 10.1016/j.jaridenv.2006.02.006.
Nielsen UN, Osler GHR, Campbell CD,et al., 2010. The influence of vegetation type, soil properties and precipitation on the composition of soil mite and microbial communities at the landscape scale. Journal of Biogeography, 37: 1317-1328. DOI: 10.1111/j.1365-2699.2010.02281.x.
Pen-Mouratov S, Rakhimbaev M, Barness G,et al., 2004. Spatial and temperal dynamics of nematode populations under Zygophyllum dumosum in arid environments. European Journal of Soil Biology, 40: 31-46. DOI: 10.1016/j.ejsobi.2004.01.002.
Peterson AC, Hendrix PF, Haydu C,et al., 2001. Single-shrub influence on earthworms and soil macroarthropods in the southern California chaparral. Pedobiologia, 45(6): 509-522. DOI: 10.1078/0031-4056-00103.
Pétillon J, Georges A, Canard A,et al., 2008. Influence of abiotic factors on spider and ground beetle communities in different salt-marsh systems. Basic and Applied Ecology, 9(6): 743-751. DOI: 10.1016/j.baae.2007.08.007.
Polis GA, 1991. The Ecology of Desert Communities. The University of Arizona Press, Tucson.
Pugnaire FI, Lázaro R, 2000. Seed bank and understorey species composition in a semi-arid environment: the effect of shrub age and rainfall. Annals of Botany, 86(4): 807-813. DOI: 10.1006/anbo.2000.1240.
Ren GD, Yu YZ, 1999. The Darking Beetles from Deserts and Semideserts of China (Coleoptera: Tenebrionidae). Hebei University Publishing House, Baoding.
Rogers LE, Woodley NE, Sheldon JK,et al., 1988. Diets of darkling beetles (Coleoptera: Tenebrionidae) within a shrub-steppe ecosystem. Annals of the Entomological Society of America, 81(5): 782-791.
Rostagno CM, Del Valle HF, Videla L, 1991. The influence of shrubs on some chemical and physical properties of an aridic soil in north-eastern Patagonia, Argentina. Journal of Arid Environments, 20(2): 179-188.
Sackmann P, Flores GE, 2009. Temporal and spatial patterns of tenebrionid beetle diversity in NW Patagonia, Argentina. Journal of Arid Environments, 73: 1095-1102. DOI: 10.1016/ j.jaridenv.2009.05.007.
Sanchez BC, Parmenter RR, 2002. Patterns of shrub-dwelling arthropod diversity across a desert shrubland grassland ecotone: a test of island biogeographic theory. Journal of Arid Environments, 63: 247-265. DOI: 10.1006/jare.2001.0920.
Shelef O, Groner E, 2011. Linking landscape and species: Effect of shrubs on patch preference of beetles in arid and semi-arid ecosystems. Journal of Arid Environments, 75: 960-967. DOI: 10.1016/j.jaridenv.2011.04.016.
Shumway SW, 2000. Facilitative effects of a sand dune shrub on species growing beneath the shrub canopy. Oecologia, 124: 138-148. DOI: 10.1007/s004420050033.
Stapp P, 1997. Microhabitat use and community structure of darkling beetles (Coleoptera: Tenebrionidae) in shortgrass prairie: effects of season, shrub cover and soil type. American Midland Naturalist, 137: 298-311.
Sylvain ZA, Wall DH, 2011. Linking soil biodiversity and vegetation: implications for a changing planet. American Journal of Botany, 98: 517-527. DOI: 10.3732/ajb.1000305.
Vetaas OR, 1992. Micro-site effects of trees and shrubs in dry savannas. Journal of Vegetation Science, 3: 337-344. DOI: 10.2307/3235758.
Weeks RD, Holtzer TO, 2000. Habitat and season in structuring ground-dwelling spider (Araneae) communities in a shortgrass steppe ecosystem. Environmental Entomology, 29: 1164-1172. DOI: http://dx.doi.org/10.1603/0046-225X-29.6.1164.
Whitham TG, Martinsen GD, Floate KD,et al., 1999. Plant hybrid zones affect biodiversity: Tools for a genetic-based understanding of community structure. Ecology, 80: 416-428.
Wilder SM, Holway DA, Suarez AV,et al., 2011. Macronutrient content of plant-based food affects growth of a carnivorous arthropod. Ecology, 92: 325-332.
Yin XQ, Song B, Dong WH,et al., 2010. A review on the eco-geography of soil fauna in China. Journal of Geographical Sciences, 20(3): 333-346. DOI: 10.1007/s11442-010-0333-4.
Zhang DZ, Zhang FJ, Yu YZ, 2002. Preliminary study on the behavior of six species of Tenebrionidae. Journal of Ningxia University (Natural Science Edition), 24(1): 94-96. DOI: 10.3969/j.issn.0253-2328.2003.01.024.
Zhao HL, Liu RT, 2013. The "bug island" effect of shrubs and its formation mechanism in Horqin Sand Land, Inner Mongolia. Catena, 105: 69-74. DOI: 10.1016/j.catena.2013.01.009.
Zheng LY, Gui H, 1999. Classification of Insects in China. Nanjing Normal University Publishing, Nanjing.
Ziesche TM, Roth M, 2008. Influence of environmental parameters on small scale distribution of soil dwelling spiders in forests: what makes the difference, tree species or microhabitat? Forest Ecology and Management, 255: 738-752. DOI: 10.1016/j. foreco.2007.09.060.
Zotov VA, Alpatov AM, 2005. Adaptive significance of the circadian rhythm of activity in Omniseasonal desert beetles (Coleoptera, Tenebrionidae). Entomology Review, 85: 357-360.
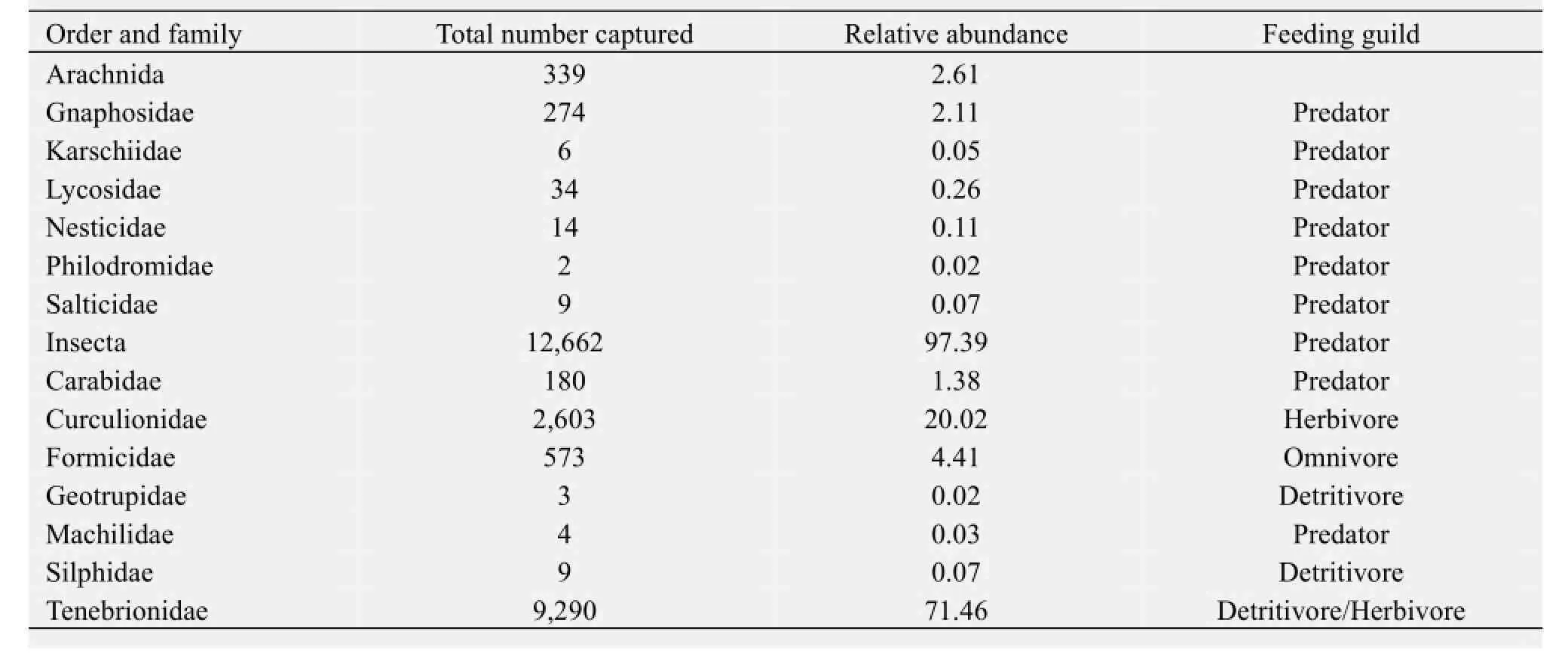
Appendix 1 Total number of arthropods captured and relative abundance (as a percentage of the total capture) for all 13 families in the ground arthropod community of the sandy desert shrubland
: Liu JL, Zhao WZ, Li FR, 2014. The effects of the identity of shrub species on the distribution and diversity of ground arthropods in a sandy desert ecosystem of northwestern China. Sciences in Cold and Arid Regions, 6(6): 0587-0596.
10.3724/SP.J.1226.2014.00587.
Received: April 15, 2014 Accepted: July 13, 2014
*Correspondence to: Dr. JiLiang Liu, Assistant professor of Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences. No. 320, West Donggang Road, Lanzhou, Gansu 730000, China. Tel: +86-931-4967336; E-mail: liujl707@lzb.ac.cn
 Sciences in Cold and Arid Regions2014年6期
Sciences in Cold and Arid Regions2014年6期
- Sciences in Cold and Arid Regions的其它文章
- A discussion on improving typhoon observation through radar by scanning the negative elevation angle
- The effects of the identity of shrub species on the distribution and diversity of ground arthropods in a sandy desert ecosystem of northwestern China
- An analysis on ecological civilization construction in Gansu based on a quantified SWOT framework
- An estimation of groundwater storage variations from GRACE gravity satellites in the Heihe River Basin, northwestern China
- Study on dynamic changes of land desertification in the circum-lake zone of the Qinghai Lake in the past 30 years supported by Remote Sensing and Geographical Information System
- Impacts of reduced wind speed on physiology and ecosystem carbon flux of a semi-arid steppe ecosystem
