Comparative pharmacokinetics of six major compounds in normal and insomnia rats after oral administration of Ziziphi Spinosae Semen aqueous extract
Chenhui Du,Yn Yn,Chenxi Shen,Xiofng Cui,Xingping Pei,Xuemei Qin,*
aSchool of Chinese Materia Medica,Shanxi University of Chinese Medicine,Taiyuan,030619,China
bModern Research Center for Traditional Chinese Medicine,Shanxi University,Taiyuan,030006,China
Keywords:
Ziziphi Spinosae Semen
Pharmacokinetics
Insomnia
UHPLC-Q-orbitrap-MS
Six compounds
ABSTRACT
Ziziphi Spinosae Semen(ZSS),a traditional Chinese medicine,is used in clinics for the treatment of insomnia in China and other Asian countries.Herein,we described for the first time a comparative pharmacokinetics study of the six major compounds of ZSS in normal control(NC)and para-chlorophenylalanine(PCPA)-induced insomnia model(IM)rats that were orally administered the aqueous extract of ZSS.An ultra-high-performance liquid chromatography coupled with quadrupole orbitrap mass(UHPLC-Q-Orbitrap-MS)method was developed and validated for the simultaneous determination of coclaurine,magno florine,spinosin,6′′′-feruloylspinosin,jujuboside A(JuA),and jujuboside B(JuB)in ZSS in rat plasma.The established approach was successfully applied to a comparative pharmacokinetic study.The systemic exposures of spinosin and 6′′′-feruloylspinosin were decreased in the IM group compared to the NC group,while plasma clearance(CL)was significantly increased.The Tmaxvalues of JuA and JuB in IM rats were significantly lower than those in NC rats.The T1/2of JuA in the IM group was significantly accelerated.The pharmacokinetic parameters of coclaurine and magno florine were not evidently affected between the two groups.These results indicate that the pathological state of insomnia altered the plasma pharmacokinetics of spinosin,6′′′-feruloylspinosin,JuA,and JuB in the ZSS aqueous extract,providing an experimental basis for the role of ZSS in insomnia treatment.The comparative pharmacokinetics-based UHPLC-Q-Orbitrap-MS using full-scan mode can therefore provide a reliable and suitable means for the screening of potentially effective substances applied as quality markers of ZSS.?2020 Xi'an Jiaotong University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
For 2000 years,Ziziphi Spinosae Semen(ZSS),the semen of Ziziphus jujuba Mill.var.spinose(Bunge)Hu ex H.F.Chou,has been widely used in many patented medicines and functional foods in China and other Asian countries,such as Korea and Japan.ZSS was first listed in the classical book Ming Yi Bie Lu in Han Dynasty of the Chinese history for the treatment of insomnia.Recent publications have reported that ZSS has many attractive pharmacological activities,including protection of the cardiovascular system,antihyperlipidemia,and anxiolytic effects[1—3].A pharmacological study also revealed that ZSS aqueous extract increased the content of serotonin(5-HT),gamma-aminobutyric acid(GABA)and dopamine(DA),decreased noradrenaline(NE)and glutamic acid(Glu)in the brain of insomnia model rats,and thus produced a sedative hypnotic effect[4].Because more in-depth phytochemical studies have been performed,the chemical compositions of ZSS have been extensively studied.In addition,through liquid chromatography high resolution mass spectrometry(LC-HR-MS)analysis,25 compounds in its aqueous extract have been characterized[5].In our previous experiment,spinosin,6′′′-feruloylspinosin,jujuboside A(JuA),jujuboside B(JuB),magno florine,and coclaurine could be determined in plasma after the ZSS aqueous extract was orally administered to rats[6].
Some scholars found that these components were related to the hypnotic and antianxiety effects of ZSS[7,8].As the major flavonoid in ZSS,spinosin has been widely used as one of the marker compounds for assessing the quality of ZSS in the Chinese Pharmacopoeia.Spinosin has also been reported to potentiate pentobarbitalinducedsleepviaaserotonergicmechanismwhile6′′′-feruloylspinosin has been proven to induce the prolongation of hexobarbital sleeping time in mice[9,10].JuA and JuB are the major saponins,and both exhibit the hypnotic effect by adjusting the mRNA expression of GABA receptor subunit and partially regulating the amino-acid metabolism pathway[11—13].Recent studies indicate that magno florine has sedative and anxiolytic effects,and coclaurine causes sedative bioactivity by interacting with melatonin receptors[6,14].Pharmacokinetic studies could also aid in elucidating the actual therapeutic material basis which is closely related to the identification of“quality-markers”(Q-markers)[15].Therefore,studying their pharmacokinetic properties would be meaningful in evaluating the use of ZSS for insomnia treatment.
To date,most researchers have mainly performed pharmacokinetic studies of spinosin,6′′′-feruloylspinosin,JuA,and JuB in plasma after intravenous administration to rats[16—18].Besides,a report moderately analyzes spinosin in rat plasma after oral administration of the ZSS ethanol extract[19].However,no analytical method has been reported for the simultaneous determination of flavonoids,saponins,and alkaloids in rat plasma after oral administration of this extract.Although the above research also focused on the pharmacokinetic properties of these compounds in normal animals,no study has used pathological models.Therefore,understanding the differences in the pharmacokinetic properties of the ZSS aqueous extract in the body with different statuses would be beneficial.
Giventheabove,wedevelopedaUHPLC-Q-Orbitrap-MS method for the simultaneous determination of coclaurine,magno florine,spinosin,6′′′-feruloylspinosin,JuA,and JuB in normal rats and rats with para-chlorophenylalanine(PCPA)-induced insomnia that were orally administered the ZSS aqueous extract.The results obtained herein provide a better understanding of the in vivo exposure of complex TCM to support further drug development and discovery of an effective screening strategy for tracking effective substances applied as Q-markers of ZSS.
2.Materials and methods
2.1.Reagents,chemicals,and materials
Acetonitrile(MS grade)and formic acid(MS grade)were purchased from Fisher Scientific(USA).Deionized water was produced with a Milli-Q water purification system(Millipore,USA).All other reagents were of analytical grade.
The reference standards for coclaurine,magno florine,spinosin,6′′′-feruloylspinosin,and JuA were purchased from the Baoji Herbest Biological Technology Co.,Ltd.(Shaanxi,China).JuB was supplied by the Nanjing Spring&Autumn Biological Engineering Co.(Jiangsu,China).The internal standards(ISs),palmatine hydrochloride(IS1),daidzin(IS2)and astragaloside IV(IS3)were obtained from Chengdu WeikeqiBiologicalTechnologyCo.(Chengdu,China).5-HTand PCPAwereprovided by Tokyo Chemical Industry Co.(Tokyo,Japan).3,4-Dihydroxybenzyl amine(DHBA)was supplied by Sigma-Aldrich(USA).Purities were above 98% as determined by HPLC.The structures of the compounds are presented in Fig.1.
ZSS was provided by Shanxi Zhendong Chinese Herbal Development Co.(Shanxi,China),and authenticated by Prof.Chenhui Du as the dried seeds of Ziziphus jujuba Mill.var.spinosa(Bunge)Hu ex H.F.Chou according to the Chinese Pharmacopoeia(2015 version).The voucher specimens were preserved at the Modern Research CenterforTraditionalChinese Medicine,ShanxiUniversity,Taiyuan,China.
2.2.Preparation of standardized ZSS aqueous extract
ZSS(0.5 kg)was pulverized into a suitable powder,immersed in 5 L distilled water for 30 min,and then extracted twice by heatreflux for 2 h per extraction.The extracts were filtered through eight layers of gauze,combined and then evaporated under vacuum,and lyophilized to generate freeze-dried powder(yield:22.7% ).
2.3.Quality control of ZSS aqueous extract
2.3.1.Standard solution preparation
Accurately weighed reference standards,including coclaurine,magno florine,spinosin,6′′′-feruloylspinosin,JuA,and JuB,were dissolved in methanol-water(70:30,V/V)to prepare stock solutions at a concentration of 0.2 mg/mL each.The mixed stock solution of the six compounds was then prepared from the stock solutions.Working solutions were obtained by serially diluting the mixed stock solution with methanol to six different concentrations in the range of 1—100μg/mL for coclaurine,1—25μg/mL for magno florine,0.6—60 μg/mL for spinosin,1.75—35 μg/mL for 6′′′-feruloylspinosin,1—50 μg/mL for JuA and 0.2—10 μg/mL for JuB.All the above solutions were stored at 4°C until use.
2.3.2.Sample solution preparation
The freeze-dried powder(0.5 g)was extracted with 70% ethanol(25 mL)for 30 min under ultrasonication.After centrifugation(13,000×g,5 min,25°C),the supernatant was injected for further analysis.
2.3.3.Quantitative analysis by UPLC-MS/MS
UPLC-MS/MS analysis was performed according to our previous method with some modifications[20].All chromatographic measurements were performed on a Shimadzu triple quadrupole LCMS 8050 system(Kyoto,Japan)equipped with a system controller(CBM-20A),column oven(CTO-20AC),autosampler(SIL-30AC),and two pumps(LC-30AD).Chromatographic separation was achieved on an Atlantis T3 C18column(2.1 mm ×150 mm,1.8μm)maintained at 40°C.The mobile phase consisted of 0.1% formic acid in water(A)and acetonitrile(B),and the following gradient was employed:0—2 min,17% B;2—4 min,17% —19% B;4—10 min,19% —33% B;10—15 min,33% —100% B.Flow rate was 0.2 mL/min and injection volume was 3μL.
ESI source was operated in a positive and negative voltageswitching mode.The optimal MS parameters were as follows:nebulizing gas flow,2 L/min;heating gas flow,10 L/min;drying gas flow,10 L/min;interface temperature,300°C;heat block temperature,400°C;and DL temperature,250°C.Mass spectrum parameters of six compounds are shown in Table 1.
2.4.UHPLC-Q-orbitrap-MS for pharmacokinetic analysis
Chromatographic analysis was performed on a Dionex UltiMate 3000 UHPLC system(Thermo,Germany)equipped with an HPG-3400RS pump,a TCC-3000RS column oven,a DAD-3000 detector,and a WPS-3000TRS autosampler.Samples were separated by using an ACQUITY UPLC?HSS T3 C18column(150 mm ×2.1 mm,1.8 μm,Waters,Ireland)maintained at 30°C.The mobile phase consisted of 0.1% formic acid-water(A)and 0.1% formic acidacetonitrile(B).The gradient elution was optimized as follows:0—1.5 min,17% B;1.5—3 min,17% —19% B;3—7 min,19% —33% B;and 7—12 min,33% —98% B.Flow rate was set at 0.3 mL/min.

Fig.1.Chemical structures of the six compounds and three internal standards(ISs).

Table 1 MS/MS detection parameters for six compounds.
Quantitative analysis was performed on a Q-Orbitrap-MS using full scan mode(resolution 70,000).The MS was equipped with a heat electrospray ionization(HESI)source and operated in the(-)-ESI and(+)-ESI switching mode.The parameters were as follows:spray voltage,+3.5 kV and-2.7 kV;sheath gas flow rate,35 arbitrary;Auxiliary gas flow rate,10 arbitrary;capillary temperature,320°C;heater temperature,300°C;S-lens RF level,55 V;NCE,20% ,30% ,50% for positive ion mode;NCE,30% ,45% ,60% for negative ion mode;and scan range,m/z 150—1500 Da.Data were processed using Xcalibur?3.0.63 software(Thermo,CA,USA).
2.5.Animal experiment
Male Sprague-Dawley(SD)rats(220±20 g)supplied by Beijing Vital River Laboratory Animal Technology(Beijing,China)were housed at controlled temperature(25± 3°C)and humidity(45±5% ),and granted free access to standard diet and water before the experiment.
Insomnia in rats was induced by intraperitoneal injection of PCPA at a dose of 400 mg/kg every day for three days[21,22].PCPA,an inhibitor of 5-HT biosynthesis,was suspended in 0.5% CMC-Na.After three days of treatment,serum was collected via the postorbital venous plexus veins and the 5-HT in serum was determined by LC-MS/MS[4].The concentration of 5-HT in PCPA-induced rats was significantly lower than that in normal control(NC)rats(Fig.2),which was consistent with that of previous studies[23].Meanwhile,rats in the PCPA group lost their circadian rhythm and were thus sleepless for the entire day.Such findings suggested that the insomnia model(IM)was successfully duplicated.
2.6.Pharmacokinetic study

Fig.2.The content of 5-HT in rat serum of the normal control(NC)group,insomnia model(IM)group,and ZSS group(30 g/kg).***P<0.001 compared to NC,###P<0.001 compared to IM.
NC and IM rats(six per group)were employed to investigate the pharmacokinetic properties of coclaurine,magno florine,spinosin,6′′′-feruloylspinosin,JuA,and JuB after oral administration of the ZSS aqueous extract.After IM was successfully induced,the ZSS aqueous extract,dissolved in normal saline,was administered to NC and IM rats by intragastric gavage at a dose of 6.8 g/kg(equivalent toa crude drug dose of 30 g/kg).Blood samples were collected from each rat in heparinized tubes via the postorbital venous plexus veins before drug administration and at 0.083,0.167,0.333,0.5,0.75,1,2,4,6,and 10 h after drug administration.Time of recovery from feeding was 4 h post-dose.Blood samples were then immediately centrifuged at 3500×g for 10 min at 4°C and plasma was stored at-80°C until use.
2.7.Preparation of calibration standard and quality control samples
Stock solutions of coclaurine and magno florine were prepared with the initial mobile phase at a concentration of 2 mg/mL,respectively.Stock solutions of spinosin,6′′′-feruloylspinosin,JuA,and JuB wereprepared with methanol at the concentration of 2 mg/mL each.The mixture working solutions were serially diluted with methanol to provide standard working solutions of the desired concentrations.Final concentrations were 0.8,1.6,16,80,128,and 160 ng/mL for coclaurine;45.2,90.4,452,2260,3616,and 4520 ng/mL for magno florine;30,60,240,1200,1920,and 2400 ng/mL for spinosin,2,4,20,100,160,and 200 ng/mL for 6′′′-feruloylspinosin;8.2,16.4,65.6,328,525,and 656 ng/mL for JuA;and 5.3,10.6,42.4,212,339.2,and 424 ng/mL for JuB.The IS working solutions were diluted with methanol to final concentrations of 78.7 ng/mL for IS1,216.0 ng/mL for IS2,and 556.8 ng/mL for IS3.
Standard calibration curves were constructed by spiking 100μL of blank rat plasma with 10μL of the standard working solutions and 10μL of the IS working solution,yielding final plasma concentrations in the range,0.08—16 ng/mL for coclaurine,4.52—452 ng/mL for magno florine,3—240 ng/mL for spinosin,0.2—20 ng/mL for 6′′′-feruloylspinosin,0.82—65.6 ng/mL for JuA,and 0.53—42.4 ng/mL for JuB.
Quality control(QC)samples at four concentration levels(0.08,0.16,1.6,and 12.8 ng/mL for coclaurine;4.52,9.04,45.2,and 361.6 ng/mL for magno florine;3,6,24,and 192 ng/mL for spinosin,0.2,0.4,2,and 16 ng/mL for 6′′′-feruloylspinosin;0.82,1.6,6.6,and 52.5 ng/mL for JuA;and 0.53,1.06,4.24,and 33.9 ng/mL for JuB)wereprepared bythe same operation described above.All solutions were stored at 4°C.
2.8.Preparation of plasma samples
Each plasma sample(100μL)was mixed with a three-fold volume of acetonitrile and 10μL IS in a 1.5 mL EP tube.The mixture was then vortexed for 5 min and centrifuged at 13,000×g for 10 min at 4°C.The supernatant(350 μL)was transferred to another EP tube and evaporated to dryness under nitrogen vacuum.The residue was reconstituted with 100μL of the initial mobile phase,and the centrifugation process was repeated.Three microliters of the supernatant were then used for analysis.
2.9.Data analysis
The pharmacokinetic parameters,including the maximum plasma concentration(Cmax),the time corresponding to Cmax(Tmax),the terminal elimination half-life(T1/2),the area under plasma concentration-time curve(AUC0-t),the area under the plasma concentration-time curve from 0 to in finity time(AUC0-∞),and plasma clearance(CL),were calculated using the noncompartment model in DAS 3.2.8 software package(Shanghai,China).All values are expressed as mean±standard error.For the pharmacokinetic parameter values of the NC and IM groups,student's t-test was employed for data comparisons.P values<0.05 were considered statistically significant.
3.Results and discussion
3.1.Content determination of six compounds by LC-MS/MS
The contents of coclaurine,magno florine,spinosin,6′′′-feruloylspinosin,JuA,and JuB were 0.12% ,1.62% ,0.4% ,0.14% ,0.41% ,and 0.05% ,respectively,in the ZSS aqueous extract.The multiple reaction monitoring(MRM)chromatography result is presented in Fig.3.
3.2.UHPLC-Q-orbitrap-MS method optimization
To achieve a rapid and efficient separation,a short chromatographic column packed with 1.8μm porous particles was employed in the UPLC analysis.Some important factors such as the composition of the mobile phase and the elution program were systematically explored.Acetonitrile-water containing 0.1% formic acid was selected because of its greater separation ability and better peak shapes.The relative intensities of base ions were compared to determine the most suitable ionization conditions for six compounds.In common,flavonoid easily loses proton in ionization process.In our study,we found that the response for 6′′′-feruloylspinosin in the negative ion mode was slightly better than that in the positive ion mode.Moreover,the intensity of spinosin in the positive ion mode was slightly better than that in the negative ion mode.However,the response for daidzin(IS2)observed in the positive ionization modewas much higher than that in the negative ionization mode.Thus,spinosin and 6′′′-feruloylspinosin were detected in positive ion mode.Based on the spectral structure pattern of JuA and JuB,the detection signals of a typical solvent adduct[M-H+HCOOH]-were better in negative mode than in positive mode.Other alkaloid compounds were detected inpositive ion mode,including[M+H]+or[M]+.
The current pharmacokinetic analyses were mainly carried out on an LC-MS/MS platform in MRM mode[24].Q-Orbitrap with resolving power and accurate mass measurement capability(<5 ppm)might be more suitable for pharmacokinetic studies of complex TCM containing dozens of components that require simultaneous quantitation.Hence,a UHPLC-Q-Orbitrap-MS system using full MS dd/ms2mode was used to identify the six compounds in rat plasma by comparing their retention time and MS data to the reference standards.Thereafter,full scan MS mode was employed with the extracted ion chromatogram(EIC)method for pharmacokinetic analysis owing to its improved selectivity and sensitivity.Data for the six tested compounds are shown inTable 2.Errors were less than 1 ppm in all cases.

Fig.3.Representative MRM chromatograms of(A)mixed standard solution and(B)ZSS aqueous extract sample(1.coclaurine;2.magno florine;3.spinosin;4.6′′′-feruloylspinosin;5.jujuboside A;6.jujuboside B)
3.3.Optimization of the extraction procedure
The six components were divided into three chemical families,namely,flavonoids,saponins,and alkaloids.Palmatine hydrochloride,daidzin,and astragaloside IV were selected as the ISs for flavonoid,saponin,and alkaloid,respectively.Due to differences inpolarity among the three chemical families,different sample pretreatment procedures,such as protein precipitation(PPT)and liquid-liquid extraction(LLE),were compared to increase the extraction recovery of each compound.The LLE method using ethyl acetate revealed the limited extraction efficiency of magno florine,coclaurine,spinosin,and 6′′′-feruloylspinosin.This finding could be attributed to the poor lipophilic property of these compounds.In contrast,the PPT method using acetonitrile was found to be beneficial in the achievement of a higherextraction recoveryfor the six compounds and three ISs in the pre-treatment process.
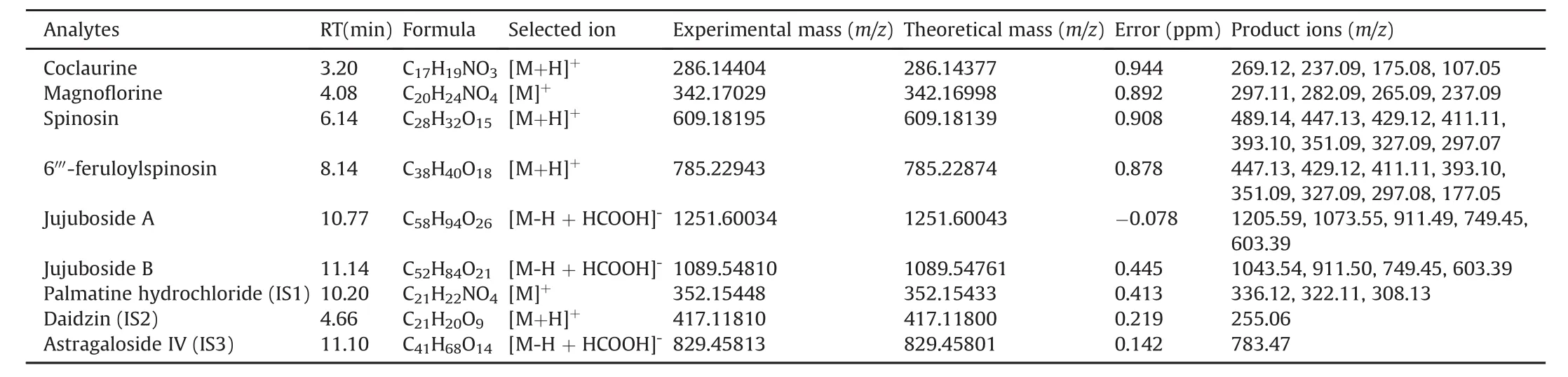
Table 2 Compounds identified from rat plasma by UHPLC-Q-Orbitrap-MS/MS.
3.4.Method validation
Blank plasma samples from six rats were prepared and analyzed to investigate the potential interferences from endogenous components.As shown in Fig.4,the chromatograms of the blank plasma samples,blank plasma samples spiked with the components and three ISs,and plasma samples after administering the ZSS aqueous extract were compared.No endogenous interference peaks were observed at the retention time of the six compounds and ISs,indicating the good specificity of the analysis method.
The calibration curves for the six compounds were established by plotting the peak area ratios of each analyte to the IS against plasma concentrations,using the least-square linear regression with weighting factor 1/concentration2.In this study,we found that the novel UHPLC-Q-Orbitrap-MS method yielded a wide dynamic range for the six compounds determined with the correlation coefficients(r)exceeding 0.992(Table 3).In addition,the lower limits of quantification(LLOQ)were defined as a signal-to-noise ratio over 10 and relative error(RE)within±20% .As shown in Table 3,the LLOQs of coclaurine,magno florine,spinosin,6′′′-feruloylspinosin,JuA,and JuB were 0.08,4.52,3.00,0.20,0.82,and 0.53 ng/mL,respectively.These results revealed that the sensitivity of the novel analysis method operated under the scan mode was much higher than that of previous studies[16—19].
Intra-day precision and accuracy were analyzed by measuring five replicate QC samples at three concentration levels within one day while inter-day precision and accuracy were investigated by determining five replicate QC samples at three concentration levels on three successive days.Precision(relative standard deviation,RSD)and accuracy(RE)for intra-and inter-day values were below 15% and within±15% for the six compounds(Table 4),respectively.Such findings suggested that all data were accepted and could be used for the analysis of suggested samples.
The extraction recoveries and matrix effects of the six compounds were evaluated by determining the QC samples at three concentration levels with five replicates.The matrix effect was expressed as the percent of post-spiked sample peak area to average peak area at the same concentration.The recovery of six analytes was measured bycomparing the peak areas of the analytes in post-extraction spiked samples to those in pre-extraction spiked samples at the same concentration.Mean extraction recoveries are shown in Table 5,with values ranging from 83.48% to 98.92% .Mean matrix effects ranged from 87.45% to 112.28% .The recovery and matrix effect of three ISs were interrogated by the same progress as shown in Table 5.The above results indicated that sample pretreatment was appropriate for obtaining stable and high extraction recovery and no evident endogenous interference.
The stability of all analytes in blank rat plasma was investigated by analyzing five replicate QC samples at three different concentrations during sample collection and the handling process.Freezethaw stability wasassessed afterthreefreeze-thaw cycles(from-20°C to 20°C).Long-term stability was studied by storing QC samples at-80°C for 30 days while short-term stability was measured by analyzing QC samples stored at 25°C for 12 h.Postpreparation stability was tested by determining the extracted QC samples stored in the auto-sampler at 4°C for 24 h.As shown in Table 6,RE values for the theoretical concentration of the QC samples were between-14.77% and 14.88% ,and RSD values ranged from0.58% to 13.56% ,indicating that all analytes werestable during the analysis.
3.5.Pharmacokinetic study
It is well known that aqueous extraction(decoction pieces)is the main prescription form of TCM.To our knowledge,the present study is the first to report the pharmacokinetics of six compounds from the ZSS aqueous extract administered orally to NC and IM rats using the above validated method.Mean plasma concentrationtime curves are presented in Fig.5,and the pharmacokinetic parameters are listed in Table 7.
3.5.1.Pharmacokinetic behaviors of the six compounds in normal control rats
Spinosin and 6′′′-feruloylspinosin were the predominant C-glycoside flavonoids,accounting for 0.10% and 0.04% ,respectively,of the ZSS content(w/w)[2].6′′′-Feruloylspinosin is a derivative of spinosin with a feruloyl group bound to the 6′′′-C of the glycoside.Here,two flavonoid C-glycosides achieved a Cmaxat 0.3 h(Tmax),suggesting that they had a rapid absorption in the gastrointestinal tract after oral administration of the ZSS aqueous extract to rats.Li et al.[19]reported that it is difficult to absorb spinosin in the ZSS ethanol extract from rat plasma,a finding that does not align with that of the current study.The quick absorption in the present study might result from coexisting constituents in the aqueous extract.Compared to that of spinosin,the CL value(928.92±309.06 L/h/kg)of 6′′′-feruloylspinosin remarkably increased(P < 0.01),indicating that 6′′′-feruloylspinosin might be rapidlyand widely distributed in rats,aligning with the finding of a previous report[10].Some studies reported that 6′′′-feruloylspinosin was first hydrolyzed to spinosin and swertisin,and spinosin could be further metabolized to swertisin in vitro by rat intestinal bacteria[25,26].Based on our knowledge,we speculate that spinosin and swertisin might be the major and high content compounds in plasma.As expected,the Cmax(45.22±7.94 ng/mL)and AUC0-tvalues(61.14±22.16μg/L?h)of spinosinwere significantly higher than the Cmax(15.83±1.54 ng/mL)and AUC0-t(20.95 ± 5.55 μg/L?h)of 6′′′-feruloylspinosin(P<0.01,P<0.05).Unfortunately,the concentration of swertisin in rat plasma was too low for detection under the present condition.Notably,a high content of swertisin was found in bile and feces(data not open),which suggested that the intestine might be the target organ of swertisin.However,the process whereby this contribution occurred requires further investigation.
As demonstrated in Fig.5,JuA and JuB showed consistent tendencies in the single and plateau absorption phase.As observed in Table 7,the CL value of JuB was much higher than that of JuA,which was consistent with a previous study[27].These phenomena might result from the hydrolysis of saponin glycosides mediated by gastrointestinal bacteria after oral administration.JuA was previously reported to be first hydrolyzed to JuB in the intestinal segments.Thereafter,JuB could be further metabolized to jujubogenin in vitro by rat intestinal bacteria[28—30].
To date,an analytical method that can be used to determine the alkaloid contents in the ZSS aqueous extract of biological samples has not been presented.As shown in Table 7,coclaurine and magno florine achieved their Cmaxat 0.3 h,demonstrating their rapid absorption from the gastrointestinal tract.Coclaurine was also rapidly eliminated from rat plasma following intragastric administration,with a T1/2of 0.45±0.17 h.This finding indicated the short action time of coclaurine in vivo.

Fig.4.Extraction ion chromatograms(EIC)of the six compounds and three internal standard(ISs):(A)blank plasma;(B)blank plasma spiked with the analytes at LLOQ and IS;(C)plasma samples 0.5 h after oral administration of the ZSS aqueous extract.3.20 min:coclaurine;4.08 min:magno florine;6.14 min:spinosin;8.14 min:6′′′-feruloylspinosin;10.77 min:jujuboside A;11.14 min:jujuboside B;10.20 min:IS1;4.66 min:IS2;11.10 min:IS3.

Table 3 The regression equations,linear range,and LLOQs for the six compounds.
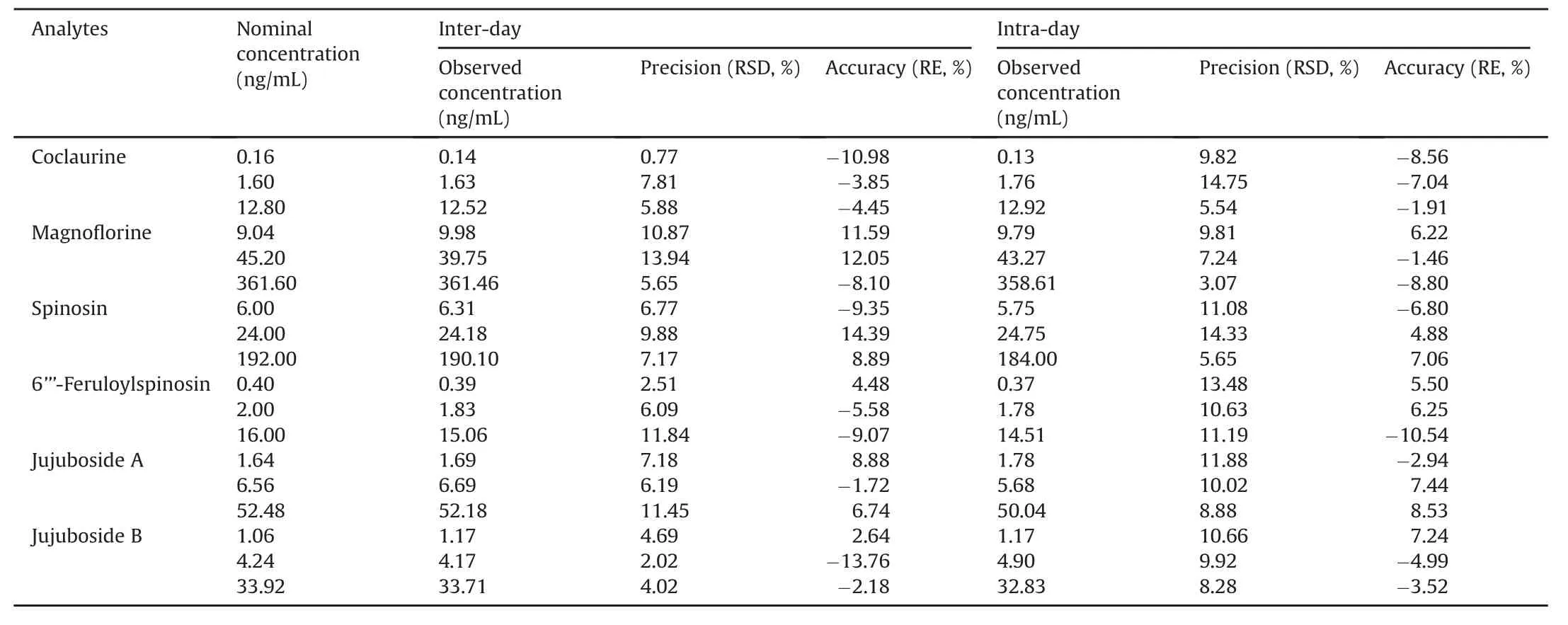
Table 4 Intra-day and inter-day precisions and accuracies for the determination of the six compounds from the assay samples(mean±SD,n=5).
3.5.2.Pharmacokinetic comparison of six ingredients in normal control rats and rats with insomnia
Studying the pharmacokinetics of active compounds of TCM in the pathological state is necessary to provide additional information and thus enhance the safety and efficacy of TCM in clinical applications[31].Many reports have demonstrated that insomnia condition would cause the alterations ofpharmacokinetic parameters.Liao group[21]has reported that the pharmacokinetic behavior of the protoberberine-type alkaloids in Jiao-Tai-Wan of IM rats had significant differences compared to NC rats.Bi group[32]found that absorptions of six sedative and hypnotic lignans in insomnia group were all significantly higher than those in normal group.In the present study,rats treated with PCPA for three days lost their circadian rhythm and were thus sleepless for the entireday and the concentration of 5-HT in serum was significantly reduced in IM rats.Meanwhile,ZSS aqueous extract(30 g/kg)had significantlyelevated the concentration of 5-HT in serum compared to IM rats(Fig.2).The results indicated that ZSS was an effective anti-insomnia drug.
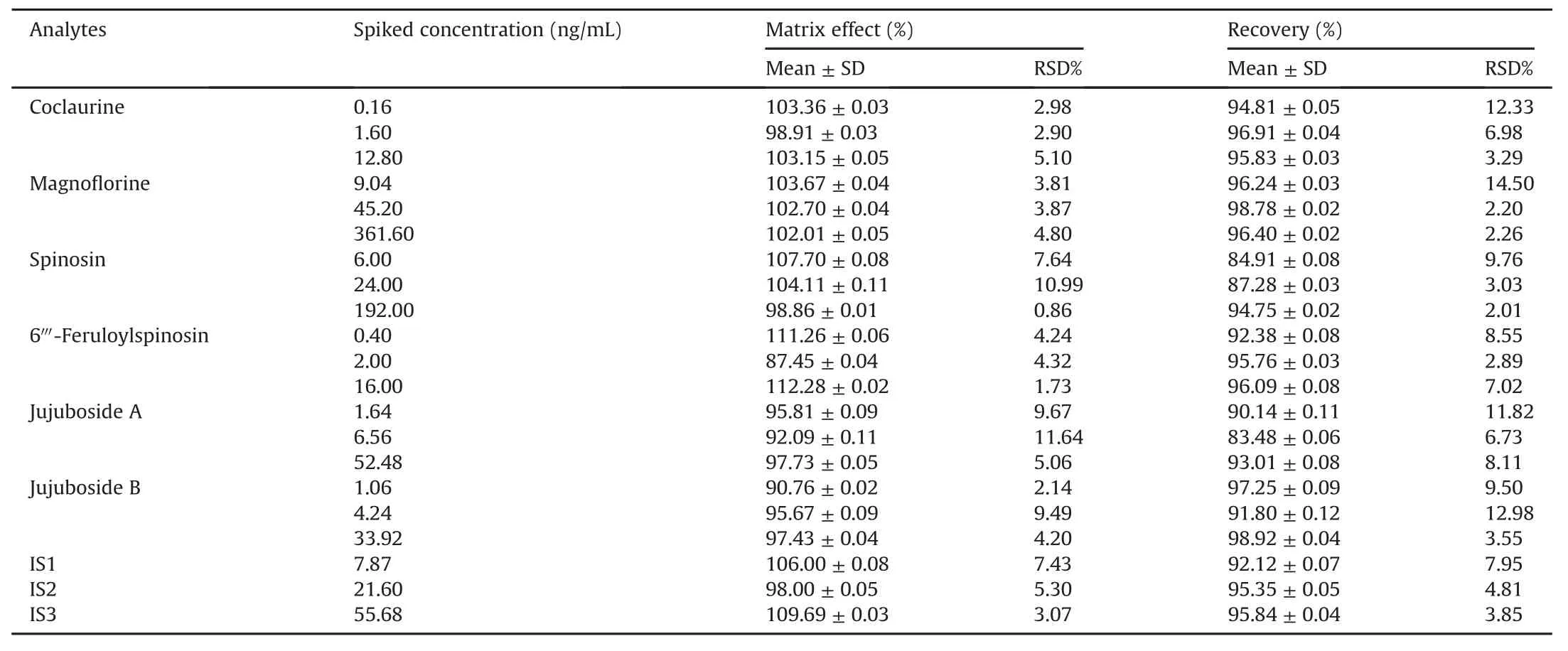
Table 5 Matrix effects and extraction recoveries for the analytes and three internal standards in rat plasma(mean±SD,n=5).

Table 6 The stability of six compounds in rat plasma under different storage conditions.
The non-compartmental model was applied to calculate the pharmacokinetic parameters in the NC and IM groups.The pharmacokinetic parameters are summarized in Table 7 and mean concentration-time profiles are presented in Fig.5.The results demonstrated that significant differences existed in these pharmacokinetic parameters(P< 0.01),including AUC0-t,AUC0-∞and CL for 6′′′-feruloylspinosin.The AUC0-tand AUC0-∞values of 6′′′-feruloylspinosin in the IM group significantly decreased(P<0.01).By contrast,the CL value of 6′′′-feruloylspinosin significantly increased in the IM group compared with NC group(P<0.01).Although no significant differences were found,an increasing trend for CL and the decreasing trend for the AUC0-tand AUC0-∞of spinosin were observed in IM group compared with NC group.After oral administration of the ZSS decoction in a previous study,the AUC0-tand Cmaxof spinosin markedly decreased in the IM group,aligning with theresultsof the current study[22].These results indicated that the absorption of two flavonoids was faster and poorer in IM rats than in NC rats after oral administration of ZSS aqueous extract.Moreover,the elimination of two compounds was higher in IM rats than in NC rats.Furthermore,a shorter Tmaxfor JuA and JuB and a longer T1/2for JuA were observed in the IM group compared with the NC group(P<0.05),whereas no significant change in Tmaxor T1/2was observed forothercompounds between the two groups.The results indicated that the oral administration of the ZSS aqueous extract could lead to quicker absorption of JuA and JuB and slower elimination of JuA in IM rats.Additionally,a weak variation tendency for Cmax,AUC0-t,and CL was observed for the two alkaloid compounds,including no evident differences between the two groups.

Fig.5.Mean concentration-time curves of six compounds in NC and IM rat plasma after oral administration of the ZSS aqueous extract.Values are presented as mean±SD of 6 rats.
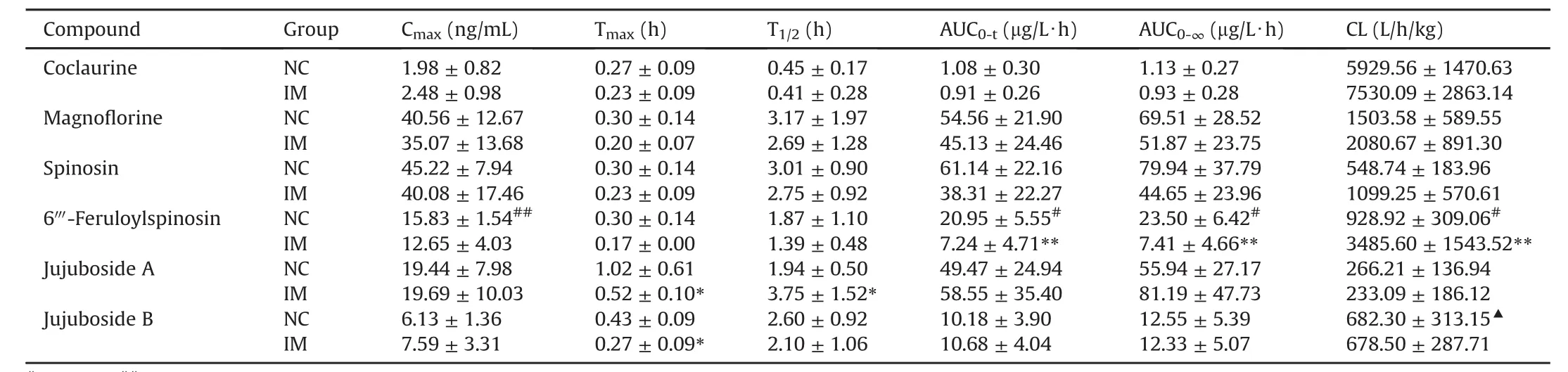
Table 7 Pharmacokinetic parameters of six compounds after oral administration of the ZSS aqueous extract to normal control(NC)rats and insomnia model(IM)rats(mean±SD,n=6).
These findings may be attributed to the body functional disorders under insomnia state.It was reported that the counts,composition,and diversity of the gut microbiota were altered in insomnia patients[33].Thus,the decreased systemic exposure for spinosin and 6′′′-feruloylspinosin in IM rats may be related to the gastrointestinal microbiota dysbiosis in insomnia condition.As mentioned earlier in the paper,6′′′-feruloylspinosin and spinosin can be transformed to swertisin in vitro by rat intestinal bacteria.Therefore,we speculated that the biotransformation of spinosin and 6′′′-feruloylspinosin was increased owing to the imbalance of gastrointestinal microbiota in IM rats,which might lead to the decreased absorption of them in blood circulation and the increased contentof swertisin in intestinal afteroral administration of ZSS aqueous extract.In addition,JuA and JuB were quickly absorbed in IM rats than in NC rats,which might be beneficial for the therapy efficacy.Second,the poorer absorptions and higher eliminations of 6′′′-feruloylspinosin and spinosin in IM rats might be ascribed to the impaired intestinal function induced by insomnia.In insomnia patients,the host's normal intestinal microbiota was changed and these changes will cause host inflammatory reactions,metabolic disorders,and impaired immune function[34].These pathological changes reduce drug absorption from the intestinal tract,leading to alteration of drug concentration and efficacy in vivo[35].
Based on the above results,spinosin,6′′′-feruloylspinosin,JuA,and JuB might be the effective compounds identified as Q-markers in the ZSS aqueous extract,and contribute to the treatment of insomnia.Because insomnia is a central nervous disease,it is regulated in a specific area of the brain and the intestine according to the “microbiome-gut-brain axis”theory[36].Therefore,further studies are necessary to explore the tissue distribution of the six compounds of the ZSS aqueous extract in pathological animals and evaluate the pharmacokinetic mechanisms of these compounds after multiple-dose oral administration.
4.Conclusions
We conducted a multi-component pharmacokinetic study of ZSS aqueous extract in this study.Six compounds in rat plasma were monitored using a fully validated UHPLC-Q-Orbitrap-MS method,and their pharmacokinetic profiles were obtained after administeringthe ZSS aqueous extract tonormal and PCPA-induced IM rats.Different structural types of compounds(flavonoids,saponins,and alkaloids)exhibited characteristic pharmacokinetic behaviors in NC rats.In fact,there were statistically significant differences among the pharmacokinetic parameters of 6′′′-feruloylspinosin,JuA,and JuB,while a weak variation tendency was exhibited by spinosin,including Tmax,T1/2,AUC0-t,and CL between NC and IM rats.Such findings demonstrate that the pathological state of insomnia alters the plasma pharmacokinetics of these four compounds.By using a comparative pharmacokinetics-based UHPLC-Q-Orbitrap-MS with full-scan mode,a reliable and suitable protocol can be obtained for screening potentially effective substances for further quality control.The findings presented herein might provide a better understanding of the in vivo exposure of complex TCMs to support further drug development and clinical application.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China(No:81603289,81603251),Key Laboratory of Effective Substances Research and Utilization in TCM of Shanxiprovince(No:201605D111004),andKeyTechnology Research Zhen Dong Special Project from Shanxi Science and Technology Department(No:2016ZD0105).We would like to thank Editage(www.editage.cn)for English language editing.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.03.003.
 Journal of Pharmaceutical Analysis2020年4期
Journal of Pharmaceutical Analysis2020年4期
- Journal of Pharmaceutical Analysis的其它文章
- Electrochemical and in silico approaches for liver metabolic oxidation of antitumor-active triazoloacridinone C-1305☆
- Magnetic metal organic framework for pre-concentration of ampicillin from cow milk samples
- An integrated spectroscopic strategy to trace the geographical origins of emblic medicines:Application for the quality assessment of natural medicines
- TGA/Chemometrics addressing innovative preparation strategies for functionalized carbon nanotubes
- Identification of impurities in nafamostat mesylate using HPLC-ITTOF/MS:A series of double-charged ions
- Solid and liquid state characterization of tetrahydrocurcumin using XRPD,FT-IR,DSC,TGA,LC-MS,GC-MS,and NMR and its biological activities
