Magnetic metal organic framework for pre-concentration of ampicillin from cow milk samples
Ahmad Reza Bagheri,Mehrorang Ghaedi
Chemistry Department,Yasouj University,Yasouj,75918-74831,Iran
Keywords:
Magnetic metal organic framework
Ultrasound assisted magnetic solid phase extraction
Ampicillin
Cow milk samples
ABSTRACT
The aim of this study is a present of a simple solvothermal synthesis approach to preparation of Cu-based magnetic metal organic framework(MMOF)and subsequently its application as sorbent for ultrasound assisted magnetic solid phase extraction(UAMSPE)of ampicillin(AMP)from cow milk samples prior to high performance liquid chromatography-Ultraviolet(HPLC-UV)determination.Characteristics of prepared MMOF were fully investigated by different techniques which showed the exclusive properties of proposed sorbent in terms of proper functionality,desirable magnetic property and also high specific surface area.Different influential factors on extraction recovery including sorbent dosage,ultrasonic time,washing solvent volume and eluent solvent volume were assessed using central composite design(CCD)based response surface methodology(RSM)as an operative and powerful optimization tool.This is the first report for determination of AMP using MMOF.The proposed method addressed some drawbacks of other methods and sorbents for determination of AMP.The presented method decreases the extraction time(4 min)and also enhances adsorption capacity(250 mg/g).Moreover,the magnetic property of presented sorbent(15 emu/g)accelerates the extraction process which does not need filtration,centrifuge and precipitation procedures.Under the optimized conditions,the proposed method is applicable for linear range of 1.0—5000.0 μg/L with detection limit of 0.29 μg/L,satisfactory recoveries(≥95.0% )and acceptable repeatability(RSD less than 4.0% ).The present study indicates highly promising perspectives of MMOF for highly effective analysis of AMP in complicated matrices.
1.Introduction
AMP is a semisynthetic antibiotic which belongs to penicillin groups.It generally has been applied for treatment of bacterial infections caused by both gram-positive and gram-negative bacteria in both humans and animals[1].AMP acts by inhibiting the proteinsynthesis of the bacterial cell wall.It is comparatively less toxic than other antibiotics,and side effects are more likely in those who are sensitive to penicillins and those with a history of asthma or allergies.In very rare cases,it causes severe side effects such as angioedema,anaphylaxis,and C.difficile infection.In addition,it can cause stomach cramps,diarrhea,dizziness,nausea,rashes and peeling of the skin when consumed by humans[2].AMP overdose can cause behavioral changes,confusion,blackouts,and convulsions,as wellas neuromuscular hypersensitivity,electrolyte imbalance,and renal failure.The maximum residue limits(MRLs)for ampicillin in milk and meat have been set by the European Union(EU)at 40 and 50μg/kg,respectively[3].In 1999,the EU forbade the use of antibiotics as additives in animal feed,but they are still use in some countries.Therefore,considerable interest has been devoted to monitoring and determination of AMP in different samples,especially cow milk samples.On the other hand,cow milk samples contain complicated matrices such as proteins,hormones,and long chain fatty acids which can significantly pollute chromatography column and/or overlap with peak of AMP during quantitative analysis of AMP(in chromatographic determination).Hence,for the detection of AMP at trace level,the fundamental steps can be attributed to the sample clean-up in order to eliminate complicated matrices and also pre-concentrate AMP in cow milk samples.Up to date,different sample preparation approaches have been applied for determination of AMP,among which solid phase extraction(SPE)has been paid much interest to due to its exclusive properties in terms of easy operation,low analysis cost and easy automation[4].Although different sorbents have been used in SPE,some of them have some limitations including low physical and chemical stability,hardness to modification,low specific surface area and also low adsorption capacity.To address these drawbacks,metal organic frameworks(MOFs)are very much preferred.MOFs are porous materials with high specific surface area which is achieved based on the coordination interactions between metal cations(clusters or secondary building units)and organic ligands(linkers)via coordinative bonds[5].They have arouse great interest principally due to their versatility,diverse structures,tunable porosity and specific surface area,ease of functionalization,unsaturated metal sites,and also biocompatibility[6].Among the MOFs,copper-basedMOFsparticularly draw great attention because they can be synthesized with available commercially reagents and possess high surface area[7].The strong Cu(II)—O bonds from copper metal nodes and organic ligands endow Cu-MOFs with excellent stability in aqueous solution over a wide pH range.Cu based MOFs draw interest in many areas due to their unique structure which is made an interconnected channel of square-shaped pores(9 ? by 9 ?)[8].It is noteworthythat in spite of all SPE benefits,it is faced with some main drawbacks such as long extraction time and also consumption of large volume of solvents,which forced researchers to use an alternative method.Magnetic solid phase extraction(MSPE)is a versatile technique in which samples are gathered using an external magnetic field without using filtration,centrifuge and precipitation procedures.In addition,MSPE does not need high volume of organic solvent or conditioning the sorbent and column packing elimination[5,9],which not only decreases extraction time but also enhances the contact surface area and also repeatability[10].Ultrasound irradiation can acceleratechemical process which can be attributed to formation of microbubbles via sound waves through the solution[11,12].Formation of bubbles in solution can accelerate and improve mass transfer process that can be related to best immersion of sorbent in balk solution and also strong enhancement of diffusion coefficient[13].Hence,in this study,we synthesized Cu based MMOF through a simple and facile solvothermal method and applied it as sorbent for the first time for UAMSPE of AMP from cow milk samples.The performance of the proposed method was assessed using CCD based RSM in ordertominimize experimental runs,time,chemicals consumption,reagents,and attain more accurate results.The prepared MMOF was fully characterized and the effects of influential factors on extraction were also evaluated.In addition,under the optimized conditions,the fully validated developed method was applied for the analysis of AMP in different cow milk samples.
2.Experimental
2.1.Materials and instruments
AMP pure powder was obtained from the Ministry of Health and Medical Education(Tehran,Iran).Iron(III)chloride hexahydrate(FeCl3?6H2O),iron(II)chloride tetrahydrate(FeCl2?4H2O),ammonium hydroxide(NH4OH),Cu(II)nitrate threehydrate Cu(NO3)2,terephthalic acid(H2BDC),tetrahydrofuran(THF),NaOH,HCl,ethanol,methanol,hexane,acetone,and acetonitrile(HPLC grade)were purchased from Merck(Darmstadt,Germany).Ultrapure water was prepared from a MilliQ gradient water purification system.Fourier transform infrared spectroscopy(FT-IR-8300,Shimadzu)via KBr pellet was used to give an idea about MMOF functional groups.Morphology of prepared MMOF was examined by scanning electron microscope(SEM)(SIGMA VP microscope from ZEISS,Germany).Transmission electron microscopy(TEM)image on a Hitachi S-570 microscope from Hitachi(Tokyo,Japan)was applied for investigation the structure of prepared MOF.X-ray diffraction(XRD,Philips PW 1800)was performed to characterize the phase and structure of the prepared MOF using Cukaradiation(40 kV and 40 mA)at angles ranging from 5°to 80°.Vibrating sample magnetometer(VSM)(LDJ 9600-1,USA)was employed to evaluatethemagneticpropertyofMMOF.TheBrunauer—Emmett—Teller(BET)surfaceareawascalculated from N2adsorption-desorption isotherms at 77 K using a BElSORP Mini(Microtrac Bel Corp,USA).A Tecno GAZ SPA Ultra Sonic System(Parma,Italy)ultrasound bath was used at 130 W at 40 kHz frequency with a maximum of about 3 L of water throughout the experiment.A Metrohm 780 pH meter(Metrohm Co.,Herisau,Switzerland)was used for monitoring the pH values.STATISTICA version 10.0(Stat soft Inc.,Tulsa USA)and Design-Expert version 7.0.0 software were used for experimental design analysis.AMP determination was done using an Agilent 1100 liquid chromatography system equipped with a micro vacuum degasser(model G1379A),a quaternary pump(model G1311A),a multiple wavelength detector(model G13658:220 nm for HCT),a sample injection valve with a 20μL of sample loop,and a Knauer C18column(4.6 mm i.d.×250 mm,5 μm)at room temperature using mobile phase that consisted of acetonitrile:ammonium acetate(15:85,V/V)filtered through a 0.45μm filter,degassed under vacuum and passed with the flow rate of 0.7 mL/min.Separation process was carried out at ambient temperature and detector wavelength was set at 280 nm.
2.2.Synthesis of MMOF
First,Fe3O4nanoparticles were synthesized using chemical coprecipitation according to our previous work[9,10].In the next step,0.5 g of prepared Fe3O4nanoparticles were fully dispersed in 75 mL(DMF(40 mL):ethanol(35 mL)under ultrasonic irradiation for 1 h.Then,1 g H2BDC and 4 g Cu(NO3)2were added to the mixture and dispersed uniformly.The mixture was transferred to a 100 mL polytetra fluoroethylene lined stainless steel autoclave and placed in an oven at 120°C.After 12 h,it was taken out and cooled down at room temperature.Then,the products were separated using an external magnet and washed several times with DMF and water.Finally,MMOF was obtained by drying the product in 80°C oven for 12 h.
2.3.Adsorption test
To investigate the adsorption capacity of MMOF,30.0 mg of MMOF was dispersed in 15 mL of AMP solution with different concentrations(5.0—50.0 mg/L)bystirring for 30 min.To obtain the adsorption capacity of prepared MMOF,it was separated from solution by an external magnet and residual(non-sorbed)concentrations of AMP in the supernatant were analyzed by HPLC.The equilibrium adsorption capacity(Q,mg/g)of MMOF was calculated according to the following equation:

where C0(mg/mL)and Ct(mg/mL)are the initial and the supernatant concentration of AMP after adsorption,respectively;V(mL)is the sample solution volume and m is the mass of the MMOF.
2.4.Milk samples pre-treatment protocol,calibration standard preparation and MMOF-MSPE procedure for determination of AMP from cow milk samples
Fresh cow milk samples were collected from paddocks at(Shiraz,Iran).Cows had not received antibiotic treatment within the last 6 weeks.The protocol used for pretreatment of milk samples is detailed below.20 mL of acetonitrile was added to 5 mL of cowmilk sample to precipitate the proteins and placed in a refrigerator for 20 min to allow the interaction of the solvent with the sample and facilitate proteins and fat removal.The mixture was then centrifuged for 10 min at 12,000 rpm,resulting in a top layer of fat and a solid protein precipitation at the bottom.Fat was extracted by suctionwith the aid of a syringeand the supernatant containing the analyte was collected using a Pasteur Pipette and was used for MMOF-MSPE-HPLC/UV procedure.A standard AMP stock solution(100.0 mg/L)was prepared by dissolving 10.0 mg of AMP in 100.0 mL of deionized water and its subsequent appropriate dilution applied as working solution.Fig.1A shows the MMOF-MSPEHPLC/UV procedure for extraction of AMP from cow milk samples.In this regards,15 mL of extracted cow milk sample solution with the AMP spiked concentration of 200.0μg/L was adjusted at pH 7.0 using HCl and NaOH and the solution was mixed with 30.0 mgof MMOF in the beaker under the ultrasound irradiation for 4.0 min.Subsequently,the MMOF was separated with an external magnet and eluted by 3 mL of acetone as washing solvent and subsequently with 3 mL of methanol:ethanol(50:50,V/V)as eluent.The eluent was evaporated to dryness under nitrogen stream in water bath(35°C)and the residue was subsequently reconstituted in 100μL mobile phase(acetonitrile/ammonium acetate)(15:85,V/V))and 20μL of it ready for HPLC-UV analysis.
3.Results and discussion
3.1.Synthesis and characterization of MMOF
The solvothermal synthesis process of MMOF is schematically shown in Fig.1B.First,Fe3O4magnetic nanoparticles(MNPs)were synthesized by co-precipitation method.This method was selected to synthesis of Fe3O4-MNPs due to its exclusive properties in terms of less consumption of hazardous materials and an organic solvents compared to other methods[14].Fe3O4nanoparticles have Fe—O groups in which the oxygen groups have the ability to chelate with metal ions(Cu2+).Next,the conjugated central metal Cu2+,H2BDC and Fe3O4were combined to form a MMOF material by simple solvothermal method.It is noteworthy that the preparation of common MMOF uses more complicated experimental equipment,more amounts of(toxic)reagents and higher temperature control.Happily,our synthesis method represents significant advantages of eco-friendliness,cost-savings,simplicity,rapidity and efficiency.The prepared MMOF was fully characterized by FT-IR,XRD,EDX,SEM,TEM,VSM and BET analyses as follows.
Fig.2 presents the FT-IR spectra of Fe3O4(A),MOF(B)and MMOF(C),respectively.In Fig.2A,the peak at 578 cm-1is related to Fe—O peak,and the broad peak around 3400 cm-1could be attributed to OH functional groups of Fe3O4.The Fe—O peak was also observed in MMOF with lower intensity which showed the successful decoration of Fe3O4nanoparticles with MOF(Fig.2C).In Figs.2B and C,the bands obtained at 488 cm-1and 721 cm-1may be due to the bending and stretching modes of Cu—O,respectively[15].The observed peaks between 663 and 766 cm-1are assigned as ring in and out of plane bending vibration of aromatic ring of H2BDC.In addition,aromatic carbon C—C vibrational mode resulted in the bands at about 1390 cm-1[16].Also,the peaks at 970 cm-1,1500 cm-1and 1640 cm-1are related to C—O,—C═C— and C═O groups of H2BDC,respectively[16].The obtained results proved the successful synthesis of proposed materials.
Fig.3A presents the XRD pattern of Fe3O4in which the peaks at 2θ of 31.0°,35.5°,43.5°,53.0°,57.5°,62.5°and 74.0°were corresponded to(220),(311),(400),(442),(511),(440)and(533)crystal planes[17].Fig.3B shows the XRD pattern of MOF in which the peaks at 2θ of 7.0°,13.8°,15.7°,19.6°,30.0°,37.0°42.5°,62.5°and 74.0°are related to(220),(222),(333),(420),(422),(773),(882),(440)and(533)crystal planes respectively and are consistent with those in previous reported literature(Cambridge crystallographic information data with deposit number of 112,954)[15,18].Fig.3C presents the XRD pattern of MMOF which shows the related peaks,indicating that the crystalline structure of MOF was not changed and confirmed the successful synthesis of MMOF[19,20].

Fig.1.The MMOF based MSPE procedure for ampicillin extraction(A)and the basic preparation procedure of MMOF(B).

Fig.2.The FT-IR spectra of Fe3O4(A),MOF(B)and MMOF(C).
Figs.4A and B show the EDX analysis of MOF and MMOF respectively in which the presence of C,O and Cu elements in MOF and C,O,Cu and Fe elements in MMOF confirmed the successful synthesis of these materials.
SEM images of MOF(Figs.4C and D)and MMOF(Fig.4E)present the regular and ordered structure of MOF and composite structure of MMOF which provides a direct evidence for successful synthesis of the MMOF composites.The SEM image of prepared MOF is presented in different magnitude.Based on Figs.4C and D,some of the prepared MOF is octahedral in shape while others are leaf-like sheets.
Fig.5 shows the TEM images of prepared MMOF in which the dark region is related to Fe3O4nanoparticles and light region is related to MOF,which confirmed the successful preparation of MMOF.The TEM image proved the composite structure of prepared MMOF.
According to Figs.6A and B,the magnetic saturation strength of Fe3O4and MMOF was 66.5 emu/g and 15 emu/g,respectively.The magnetic saturation strength of MMOF is higher than those in some other reports[21—23].Seen from the picture in the bottom right of Fig.6,the MMOF was dispersed in the water sample homogeneously and the turbid water sample could become clear quickly under an external magnetic field.Owing to high magnetic saturation strength,the MMOF could be easily isolated by wasting the least of energy without any complex equipment.
The specific surface area and porous structure of MOF and MMOF were checked byBET(Figs.7A and B)and exhibited a type IV isotherm and strongly supported a mesoporous characteristic.The specific surface area of MOF and MMOFs was achieved to be 400 and 300.00 m2/g,respectively.This case can be explained that Fe3O4particles partially filled the pores of MOF.In addition,pore sized distribution of MOF and MMOF were investigated and were obtained to be 10.64 nm and 5.35 nm,respectively.Porosity and high specific area of MMOF significantly facilitated mass transfer of AMP and the binding sites were more accessible to interact with AMP molecules.
3.2.Parameter optimization for UASPE based MMOF of AMP from cow milk samples
To attain maximum extraction yield of AMP in cow milk samples,the elementary effects of qualitative parameters such as pH and kind of washing and eluent solvents were evaluated at the spiked concentration level of 500.0μg/L.

Fig.3.The XRD pattern of Fe3O4(A),MOF(B)and MMOF(C).
The pH of solution can affect the ionic or neutrality structure of both AMP and MMOF and has a noticeable role in the extraction of AMP.In order to find the optimum pH value,the effect of sample pH was assessed in the range of 2.0—8.0,individually.Based on the structures of AMPand MMOF,the adsorption of AMP fromcowmilk samples by MMOF might be attributed to the combined action of hydrogen bonding,π-π interaction,and also coordination bonds.On the other hand,we envisioned that the dominant adsorption mechanism is hydrogen bonding.Indeed,AMP has two pKas(2.5 and 7.3)[24]which are related to its carboxylic acid and amine functional groups,respectively.At pH value lower than 7.3,the amine group of AMP induces positive charge and changes to NH3+.On the other hand,in pH values between 2.5 and 7.3,the amine group is remained in NH3+form,while carboxylic groups get negative charge and transfer to carboxylate species.Also,at pH values higher than 7.3 the amine group and carboxylic groups remain in neutral and carboxylate forms,respectively.In pH values between 2.5 and 7.3,carboxylate groups of AMP can act as donor electrons and react with Cu2+cations through coordination bonds while COO-groups of H2BDC can react with NH3+groups of AMP through hydrogen bonding.In addition,the adsorption of AMP by the MMOF can occur viz.π-π interaction between benzene ring of AMP and H2BDC.In the pH values between 2.5 and 7.3,due to the highest negative charge density and presence of utmost donor groups and also due to presence of amine group in NH3+form,we expected that the best recovery was obtained since experimental results confirm this phenomenon.Overall,at sample pH of 7.0,the maximum extraction recovery was achieved,which is logical(Fig.S1).Therefore,the sample pH of 7.0 was set for the rest of experiments.
Cow milk samples have complicated matrices such as proteins,hormones and other metabolites which not only can retain on MMOF and HPLC column but also can overlap with AMP peak and subsequently decrement the performance of the proposed method.Therefore,to decrease the effect of interfering species,different washing solvents with different polarity including deionized water,methanol,acetonitrile,acetone and hexane were examined to obtain the cleanest extracts.The results showed that the cleanest extraction was achieved when acetone was applied as washing solvent.This phenomenon could be related to rinsing strength of acetone as nonpolar solvent for washing nonpolar interferents while adsorbed AMP retains on MMOF.
Another key factor in MSPE is selection of eluent solvent which can completely desorb target analyte from sorbent.Hence,the effects of different eluents solvents as well as their mixture with different polarity including methanol,ethanol,acetonitrile and methanol:ethanol(50:50,V/V),were assessed.Experimental results confirmed that maximum extraction efficiency was achieved when methanol/ethanol(50:50,V/V)was applied as eluent and that this result might be due tothe likeness between the polarityof AMPand methanol and ethanol and high rinsing strength of them.
3.3.CCD multivariate approach for assessment and optimization of an influential factors

Fig.4.EDX analysis of MOF(A)and MMOF(B)and SEM images of MOF(C and D)and MMOF(E).

Fig.5.TEM images of prepared MMOF.
Investigation and optimization of an effective factor on extraction recovery is a critical step in analytical methods which not only can monitor the effect of each factor individually but also can investigate the factor interactions as well as obtain true optimum levels.In this regard,different methods have been extensively applied for this purpose,among which one-factor-at-a-time(OFAT)is a common and popular approach in which the influence of one factor is monitored at a time,while other factors are fixed.In spite of simplicity and applicability,OFAT is faced with some main drawbacks such as a large number of experimental runs,labor effort,high consumption of chemicals and solvents,time consumption and high cost.Most importantly,it cannot distinguish the importance of parameters and their interactions and also it is unable to attain the true optimum levels.To address these obstacles,multivariate optimization techniques (MOT)are alternative methods in which the effects of several factors are evaluated concurrently.The reasons that distinguished MOT from OFAT are that MOT can eliminate the drawbacks of OFAT and more interestingly MOT is able to estimate the interactions between different factors,yielding a moreefficientoptimization and also attaining the true optimum levels[25—28].CCD based RSM is an efficient and powerful statistical and mathematical MOT that is able to evaluate the significance of factors,their interactions,relationships between target response and independent parameters,and most importantly it is able to attain the true optimum levels[29—32].Therefore,in this work,the effects of four main qualitative factors such as MMOF dosage(A:15.0—35.0 mg),ultrasonic time(B:1.0—5.0 min),washing solvent volume(C:1.5—3.5 mL)and eluent solvent volume(D:1.5—3.5 mL)as well as their interactions affecting extraction efficiency of AMP were performed through CCD based RSM according to 21 experimental runs,as listed in Table S1.To evaluate the significance of factors,their interactions,reliability and accuracy of the model,the experimental results were fitted to the quadratic model and analysis of variance(ANOVA)parameters(Table S2)including P and F values,lack-of-fit test,coefficients of determination(R2),adjusted R2,adequate precision,standard deviation and coefficient of variation at a certain confidence level(α=0.05)were considered.The factors with higher values of F and P values less than 0.05 have significant contribution to extraction recovery.Based on Table S2 and Fig.S2(Pareto chart)A,B,C,D,AB,AD,BD,and A2factors had the P values less than 0.05,which confirmed their significant contribution on extraction recovery while AC,BC,CD,B2,C2and D2factors had P values more than 0.05 that proved they were not significant and did not have significant contribution toextraction recovery.In addition,the lack-of-fit value higher than 0.05(0.1028)and the correlation coefficient(R2)higher than 0.98(0.9837)demonstrated the adequacy,reliability and fitness of the suggested model.R2was calculated according to Eq.(1)and considered as a measure of the variation around the mean calculated by the model:

Fig.6.VSM analysis of Fe3O4(A)and MMOF(B).

Fig.7.The BET and pore size distribution analysis of MOF(A)and MMOF(B).

while adjusted R2(0.9456)was determined using Eq.(2)and applied as a measure of the variation around the mean determined through the experiments,adjusted for the number of terms in the model:

Where SS and DF are the sum of squares and the degrees of freedom,respectively.Adequate precision represents the signal-tonoise ratio calculated based on Eq.(3)and had the value of 21.055 that confirmed a desirable adequate signal[33]:

Where?y is the response predicted by the model,P is the number of parameters applied in the model,σ2is the residual mean square,and n is the number of experiments.The standard deviation and coefficient of variation of model were obtained to be 2.70 and 3.28,respectively,which shows the adequacy and reliability of the proposed model.Fig.S3 Shows good agreement between experimental extraction recovery(ER(% ))and calculated values.A second-order polynomial Eq.(5)was used toexpress responseas a function of the independent variables as follows:

where β0is the constant terms,βi,βiiand βijare the regression coefficients for intercept,linear,quadratic and interaction terms,respectively;Xiand Xjare the independent variables and ε is the residual associated to the experiments.The polynomial function allows to predict the value of the response Y based on specific values of the selected factors.Eq.(6)shows the effect of factors and their influences on ER(% )of AMP.

Equation(6)represents the appropriate relation between ER(% ),factors,their interactions and also quadratic model in which the coefficients show the intensity of each factor on ER(% ).In addition,the positive signs show the growth of ER(% )while negative signs have an opposite effect and show decrease of ER(% ).In the following part,to investigate the simultaneous effect of factors on ER(% ),RSM 3D plots were applied.Fig.S4A shows the interaction between sorbent dosage and an ultrasonic time in which concurrent increase of them enhances ER(% ).Increase of sorbent dosage enhances the specific surface area and available sites of MMOF which enhances the adsorption of AMP molecules onto MMOF and subsequently increase ER(% ).In addition,an ultrasonic irradiation can enhance the dispersion of sorbent in sample solution and increase the contact between AMP molecules and MMOF.An adequate ultrasonic time increases mass transfer of AMP molecules from sample solution to MMOF surface,while high ultrasonic time might have an opposite effect and cause desorption of AMP molecules from MMOF surface which decreases ER(% ).As the cow milk samples contain interfering species and accumulated matrix interferences,it is necessary to remove them from sample matrix.Therefore,an adequate volume of washing solvent can remove impurities and enhance ER(% ).The effect of washing solvent volume was assessed in the range of 1.5—3.5 mL.As shown in Figs.S4B,D,and F,an adequate volume of washing solvent can remove impurities while an inadequate volume of washings solvent may not be able to eliminate impurities from the sample.In addition,high volume of washing solvent notonlycan remove impurities,but also can remove AMP molecules from sample matrix.Figs.S4C,E and F represent the effect of eluent solvent volume on ER(% ).The appropriate volume of eluent solvent can successfully elute the adsorbed AMP molecules from the MMOF surface.The effect of eluent solvent volume was investigated in the range of 1.5—3.5 mL and the best ER(% )was achieved in 2.0 mL of eluent solvent.Actually,2.0 mL of eluent solvent was enough to desorb AMP molecules from cow milk samples.After RSM evaluation,for concurrent optimization of the analyzed factors,desirability function(DF)was applied based on Eq.(7)[34]:

DF is a useful and conventional approach to determine the global optimum conditions which can convert the predicted and experimental response of each factor into a desirability score whereas it takes values between 0.0 and 1.0,which shows the completely undesirable and fully desired response,respectively.On the basis of the desirability score of 0.89,maximum recovery(92.9% )was achieved at optimum conditions set as the sorbent dosage(30 mg),ultrasonic irradiation(4.0 min),washing solvent volume(3.0 mL),and eluent solvent volume(2.0 mL)(Fig.S5).
3.4.Evaluation of adsorption isotherms for determination of AMP by MMOF
Adsorption isotherm is an important aspect in adsorption study whichshowstheaffinity,tendencyaswellasadsorptionmechanism of analyte toward sorbent[35].To determine the adsorption isothermsofAMPontoMMOF,thequantitativeanalysiswasdoneinthe concentration range of 5.0—50.0 mg/L of AMP.To estimate the binding properties of MMOF,different isotherm models including Langmuir,Freundlich,Temkin and Dubinin—Radushkevich were evaluated as listed in Table S3.According to Table S3,qeshows the adsorbed AMP at equilibrium,Qmand Qsare the maximum adsorption capacity,Ceis the AMP concentration in sample solution at equilibrium,KL,RL,α,m,B,KTandβare the Langmuir,Freundlich,Temkin and Dubinin—Radushkevich constants,respectively.Based on Table S3,Qmfor Langmuir model was achieved to be 250.5 mg/g anditisclosetothevalueobtainedfromexperimentalresults.TheR2for Langmuir model(0.9987)was higher than that forother models,which confirms that the adsorption of AMP onto MMOF can be well described by Langmuir isotherm.The principle of Langmuir isotherm is based on the monolayer adsorption of AMP molecules onto homogenous surface of sorbent while Freundlich model assumes the multilayer adsorption of AMP molecules onto heterogeneous surface of sorbent[36].Considering the previous discussion on effect of pH,it is speculated that the AMP molecules are monolayer adsorbed on the surface of MMOF mainly through hydrogen bonding.RLisaseparationfactorthatcantakevaluesbetween0and 1,which show desired(irreversible)and undesired(reversible)adsorption,respectively.The RLfor AMP adsorption onto MMOFs was achieved to be 0.032—0.252 that is close to 0 and shows a desired(irreversibleadsorption)process.mandBaretheadsorption intensity and Temkin constant,respectively.Dubinin—Radu shkevich isotherm represents the physical and/or chemical nature of adsorption[37].Ifβvalues arebetween 8 and 16 kJ/mol,chemical mechanisms control the adsorption process while forβlower than 8 kJ/mol,the adsorption process occurs through a physical mechanism[38].The obtainedβvalue was 7 kJ/mol,which confirms the physical adsorption of AMP onto MMOFs.
3.5.Adsorption kinetic investigation for adsorption of AMP by MMOF
To examine the dynamics of adsorption process in terms of the order of rate constant,different adsorption kinetics such as Firstorder-kinetic,Pseudo-second-order-kinetic,Intraparticle diffusion and Elovich were studied.Based on Table S4,qeand qtare the adsorbed AMP at equilibrium and time t,K1,K2,Kdiff,C,βandαare the constants of models,respectively[39].According toTable S4,R2value for Pseudo-second-order-kinetic model(0.9932)was higher than that for other models,which confirms adsorption kinetic followed by Pseudo-second-order.Also,the theoretical qe(cal)(42.70)value was close to the experimental qe(exp)(38.74)value for Pseudo-second-order-kinetic model,which indicates that the second order model is in good agreement with experimental data and can be used to favorably explain the AMP adsorption on MMOF.
3.6.Method validation and real sample analysis
The developed MMOF-MSPE-HPLC/UV was fully validatedunder the optimum conditions in terms of the analytical figures of merit including linear range,limit of detection(LOD),limit of quantification(LOQ),precision,and accuracy.The calibration curve was constructedbyplottingpeakareaversusAMPconcentrationinthewide range of 1.0—5000.0 μg/L for AMP,for cow milk samples with satisfactorycorrelation coefficient(≥0.99).The LOD and LOQ values wereachievedtobe0.29μg/Land0.96μg/Lusing3σ/slopeand10σ/slope ratios,respectively,whereσis the standard deviation in the mean value for chromatograms obtained from the blank cow milk sample according to IUPAC recommendation[40].The precision of the method was investigated by performing replicate analyses of blank cow milk samples at five different concentration levels(1.0,10.0,100.0,1000.0 and 5000.0μg/L)on the same day and in the six successive days expressed as relative standard deviation(RSD).As seeninTable1,theRSDvalueswerelowerthan5.5% forallintra-and inter-day precision while supreme extraction recoveries(higher than 93.0% )were achieved,demonstrating qualified reproducibility and high accuracy of the proposed method.In order to indicate the effectofsamplepretreatmentinAMPanalysis,thecowmilksamples were directly injected to the HPLC.As shown in Fig.8,due to the complexity of the cow milk samples,AMP peak was not detected,which indicated direct analysis of AMP is impossible.More excitingly,owingtothehighpotentialofMMOF-MSPEforenrichingAMP,trace quantities of AMP have remarkable signal.Consequently,the developed MMOF-MSPE is worthy for pre-concentration of AMP from complicating cow milk samples at trace level.In order to confirm the analytical applicability of the proposed method,the quantitative analysis of AMP in two different cow milk samples was carried out.The standard addition method was performed by spikingstandardsolutionofAMPtothesamples.AslistedinTable2,the accuracy were estimated through investigation of the recovery test.The ER% was found to be in the range of 95.0% —100.8% while RSDvalueswerelowerthan4.0% fortriplicatecontinuousinjections.These results suggest that the authoritative MMOF-MSPE-HPLC/UV method was mighty for clean extraction of AMP at trace level from intricate cow milk samples with supreme precision and accuracy.
3.7.Selectivity of the proposed method
The effects of interfering compounds on the extraction and pre-concentration of AMP in real samples were examined.Experiments performed using a 500μg/L of AMP solution was performed in the presence of different concentrations of the interfering compounds(Table S5).In this study,the tolerance limit was defined as the highest amount of interfering compounds that lead to an error that is±5.0% in the determination of AMP.The results in Table S5 indicated that the presence of most of the metal ions did not interfere with the extraction and pre-concentration of trace quantities of AMP.On the other hand,due to similarity of AMP with some compounds such as amoxicillin and oxacillin,they interfered in extraction of AMP.This fact can be related to the structures and interactions between compounds and MMOF.
3.8.Reusability of the prepared MMOF
To examine the applicability of the proposed sorbent,the reuasability aspect of sorbent was evaluated.For this purpose,differentadsorption-desorption replicationsofMMOFwere completed and results showed that the sorbent was applicable for at least eight times(Fig.S6).Thus,the proposed MMOF is not only ease of synthesis,inexpensive,and simple but also is a good candidate for practical application for extraction and determination of AMP from complicated cow milk samples.
3.9.Method comparison
The developed method was compared with those in some other reports for determination of AMP in different real samples.The MMOF in the present study showed the highest adsorption capacity(250.5 mg/g)and the short adsorption equilibrium time(4 min)and outstanding reusability(8 times).In addition,because of desirable magnetic properties(15 emu/g),it is easier to separate sorbent from sample and realize recycling than nonmagnetic MOF.From the analytical aspect,the linear range and LOD of the proposed method were approximately the same or better than those of some other methods,which represents the promising future of our present MMOF for determination of AMP from complicated cow milk samples[3,41—43](Table 3).Moreover,the prepared MMOF wascomparedwith some commercial sorbents presented inTable 4[44—54].These sorbents have been applied for determination of different compounds.The presented method has exclusive properties in terms of high adsorption capacity and short extraction time which are better than those of some reported sorbents.Preparation of the proposed sorbent is easy,simple and cheap compared to some reported methods.The proposed method does not need an expensive equipment and the materials and reagents are available.Some reported methods are multi-step and time consuming while the presented method is one step.
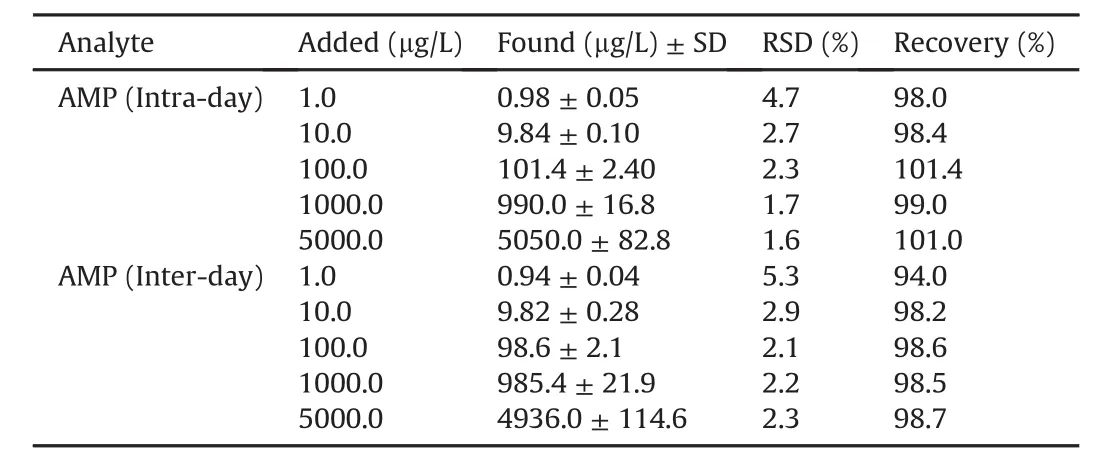
Table 1 Intra-day and inter-day precision and recovery of the MMOF-MSPE-HPLC/UV method for ampicillin determination in spiked cow milk samples(n=5).

Fig.8.Typical chromatograms of(A)direct injection of extracted cow milk sample and(B)spiked cow milk sample after MMOF-MSPE procedure.

Table 2 Accuracy of the MMOF-MSPE-HPLC/UV method(n=5).
4.Conclusion
In the present study,we prepared MMOF material by a simple and facile one pot solvothermal method and successfully applied it for the determination of AMP from cow milk samples for the first time.Compared to some reported sorbents,the proposed sorbent has high adsorption capacity and(250.5 mg/g)and also short extraction time(4 min).The magnetic property of the prepared sorbent makes it easy to separate sorbent from sample solution,which not only reduces extraction time but also improves the repeatability of sorbent.The MMOF material could be effectively regenerated by recycling it over 8 times.Under the optimized conditions,the proposed method is applicable for linear range of 1.0—5000.0 μg/L with detection limit of 0.29 μg/L,satisfactory recoveries(≥95.0% )and acceptable repeatability(RSD less than 4.0% ).Compared to methods that have been used for determination of AMP,the presented work represents the satisfactory results in terms of synthesis of sorbent and analytical aspect.In view of the combined advantages of magnetic Fe3O4and MOF,wecould foreseethat this magnetic adsorbent will have great potential for determination of AMP from cow milk samples.
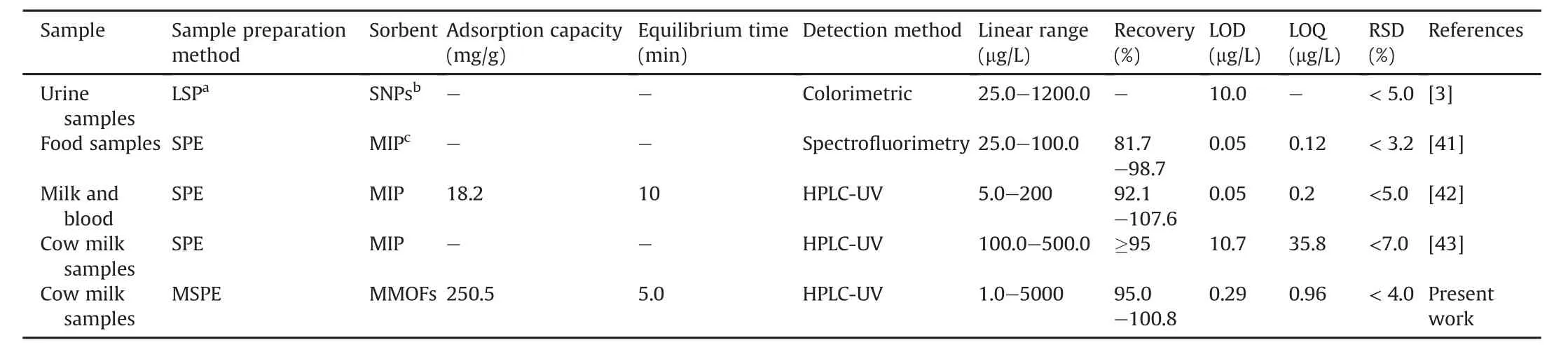
Table 3 Comparison between MMOF-MSPE-HPLC/UV and other approaches for determination of ampicillin.

Table 4 Comparison of the prepared MMOF with some commercial sorbents.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was financially supported by Graduate School and Research Council of Yasouj University.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.02.006.
 Journal of Pharmaceutical Analysis2020年4期
Journal of Pharmaceutical Analysis2020年4期
- Journal of Pharmaceutical Analysis的其它文章
- Comparative pharmacokinetics of six major compounds in normal and insomnia rats after oral administration of Ziziphi Spinosae Semen aqueous extract
- Electrochemical and in silico approaches for liver metabolic oxidation of antitumor-active triazoloacridinone C-1305☆
- An integrated spectroscopic strategy to trace the geographical origins of emblic medicines:Application for the quality assessment of natural medicines
- TGA/Chemometrics addressing innovative preparation strategies for functionalized carbon nanotubes
- Identification of impurities in nafamostat mesylate using HPLC-ITTOF/MS:A series of double-charged ions
- Solid and liquid state characterization of tetrahydrocurcumin using XRPD,FT-IR,DSC,TGA,LC-MS,GC-MS,and NMR and its biological activities
