Electrochemical and in silico approaches for liver metabolic oxidation of antitumor-active triazoloacridinone C-1305☆
Agnieszk Pote?g,Dorot˙Zelszczyk,Zo fi Mzersk
aDepartment of Pharmaceutical Technology and Biochemistry,Faculty of Chemistry,Gdańsk University of Technology,Gabriela Narutowicza St.11/12,Gdańsk,80-233,Poland
bDepartment of Organic Chemistry,Faculty of Pharmacy,Jagiellonian University,Medyczna St.9,Krakw,30-688,Poland
Keywords:
Antitumor triazoloacridinone
P450-catalyzed reactions
Electrochemistry/mass spectrometry
In silico metabolism Prediction
Liver microsomal assay
ABSTRACT
5-Dimethylaminopropylamino-8-hydroxytriazoloacridinone(C-1305)is a promising antitumor compound developed in our laboratory.A better understanding of its metabolic transformations is still needed to explain the multidirectional mechanism of pharmacological action of triazoloacridinone derivatives at all.Thus,the aim of the current work was to predict oxidative pathways of C-1305 that would reflect its phase I metabolism.The multi-tool analysis of C-1305 metabolism included electrochemical conversion and in silico sites of metabolism predictions in relation to liver microsomal model.In the framework of the first approach,an electrochemical cell was coupled on-line to an electrospray ionization mass spectrometer.The effluent of the electrochemical cell was also injected onto a liquid chromatography column for the separation of different products formed prior to mass spectrometry analysis.In silico studies were performed using MetaSite software.Standard microsomal incubation was employed as a reference procedure.We found that C-1305 underwent electrochemical oxidation primarily on the dialkylaminoalkylamino moiety.An unknown N-dealkylated and hydroxylated C-1305 products have been identified.The electrochemical system was also able to simulate oxygenation reactions.Similar pattern of C-1305 metabolism has been predicted using in silico approach.Both proposed strategies showed high agreement in relation to the generated metabolic products of C-1305.Thus,we conclude that they can be considered as simple alternatives to enzymatic assays,affording time and cost efficiency.
1.Introduction
Tumor diseases are common and usually rapidly progressive disorders known worldwide[1].Although current medicine is developing very dynamically,the possibilities of treating tumors are still very limited.The success of antitumor therapy with the chemotherapeutic agent depends on the selection of the medication characterized by well-balanced profile between efficacy and safety[2].In this light,the studies on the possible metabolic pathways of a potent drug are extremely important for assessing its bioavailability,activity and toxicity in the human body.Insufficient knowledge about metabolic transformations of the drug is one of the most common causes of failure and the lack of complete results during clinical trials[3].
In recent years the tools for the prediction of metabolic status of drugs have been developed and improved.The liver microsomal system is one of the best characterized models for drug biotransformation research which is simple to use[4,5].This subcellular fraction contains major drug-metabolizing enzymes,including the cytochrome P450(P450)family capable of catalysing the oxidative biotransformation of most drugs,including antitumor agents,and other xenobiotics[6].Thus,the compound incubation with liver microsomes generates oxidative phase I metabolites.However,the efficiency of the microsomal approach strongly depends on the specific expression of different P450 isoforms in each organism.Consequently,some differences between the metabolites predicted from liver microsome experiments and the metabolites found in the patient's body fluids after the intake of a drug are usually observed[6,7].Moreover,a targeted drug may be metabolized in the human body through different pathways and processes.
In our studies we considered that in enzymatic metabolism systems,the processes of purification and separation prior to identification and quantification of metabolic products are expensive,laborious,and time-consuming[4].To replace these procedures,otherapproaches,which are able tosupport and improve drug metabolism studies,were developed and they can be classified in two categories:(i)electrochemical simulation of P450-mediated reactions and(ii)in silico prediction of drug metabolism.Electrochemistry(EC)is one of the classical methods to induce oxidationreduction reactionscatalyzed by liverP450 isoforms[8,9].Compared to the enzymatic assays,there are many advantages of this approach,such as low cost,fast response,simplicity,clean system,and ease of automation.These are universally valid and make electrochemical method extremely powerful in drug metabolism studies[10,11].Nowadays,electrochemical cells coupled online to analytical techniques such as mass spectrometry(MS)with or without previous liquid chromatographic(LC)separation(Fig.1)hold a great potential for simulation experiments and are intensively developed.EC(/LC)/MS techniques may provide exhaustive information about the properties of the electrochemically generated transformation products,even though the enzymatic mechanism of transformation differs from the electrochemical oxidative pathway[9,12,13].In turn,in silico screening strategy aims at predicting the most probable sites of metabolism for the investigated compound,without any need for experimental data.Therefore,this approach provides the main metabolite structures which can be formed in various human tissues(liver,skin,brain,and lungs).Then,the metabolites predicted are listed with a likelihood ranking[14,15].
The aim of the present research was to investigate C-1305(5-dimethylaminopropylamino-8-hydroxytriazoloacridinone)oxidative transformations by the application of three different strategies for studying drug metabolism:experimental EC,in silico prediction,and incubation with rat liver microsomes(RLMs).The unknown products of phase I metabolism were subsequently identified by using electrospray ionization(ESI)MS.
C-1305(a structure presented in the frame in Fig.1)is the most potent derivative among the antitumor-active triazoloacridinones developed in our laboratory[16].It exhibited significant and clearly differentiated cytotoxicity against a number of tumor cell lines of human and animal origin in tissue cultures[16,17],as was also shown in vitro screening scheme(panel of 60 different human tumor cell lines)in the National Cancer Institute(Bethesda,MD,USA).Moreover,C-1305 displayed high antitumor potency against P388 leukemia in mice and towards a wide spectrum of experimental transplantable solid tumors in animals such as colon and breast cancers[17,18].C-1305 is the close structural analogue of the clinically tested imidazoacridinone antitumor agent,C-1311[19-21];however,some differences in their chemical structure may determine the more advantageous pharmacological properties of the first one.Both compounds were synthesized as a result of rational drug design strategy based on several circumstances connected with combined characteristics of anthracyclines and acridines essential for biological activity[22,23].Overall,the specific structure of C-1305 determines its mode of action that involves several routes as physicochemical interaction with DNA[24],influence on cell cycle progression[25],induction of cell death[25],and others,in particular topoisomerase II inhibition[26,27]and covalent DNA crosslinking[24].The leading concept of the antitumor effect of C-1305,similar to the case of C-1311,is considered as the relationship between its biological activity and the susceptibility to enzymatic oxidation.
The results regarding the capability of the EC to simulate the oxidative metabolism of C-1305 are discussed here for the first time.Then,electrochemical findings related to phase I metabolites of C-1305 were compared with those predicted from in silico manner and a classical enzymatic approach employing RLMs.Elucidation of the mechanism of the electrochemical oxidation would provide a deeper insight into the chemistry and EC of triazoloacridinones.What is more,the identification of the obtained products would enable their comparison with the C-1305 metabolites eliminated from the body.The results obtained from the proposed multi-tool analysis of C-1305 metabolism would be of great help in designing new compounds with a better pharmacokinetic profile,or in designing prodrugs where the compound needs to be metabolized in order to become active.
2.Materials and methods
2.1.Chemicals,reagents,and enzymes
C-1305[16]was synthesized as dihydrochloride in our laboratory.The compound was of more than 98% pure as determined by liquid chromatography(LC)and nuclear magnetic resonance analyses.The following chemicals were purchased from Sigma-Aldrich(St.Louis,MO,USA):dipotassium phosphate(K2HPO4),and formic acid(HCOOH),magnesium chloride anhydrous(MgCl2),monopotassium phosphate(KH2PO4)and potassium hydroxide(KOH).Methanol(CH3OH;gradient grade for LC)andβ-nicotinamide adenine dinucleotide 2′-phosphate tetrasodium salt(NADPH)were obtained from Merck KGaA(Darmstadt,Germany).Ammonium formate(HCOONH4;reagent grade)was ordered from Fisher Scientific(Loughborough,UK).All other commercially available chemicals and reagents were of the highest possible grade available.Ultrapure water of 18 MΩ?cm of resistivity,used in all the experiments,was passed through a Milli-Q water purification system from Merck KGaA(Darmstadt,Germany).

Fig.1.Schematic set-up of the system used for the electrochemical generation and identification of C-1305 oxidation products.Molecular structure and atomic numbering of antitumor-active triazoloacridinone C-1305 is shown in the frame.LC,liquid chromatography.
Pooled RLMs from untreated,male Sprague-Dawley rats(protein concentration,20 mg/mL;P450 content,680 pmol/mg protein)were purchased from Tebu-bio(Le Perray-En-Yvelines,France).
2.2.Instrumentation
The simulation of the oxidative metabolism of C-1305 was accomplished in an amperometric electrochemical thin-layer cell equipped with a disc glassy carbon(GC)working electrode(φ=8 mm;A=0.502 cm2)and a Pd/H2reference electrode(reactor cell;Antec Leyden,Zoeterwoude,the Netherlands).Carbon-loaded polytetra fluoroethylene(PTFE)served as auxiliary electrode.The cell potentials were applied using a ROXY EC System(Antec Leyden).All potentials mentioned in this work were based on the reference electrode.The software used for controlling EC was Dialogue(Antec Leyden).
The outlet of the electrochemical cell was interfaced into an ESI source of a quadrupole-time of flight(Q-TOF)mass spectrometer(Agilent Technologies,Santa Clara,CA,USA)inlet for on-line EC/MS analysis using PEEK tubing.For controlling the MS,MassHunter software(Agilent Technology)was used.The electrochemically generated oxidation products were also off-line injected onto the LC column,separated,and detected by ESI-MS.LC separations were performed with Waters Associates HPLC system(Waters Co.,Milford,MA,USA).It was equipped with a model 600 E system controller,a model 7725i Rheodyne injector,and a model 2996 photodiode array detector(DAD)controlled with Millennium software(Waters Co.).
2.3.Electrochemical simulation of C-1305 oxidative metabolism
The setup used for generation of C-1305 products by electrochemical oxidation and subsequent direct(on-line)MS or off-line LC/MS analysis is shown in Figs.1,2,3,4,5 and 6.For electrochemical oxidation,10μM C-1305 in 0.1% HCOOH in water/CH3OH electrolyte(50/50,V/V)was conducted through the EC cell using a dual piston syringe pump model SP2-ROXY(Antec Leyden)at a flow rate of 20μL/min.The effluent from the EC was injected directly into the ESI-MS interface or was collected for further LC/ESI-MS analysis.
2.4.Rat liver microsomal incubations
A mixture of microsomal protein(RLMs)and C-1305,dissolved in 0.1 M potassium phosphate buffer solution(adjusted to pH 7.4 with 1 M KOH),was pre-warmed for 5 min at 37°C in a shaking water bath.MgCl2and NADPH were added to the incubation mixture(to a total volume of 100μL),which was then further incubated for 60 min at 37°C.The final concentrations were as follows:2 mg/mL RLMs,0.2 mM C-1305(added from 2 mM stock solution in 0.1 M potassium phosphate buffer solution,pH 7.4),0.5 mM MgCl2,and 2 mM NADPH(added from 10 mM stock solution in 0.1 M potassium phosphate buffer solution,pH 7.4).The incubation was terminated by adding ice-cold CH3OH(1:1,V/V)to the incubation mixture for the precipitation of proteins.The sample was then vortexed and placed in ice for 10 min.After centrifugation at 10,000×g for 15 min,an aliquot of the supernatant(150μL)was then analysed directly by reversed-phase high-performance LC(RP-HPLC)with UV—Vis detection at 420 nm and/ordiode arrayand multiple wavelength detection,and monitored by MS.For each incubation,a negative control was carried out without the cofactor NADPH to prevent P450 catalysis.All assays were conducted in at least triplicate.

Table 1 EC and ESI-MS parameters as applied in direct EC/ESI-MS experiments for determination of accurate masses of product ions.
2.5.In silico prediction of C-1305 metabolism in liver
The MetaSite software tool(version 5.1.1;Molecular Discovery Ltd.,Hertfordshire,UK)is a computational algorithm that predicts the most likely metabolic transformations of the compound related to phase I reactions in the liver.Moreover,it provides the structure of the metabolites,formed with a ranking derived from the site of metabolism predictions,with exact molecular weight(MW)and calculated logarithm of partition coefficient(cLogP)to help and complement the experimental elucidation of metabolite structures.
A 2-D structure of the C-1305 was imported into the interface of MetaSite to predict metabolic soft spots for liver metabolism and structures of metabolites in the liver related to P450-mediated reactions.Only metabolites with a molecular mass higher than 150 Da and with a likelihood ranking≥50% were considered.
2.6.EC and ESI-MS conditions
ESI-MS detection of C-1305 products generated by EC was performed in the positive ion mode in the full scan mode(mass-tocharge ratio,m/z 100-1000).The detailed EC and ESI-MS parameters are presented in Tables 1 and 2.
2.7.LC conditions
Chromatographic separations of the parent drug and its transformation products were carried out using a reversed-phase 5-μm Suplex pKb-100 analytical column(C18)(Supelco Inc.,Bellefonte,PA,USA)with the following dimensions:250 mm length×4.6 mm i.d.,5-μm particle size.The column was operated at a room temperature of 25°C.The flow rate utilized was 1 mL/min.For all chromatographic separations,eluent A of the mobile phase was a solution of 0.05 M HCOONH4in water(adjusted to pH 3.4 with HCOOH).Eluent B was CH3OH.A linear gradient from 15% to 85% B in A was kept for 25 min,then followed by a linear gradient from 80% to 100% B in A for 3 min.A rate of 100% of solvent B was kept for 1.5 min before returning to initial conditions within 0.5 min.These were then kept for 10 min to allow for column equilibration.
3.Results and discussion

Fig.2.The representative MS spectrum of C-1305(m/z 338)before(reactor cell off;the inset at the top-left corner)and after(reactor cell on)reaction in the electrochemical cell(positive-ion mode).

Fig.3.The representative ion mass intensity-time curves of 10μM C-1305 oxidation at a GC working electrode(positive-ion mode).The m/z ratios shown correspond to the protonated[M+H]+C-1305 and its oxidation products(see legend).The signal is dependent on the voltage used in the EC cell(two and half cell cycles are shown).Experimental conditions:potential range 0—2.5 V;scan rate 10 mV/s,continuous;T=21 °C;φ GC working electrode 8 mm.
Any drug development process must proceed through several stages in order to produce a product that is efficacious and safe.Hence,one of the most important topics is identifying metabolic pathways of a new drug.It requires specific methods involved in the generation and characterization of various types of drug metabolites that determine its bioavailability,activity and toxicity profile in humans.In this work,three different strategies for the investigation of C-1305 oxidative metabolism were used.In the following part,the results of electrochemical simulation are discussed towards their correlation with the results from a conventional microsomal experiment and in silico model for the prediction of potential liver metabolism reactions.The C-1305 products of electrochemical and enzymatic transformations were separated and analysed by RP-HPLC with UV—Vis detection and/or diode array and multiple wavelength detection.For structure elucidation of the detected compounds,ESI-MS experiments were performed.
3.1.Electrochemical generation of C-1305 oxidation products

Fig.4.The representative EICs of C-1305 and its oxidation products found in an electrochemical simulation(EC)(A)and in an incubation mixture with rat liver microsomes(RLMs)(B).The m/z ratios shown correspond to the protonated[M+H]+C-1305 and its oxidation products.Peak names correspond to compounds presented in Table 2.
The focus of this study lied in the investigation of the metabolic pathway of C-1305 using the purely instrumental EC method.No metabolism data for this compound is yet available from such a matrix-free environment.In the beginning,in order to obtain a comprehensive overview of C-1305 oxidation products,the electrochemical method was optimized by adjusting several parameters.The obtained results(Tables S1 and S2)indicated that type of electrolyte(composition,pH),working electrode material,flow rate,and potential range influenced the number and/or amounts of products formed during the electrochemical treatment.Finally,the highest electrochemical conversion of C-1305 into its expected products was achieved for 0.1% HCOOH in water/CH3OH electrolyte(50:50,V/V)with a flow rate of 20μL/min and with GC as working electrode material operated in the range of potential 0—2.5 V.GC electrode,widely used in EC,can be applied over an extended potential range when oxidation reactions occur.Thus,due to its high overpotential for oxygen,it cannot be excluded that partial electrolysis of the aqueous solvent also took place.The working electrode surface was cleaned between each oxidation step by flushing with a solution of 50% CH3OH in water.When disassembling the working electrode after an oxidation,no adsorption residue was visible.
In order to obtain products generated electrochemically from C-1305,the parent compound was introduced into the electrochemical cell at a flow rate of 20μL/min for a designated time period.The scan mode was used for continuous synthesis of C-1305oxidation products.Fig.2 illustrates the MS spectra of C-1305 before(cell off,control measurement;Fig.2 inset)and after(cell on)reaction in the electrochemical cell.When the potentialwas not applied to the EC system,the MS spectrum shows the peak at m/z 338,that is attributed to the C-1305 ion[M+H]+.Additionally,in a solution of C-1305 without electrochemical oxidation,we also observed an intense ion at m/z 310 which might correspond to C-1305 derivative.In turn,the intensityof these peaks decreased with the increasing potential and depended on the potential value the peaks of the appropriate oxidation products appeared.The obtained results were displayed as ion mass intensity-time curves,which made it possible to perform fast screening for electrochemically generated products by showing the ion signal intensity of the relevant product(y-axis)depending on the time of the electrochemical conversion(x-axis)(Fig.3),which reflected the changes in electrochemical potential.After electrochemical reaction seven possible products of C-1305 were detected.A growth in the intensity of signals coming from oxidation products and a drop in the intensity of signal from parent ion were observed.
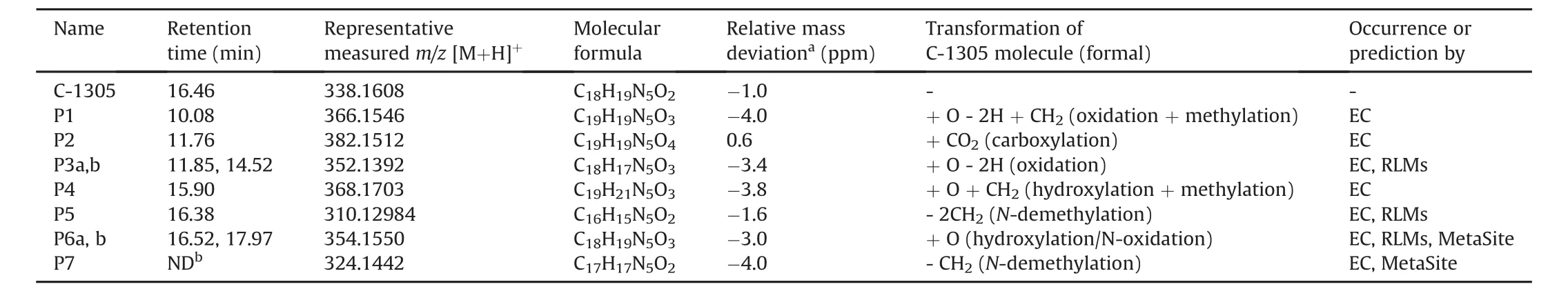
Table 2 Molecular formulas of C-1305 and its products found after electrochemical oxidation on a GC working electrode in a potential range of 0—2.5 V versus Pd/H2and predicted in incubations with RLMs or by MetaSite.The structural calculations are based on exact masses,with the relative mass deviations between measured and theoretical m/z values being provided in ppm.For peak identification refer to Fig.4A.
3.2.Separation and identification of the electrochemical products of C-1305
For characterization of C-1305 electrochemical products in more detail regarding their polarity,a separation method based on RPHPLC has been developed.The extracted ion chromatograms(EICs)of C-1305 and its generated electrochemically potential products are shown in Fig.4A.These datawere then compared with LC/ESI-MS data obtained from liver microsomal incubation.With the application of off-line EC RP-HPLC/ESI-MS method,C-1305 and its six electrochemical products were identified.Table 2 provides a list of the products of electrochemical conversion of C-1305 characterized by different m/z values,retention time and the proposed transformations of C-1305 molecule.The deviation of measured m/z to the theoretical m/z,given as relative mass deviation(ppm)=((m/zexperimental— m/zcalculated)/m/zcalculated)× 106,was for all products≤4 ppm.
P450 is the most important enzyme system that catalyses a variety of oxidative transformations of xenobiotics and endogenous compounds(phase I reactions).P450s activate molecular oxygen and transfer a single oxygen atom to a substrate while the other oxygen atom ends up inwater molecule[28].These oxygen transfer reactions include the following:aromatic/aliphatic hydroxylations,epoxidation,and heteroatom oxidations.Additionally,P450s can also promote heteroatom-dealkylations,dehalogenation,and dehydrogenation[29].As can be seen from the literature,the electrochemical system is able to successfully simulate the majority ofP450-catalyzed mixed-function oxidation reactions[9-13].During the direct electrochemical oxidations,the electrode behaves as an oxidant which can be adjusted by the applied potential in order to perform charge transfer reactions[8].The proposed electrochemical conversion of the investigated triazoloacridinone,summarised in the reaction scheme shown in Fig.5,includes mainly N-dealkylation(P5 at m/z 310 and P7 at m/z 324)and single O gain via hydroxylation(P6 at m/z 354).
N-Dealkylation is a typical biotransformation pathway in P450-catalyzed oxidation reactions that usually inactivates xenobiotics,including secondary and tertiary amines found in many drugs,and facilitates their elimination[30].It can be readily simulated by the EC system.The electrochemical mechanism resembles to a large extent a single-electron transfer mechanism mediated by P450.It consists of an initial electron abstraction,followed by deprotonation and the subsequent abstraction of another electron that results in an iminium intermediate.Following hydrolysis,the intermediate decomposes into the final N-dealkylated product[31].In the case of C-1305,N-demethylated and N-bis-demethylated products were obtained in the electrochemical cell.The chromatographic peak of the latter(P5,m/z 310)was eluted with the peak corresponding to that of substrate(C-1305,m/z 338),which indicates that these compounds may have similar polarity.In turn,product P7,which had been previously found in EC analysis,was not detected after chromatographic separation,likely due to the slightly inferior limit of detection and/or its limited chemical stability.It should be noted that reactive iminium intermediate formed during N-dealkylation process may be associated with the mechanism-based inactivation of P450 enzymes reported for C-1305[32].
Another remarkable oxidative capability of P450 is hydroxylation[30].This process can be easily induced electrochemically at positive potentials[12].The presence of an ion at m/z 354 corresponding to an increase in mass by 16 Da confirmed that successful electrochemical hydroxylation of C-1305 molecule took place.Further,two different isomers of this product(peaks P6a and P6b in Fig.4A)were found after chromatographic separation of the postelectrochemical mixture.Both are characterized by retention time slightly higher than that of C-1305.

Fig.5.Overview about all relevant products generated after electrochemical oxidation of C-1305 with proposed structure.Observed m/z value is shown.Numbers correspond to compounds presented in Table 2.
Due to the presence of an additional hydroxyl group there may be more polar substances than substrate.Therefore,it is suspected that O gain might have occurred at various positions of the C-1305 molecule by aliphatic and aromatic hydroxylation.The electrochemical oxidation potentials for hydroxylation of aliphatic C-H bonds are generally very high[12],but the application of a potential of above 2 V might have enabled oxidation reaction in this case.We suppose that the product of such hydroxylation was obtained with lower yield.For product P6,a putative structure of diol(quinol)is also considered.Electrochemical oxidation of aromatic ring is initiated by electron abstraction.This electron transfer requires a lower positive potential than for aliphatic hydrocarbons due to resonance stabilization of the aromatic radical cation.In order to be oxidized electrochemically within the potential limits of water,the aromatic ring must be activated by an electron-donating group.This requirement was made by 8-hydroxyl group present in the triazoloacridinone moiety.It can be expected that analogously as for compound C-1311[33],the activation of the triazoloacridinone ring system at position ortho to this functional group will be the most preferential.Furthermore,product P3 observed at m/z 352,indicating the mass gain of two hydrogens,was very likely generated from product P6 by dehydrogenation.Thus,the above hydroxylations might have proceed immediately to respective less polar carbonyl or quinone derivatives.
In our study we also observed signals at m/z 366(P1),368(P4),and 382(P2)which could be supposed to be the potential products resulting from the combination of different oxidative pathways(e.g.,hydroxylation,dehydrogenation)of the investigated antitumor agent.In the elution profile their peaks appeared before the C-1305 peak.When considering the structure of the product P4,it can be assumed that the addition of a single methoxy group achieved by EC could have taken place here.We found that its formation was highly dependent on the electrolyte used.So,it is suggested that this product may result from the interaction between the substrate to be oxidized and CH3OH being a component of the electrolyte solution.
3.3.Metabolism study in RLMs
In order to obtain the metabolite profiling of drug compounds,the incubationwith livermicrosomes is a conventional,well known method[4,5].Therefore,this approach was performed on C-1305 to compare the metabolic products with those detected after electrochemical conversion of the studied compound.The representativeEICsofC-1305 productsobtained from microsomal incubations are shown in Fig.4B.In the sample taken from rat liver microsomal incubation with C-1305,compared to the purely instrumental non-enzymatic approach,the presence of only three metabolites of the parent drug:C-1305+O-2H(peak P*3a at m/z=352),C-1305-2CH2(peak P*5 at m/z=310),and C-1305+O(peak P*6a at m/z=354)was observed.No additional metabolites were detected in the LC/ESI-MS analysis of the microsomal sample.Although liver microsomes contain the physiologically relevant combinations of drug-metabolizing enzymes,the expression of the different P450 isoforms may be very diverse in each organism.This may explain why some products of C-1305 generated electrochemically were not produced in liver microsomes.
The peaks of products P*3b,P*5,and P*6b were characterized byretention time identical tothat of products P3a,P5,and P6b from EC analysis.The first two were of low intensities,whereas the amount of this latter increased with longer incubation time.Based on the experimental mass ion data,the same observations as for the previously identified products of electrochemical conversion were made for the P3a and P5 structures.Thus,N-dealkylation of alkylamine,hydroxylation and dehydrogenation seem to be the main pathways in both EC and enzymatic oxidations of C-1305.The generation of metabolite P*b presumably involves the insertion of a single oxygen atom that,as it was proposed above,may take place under the formation of a hydroxyl group.However,the antitumor agent selected for this study was a good substrate for human recombinant flavin-containing monooxygenases(FMOs),FMO1 and FMO3[34].FMOs can oxidize a wide array of heteroatoms,particularly soft nucleophiles,such as amines,in the presence of an oxygen,an NADPH cofactor,and an FAD prosthetic group[35].Assuming that the active enzymes of RLMs are FMOs,it was suggested that peak P*6b at m/z 354 may be an amine-N-oxide.Nitrogen oxidation is also possible in the EC system,but unlike formation of S-or P-oxides,N-oxide formation can only be detected in low yields[12,36].
3.4.In silico prediction of C-1305 liver metabolism
MetaSite software allowed to predict the main sites of liver metabolism for C-1305 and its main metabolic reactions(Fig.6).The most probable sites of P450 attack in the C-1305 molecule proved the dialkylaminoalkylamino moiety and the carbon atom at position 9 of the triazoloacridinone ring.In view of this,five top ranked phase I metabolite structures were reported.
One of the major metabolites predicted was that derived by the cleavage of the N-C bond leading to N-demethylation(M1;100% of likelihood).The identical product ion P7 at m/z 324 was also identified by EC/ESI-MS analysis.In turn,further degradation of the side chain may result in a complete N-dealkylated metabolite M4(50% of likelihood)which,in contrast to M1,was not generated from electrochemical oxidation. Another metabolic reaction predicted for C-1305,common to the P450 catalytic activity,was also dehydrogenation in the propylamino chain(M5;50% of likelihood).The N-dealkylation and dehydrogenation reactions in the dialkylaminoalkylamino side chain,analogous to those described above,were also predicted by in silico analysis for imidazoacridinone derivative,C-1311[37].Such substituent is flexible and,after metabolic activation,it may be able to induce interstrand covalent crosslinks in DNA of tumor cells playing a key role in biological(antitumor)activity of these compounds[24,38,39].Such mechanism mediating the cytotoxicity was also demonstrated in the case of the clinically used antitumor drugs,mitoxantrone and ametantrone[22].Further,MetaSite indicated the possibility of the formation of two isomeric hydroxylated metabolites(MW=353.15)with various ranking that can be linked with product ion P6 at m/z 354 found after electrochemical simulation.The most probable hydroxylation site in the C-1305 molecule appears to be the ortho position to the hydroxyl group in the aromatic ring,giving metabolite M2(66% of likelihood).The resulting diol(quinol)and its putative quinone derivative,which may correspond to product ion P3 obtained at m/z 352 in the EC analysis,are the preffered structures for the substitution by nucleophiles existing in a living organism(i.e.,reduced glutathione,thiols of proteins or purine and pyrimidine bases of DNA).The formation of stable adducts may alter biological functions of cellular biomolecules,which ultimately leads to a toxic response[5].Thus,both diol and quinone metabolites may be responsible for cytotoxic and antitumor actions of triazoloacridinones.Moreover,they may be related to the observed inactivation of P450 1A2 and 3A4 by C-1305 by the covalent apoprotein modification of P450 isoenzymes[32].In addition to aromatic hydroxylation,aliphatic hydroxylation is also possible,as in the case of metabolite M3(50% of likelihood).
The ability of the drugs to penetrate various biological membranes,tissues and barriers is determined by the hydrophilic or hydrophobic properties of molecules,for which the partition coefficient,P,of a molecule is used[40,41].In the case of all MetaSite predicted metabolites of C-1305 formed by liver P450s,the calculated cLogP values were in the range of 0.90—1.88.It means that they are rather more hydrophilic substances than the parent compound(cLogP=2.06),so they may be preferentially localized in hydrophilic compartments,such as blood serum,and easily excreted from the body.
To sum up,in silico study has demonstrated the possibility to theoretically predict the main metabolites of antitumor active triazoloacridinone C-1305.In silico approach proposed here appears very promising,allowing the identification of main metabolites of C-1305,which are extensively metabolized as demonstrated by electrochemical(non-enzymatic)and microsomal(enzymatic)experiments performed.
4.Conclusions

Fig.6.Liver metabolism prediction by MetaSite for C-1305.Ranking of the metabolites(A)and plot of the most probable sites of C-1305 metabolism and metabolites predicted(≥50% of likelihood)(B).The functional groups in compound that most likely will be metabolized by liver P450s are marked:the darker the color and the greater the circle of marked functional group—the higher the probability of metabolism to occur.MW and cLogP are predicted values according to MetaSite software tool(version 5.1.1;Molecular Discovery Ltd.,Hertfordshire,UK).MW,molecular weight;cLogP,the logarithm of partition coefficient.
This work demonstrated three different strategies for the investigation of oxidative metabolism of antitumor triazoloacridinone C-1305.In particular,we presented here the first application of electrochemical approach for generation of potential oxidation products of the studied compound within a matrix-free environment.We found that a dialkylaminoalkylamino moiety was most susceptible to electrochemical oxidation site in the C-1305 molecule.The proposed N-dealkylated,hydroxylated,and dehydrogenated products turned out to be typical for the processes catalyzed by P450 is oenzymes.Next,the results achieved from EC/ESI-MS were correlated with those from liver microsomal experiments.On the one hand,each method was found to provide unique features not identified with those of others.However,despite some differences in the mechanisms of electrochemical and enzymatic oxidation,the products detected after electrochemical and enzymatic conversion of C-1305 were generally well comparable.This underlines the high potential of EC approach as a fast screening tool in the prediction of metabolic transformations in drug development.In the final step,MetaSite in silico analysis reported five top ranked phase I metabolite structures for C-1305 liver metabolism that helped in the interpretation of the results provided by electrochemical and enzymatic approaches.The value of this strategy consists in the possibility of gaining very useful information on the most probable metabolites to be screened in all those cases where the exact metabolism pathways of a compound are unknown.According to the above findings,the electrochemical and in silico methods,which were shown to predict metabolism patterns,can be used as simple and fast alternatives for metabolic(enzymatic)assays,affording time and cost efficiency.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Science Center(Poland)(2012/07/D/NZ7/03395).The authors wish to thank Professor Agata Kot-Wasik(Department of Analytical Chemistry,Faculty of Chemistry,Gdańsk University of Technology,Poland),for help in the field of MS analyses.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.03.011.
 Journal of Pharmaceutical Analysis2020年4期
Journal of Pharmaceutical Analysis2020年4期
- Journal of Pharmaceutical Analysis的其它文章
- Comparative pharmacokinetics of six major compounds in normal and insomnia rats after oral administration of Ziziphi Spinosae Semen aqueous extract
- Magnetic metal organic framework for pre-concentration of ampicillin from cow milk samples
- An integrated spectroscopic strategy to trace the geographical origins of emblic medicines:Application for the quality assessment of natural medicines
- TGA/Chemometrics addressing innovative preparation strategies for functionalized carbon nanotubes
- Identification of impurities in nafamostat mesylate using HPLC-ITTOF/MS:A series of double-charged ions
- Solid and liquid state characterization of tetrahydrocurcumin using XRPD,FT-IR,DSC,TGA,LC-MS,GC-MS,and NMR and its biological activities
