Efficacy, patterns of use and cost of Pertuzumab in the treatment of HER2+ metastatic breast cancer in Singapore: The National Cancer Centre Singapore experience
Sylwan Rahardja, Ryan Ying Cong Tan, Rehena Sultana, Fun Loon Leong, Elaine Hsuen Lim
Abstract
Key words: Pertuzumab; Chemotherapy; Metastatic breast cancer; Treatment cost
INTRODUCTION
Cancer is a leading cause of death worldwide, and has recently taken over cardiovascular disease as the leading cause of death in developed countries[1].Majority of adults are concerned about contracting cancer, especially the biopsychosocial aspects that accompany the diagnosis[2]. According to the Singapore Cancer Registry, breast cancer is the most common female malignancy, accounting for 29.1% of all female cancers with an age-standardized incidence rate that has increased threefold since the 1970s. A national government-subsidized breast cancer screening program (BreastScreen Singapore) estimates 7% of new breast cancer cases in Singapore are metastatic at presentation. Metastatic breast cancer (MBC) remains a lethal disease with a historical 5-year survival rate of 22% or lower. However, median survival improved over the past 25 years[3]now ranging from 33[3]to 37 mo[4]. Human epidermal growth factor receptor 2 (HER2) overexpression is seen in 20% of breast cancers and follows a clinically aggressive course with poorer prognosis[5].Pertuzumab is a humanized anti-HER2 monoclonal antibody found in a Phase III clinical trial to significantly improve median survival in HER2 positive MBC when used in combination with a taxane and Trastuzumab[6], and its clinical efficacy has transformed the therapeutic landscape of HER2-positive breast cancer[7]. Given its mortality benefit when added to a trastuzumab-based regimen as first-line therapy[8],it was approved by Singapore’s health authority (Health Sciences Authority) in 2014.Despite its prevalence, the mortality and morbidity of cancer has improved dramatically with the advent of novel therapeutic options. Thus, our study aims to describe the clinical use, efficacy and costs of Pertuzumab in HER2 positive MBC treated in a tertiary cancer centre in Singapore.
MATERIALS AND METHODS
Patient selection
We retrieved electronic medical records of 1185 consecutive patients with newly diagnosed MBC referred to the Division of Medical Oncology of National Cancer Centre Singapore (NCCS) from 1stJanuary 2011 to 31stDecember 2017 from the Joint Breast Cancer Registry. The study was reviewed and approved by the SingHealth Centralised Institutional Review Board Reference: 2018/2400.
Patients with histologically-proven breast cancer, radiological evidence of metastatic disease, and HER2 positivity on immunohistochemistry (IHC) or fluorescencein situhybridization were selected. HER2 positivity was defined as a score of 3+ on IHC or an IHC score of 2+ and a HER2/CEP17 ratio ≥ 2.0 for samples after 1 January 2014 and HER2/CEP17 ratio ≥ 2.2 for samples before 1 January 2014 on fluorescencein situhybridization testing.
After exclusion of 855 patients with HER2 negative and unknown HER2 status,clinical and treatment data were collected for 329 HER2 positive MBC patients.Patients with unknown Pertuzumab usage were then excluded, and data analysis was conducted for all 304 HER2 positive MBC patients. For further cost analyses, patients with incomplete billing data were excluded.
Source of data
Clinical data was retrieved in stages. In the first stage, patient demographics,diagnosis, date of death and clinical variables were retrieved electronically from the Department of Cancer Informatics of NCCS. Clinical variables included: Estrogen receptor (ER) status, progesterone receptor (PR) status, HER2 status, histological grade and subtype, diagnosis date, age of patient at diagnosis, TNM staging and site of metastases. Presence of visceral metastases was defined as metastases in the liver,lung or brain.
In the second stage, detailed treatment histories of each patient were constructed using the electronic medical records, supplemented by data from the NCCS MOSAIQ chemotherapy prescription database and pharmacy billing records in the SingHealth Electronic Health Intelligence System (eHINTS).
Data on the duration of treatment and date of progression was collected for every line of treatment. Duration of treatment was determined by the patients’ dates of registered visits to the outpatient chemotherapy unit as well as from the outpatient records. The date of progression was obtained based on the date of radiological findings of progressive disease.
In patients who received Pertuzumab, treatment outcomes and order of line of therapy were also noted. This included documenting best response clinically or radiologically, as well as the presence of cardiotoxic side effects. Cardiotoxic side effects was defined as Grade 3 Left Ventricular Systolic Dysfunction by the presence of resting ejection fraction of 40%–50% or 10%–19% drop from baseline on twodimensional echocardiography based on the Common Terminology Criteria for Adverse Events Version 4.03. Echocardiography and multiple-gated acquisition results of patients were systematically reviewed to asses for cardiotoxicity consistent with Common Terminology Criteria for Adverse Events Grade 3 Left Ventricular Systolic Dysfunction. Patients with no echocardiography, multiple-gated acquisition scan of the heart or clinical documentation were excluded from this analysis.
In the last stage, costs incurred by patients were retrieved based on inpatient and outpatient bills, including total treatment cost for chemotherapy and total treatment cost of Pertuzumab. Patients who had Pertuzumab treatment but did not have any corresponding bills were excluded from this analysis.
Statistical analysis
All the patients were categorized as “yes” or “no” based on status of Pertuzumab use.All demographic, clinical and histological data were summarized based on Pertuzumab use. Continuous and categorical variables were summarized as mean[standard deviation (SD), range] or median (inter-quartile range, range), whichever applicable, and frequency (percentage) respectively. Difference between statuses of Pertuzumab group was tested using 2-sample independentt-test or Mann-WhitneyU–test, whichever applicable, and fisher’s exact test for continuous and categorical data respectively.
We also plotted Kaplan-Meier curve to find a difference in overall survival (OS)between statuses of Pertuzumab use. We defined OS as duration between date of diagnosis to date of death or date of last follow up, whichever was later. Difference between Pertuzumab and non-Pertuzumab group was assessed using log-rank test and median survival time. Univariate and multivariable Cox proportional hazard regression model was used to find associated risk factors of OS this population.Quantitative association from Cox regression was expressed as hazard ratio (HR) with its corresponding 95%CI. All the tests used in this study were two sided andPvalues< 0.05 was considered as statistical significance. All statistical analyses were carried out using SAS Institute Inc 2013. SAS/ACCESS?9.4. Cary, NC: SAS Institute Inc.
RESULTS
Patient characteristics
The patient and disease characteristics of the study population are shown in Table 1.The mean ± SD age at MBC diagnosis was 58.1 (11.7) years. The majority of the patients were of Chinese ethnicity (64.5%). The median (interquartile range) duration of follow-up was 21.5 (27.8) mo.
The majority of patients had invasive ductal carcinoma (88.2%) while other histological subtypes comprised the remaining 11.8%. Most breast cancers were histological grade 3 (70.7%). Among the 304 patients, 297 (97.7%) were de novo metastatic cancers. The most common site of metastasis was to the bone, seen in 167(54.9%) patients.
Descriptive statistics of study population
In this study population, 50 (16.4%) patients received Pertuzumab. Of these 50 patients, 31 (62.0%) patients had first-line Pertuzumab therapy. Patients who received Pertuzumab were significantly younger (54.5 yearsvs58.9 years,P= 0.0133). There was also a significant difference in ethnic distribution with more patients of Indian and other ethnicities in the Pertuzumab group (P= 0.0003). There was no significant difference between hormone receptor status, grade, histology subtype and site of metastases.
Survival analysis
The Kaplan-Meier curve (Figure 1) showed statistically significant increase in OS among the patients who received Pertuzumab (P= 0.0128). The median OS of the Pertuzumab and non-Pertuzumab group were 51.5 (95%CI: 35.8–60.0) and 32.9(95%CI: 28.1–37.5) mo respectively.
All the variables were analysed to find associated risk factors of overall survival(Table 2). Univariate regression analyses revealed that Pertuzumab usage (HR = 0.515,95%CI: 0.303–0.877,P= 0.0145) and positive ER status (HR = 0.722, 95%CI:0.531–0.981,P= 0.0374) were significantly associated with increased survival, while presence of liver (HR = 1.900, 95%CI: 1.395–2.590,P< 0.0001), lung (HR = 1.393,95%CI: 1.023–1.897,P= 0.0352), and brain metastases (HR = 2.953, 95%CI: 1.844–4.730,P< 0.0001), were significantly associated with decreased survival. Although age and race of the subpopulations were different, they did not significantly affect survival.
Multivariate analysis was conducted to elucidate associations between Pertuzumab use, ER and PR status, and presence of brain, lung and liver metastases. It revealed that site of metastases (brain, liver, lung, bone) and Pertuzumab usage continued to be significantly associated with survival differences, while ER and PR difference did not result in statistically different survival outcomes.
Pertuzumab response and side effects
Two (4.88%) of the 41 patients who received Pertuzumab experienced grade 3 cardiotoxicity. Nine patients had unknown side effect status. Thirty-three (66.0%)patients achieved either complete or partial remission as best response to Pertuzumab therapy. Seven (14.0%) patients had unknown response status, while 5 (10%) patients had stable disease and 5 (10.0%) had progressive disease.
Treatment costs
For the study population, treatment costs of the chemotherapy were extracted in Singapore Dollars (SGD) and reviewed. The median cost on chemotherapy of the subgroup with Pertuzumab was higher at a median of SGD 130456 compared to SGD 34523 in the subgroup without Pertuzumab. Similarly, the median cost on all services incurred in NCCS of the subgroup with Pertuzumab was higher at a median of SGD 170875 compared to SGD 63741 in the subgroup without Pertuzumab. The median percentage of total chemotherapy costs and total services spent on Pertuzumab is 50.3% and 37.3%, respectively.
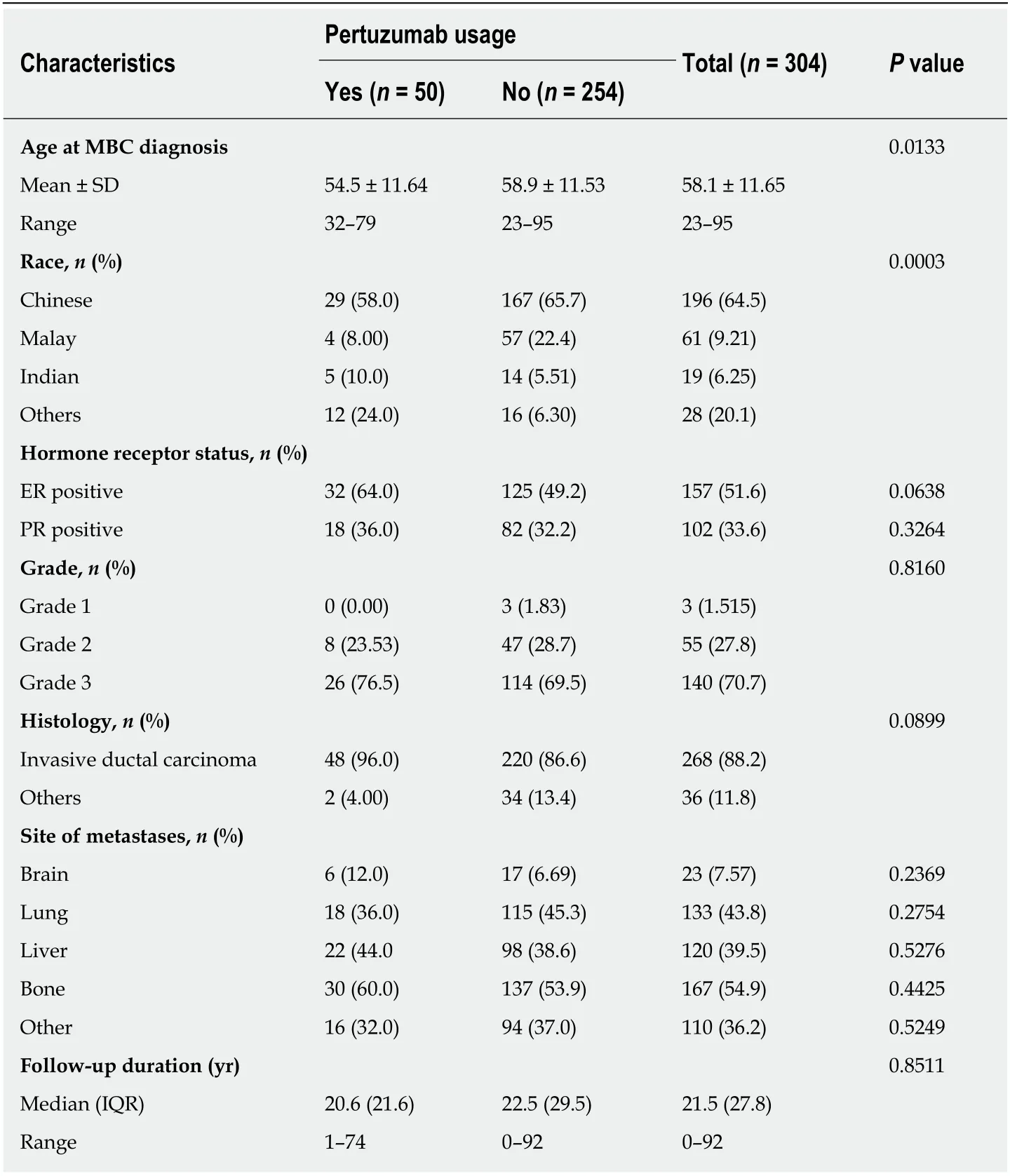
Table 1 Patient and disease characteristics
DISCUSSION
Current guidelines recommend the use of Pertuzumab, Trastuzumab and taxanes for the treatment of metastatic HER2 positive breast cancer. Dual HER2 blockade by adding Pertuzumab to Trastuzumab and a taxane backbone has significantly improved disease control, improved overall survival, duration to progression of disease and improved tumor response to therapy[9,10]. Usage of Pertuzumab as first line therapy was consistent with clinical guidelines, and has demonstrated reproducible safety and efficacy[11]. To further assess real-world efficacy of adding Pertuzumab to Trastuzumab and taxanes, we compared our study population with that of the CLEOPATRA[6]study in terms of clinical efficacy, side effects and cost effectiveness.
Comparison with CLEOPATRA trial
Clinical characteristics of this study was compared to the CLEOPATRA trial. The mean age of diagnoses was 58.1 for our study compared to 54 in the CLEOPATRA study. 55.3% of this study has ER and/or PR positive disease compared to 48.0% in the CLEOPATRA study. 68.4% of this study has visceral metastases compared to 78.0% in the CLEOPATRA study.
Notably, 96.4% of our study population were of Asian ethnicity compared with 32% in the CLEOPATRA study. Despite having a slightly inferior overall survival than the CLEOPATRA trial, we are able to conclude that Pertuzumab does significantly prolong overall survival in study with a predominantly Asian population. The cross-study comparison of complete or partial response was also remarkably similar (66% in our studyvs68.4% in CLEOPATRA).
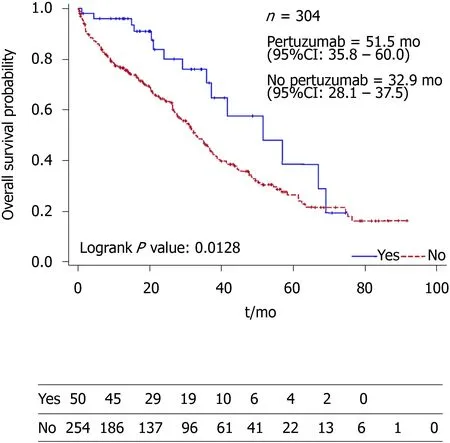
Figure 1 Kaplan-Meier survival curve comparing median overall survival between patients who had Pertuzumab treatment and who did not have Pertuzumab treatment.
The slight difference in overall survival could be explained by medical comorbidities of the different studies, difference in age, and also follow up duration.In addition, the CLEOPATRA study had a longer median follow-up duration of 50 mo compared to 21.5 mo in this study, and a younger population age of 54 compared to 58.1 in this study.
Prognostic factors
Consistent with previous studies, presence of visceral metastases (brain, liver and lung) had the strongest association with poorer prognosis[12]. Although ER and PR positivity have been reported to be associated with a better prognosis[13]and higher histologic grade is a poor prognostic factor[14], our univariate and multivariate analyses only demonstrated statistically insignificant trends. This could be attributed to insufficient power of our sample size as well as the possibility that HER2 positivity may contribute significantly more to the prognosis of this subpopulation of breast cancer patients as opposed to the aforementioned factors.
Serious adverse effects of dual HER2 therapy
One major adverse effect of HER2 therapy is cardiotoxicity. Studies on Pertuzumab have shown cardiotoxicities to be mainly asymptomatic left ventricular systolic dysfunction or symptomatic heart failure[15].
In our study population, 4.88% of patients who had undergone Pertuzumab treatment experienced significant cardiotoxic side effects. These findings in the realworld setting corroborate with rates in the CLEOPATRA trial and the JACOB[16]trial which reported 6.1% and 5.0% incidence of cardiotoxicity, respectively.
Cost effectiveness
Given that many guidelines recommend the use of Pertuzumab due to its efficacy, it would also be prudent to consider literature on value-based and cost effectiveness studies. The United Kingdom National Institute for Health and Care Excellence guidelines concluded that the Incremental Cost-Effective Ratio (ICER) of Pertuzumab exceeds the limit for cost-effective use of United Kingdom’s National Health Service resources, without special considerations as life-extending treatment for patients with incurable disease. This is based on a maximum acceptable ICER of Great Britain Pound (GBP) 30000 per Quality-adjusted Life Years (QALY) gained in treating metastatic breast cancer. Similarly, the American Society of Clinical Oncology published a cost-effectiveness study of addition of Pertuzumab to docetaxel and trastuzumab for HER2 positive metastatic breast cancer. Although median survival was 56.9 mo with Pertuzumab and 39.4 mo without, it concluded that Pertuzumab in addition to Docetaxel and Trastuzumab is unlikely to be cost-effective, with 0%probability of cost-effectiveness at United States Dollar 100000 per QALY gained[17].Lastly, a cost-effectiveness study of addition of Pertuzumab in combination with Trastuzumab and Docetaxel for HER2 positive metastatic breast cancer in Japan demonstrated that, at a higher limit of GBP 50000, it is still not cost effective as theICER of Pertuzumab addition is approximately GBP 90000 per QALY[18].
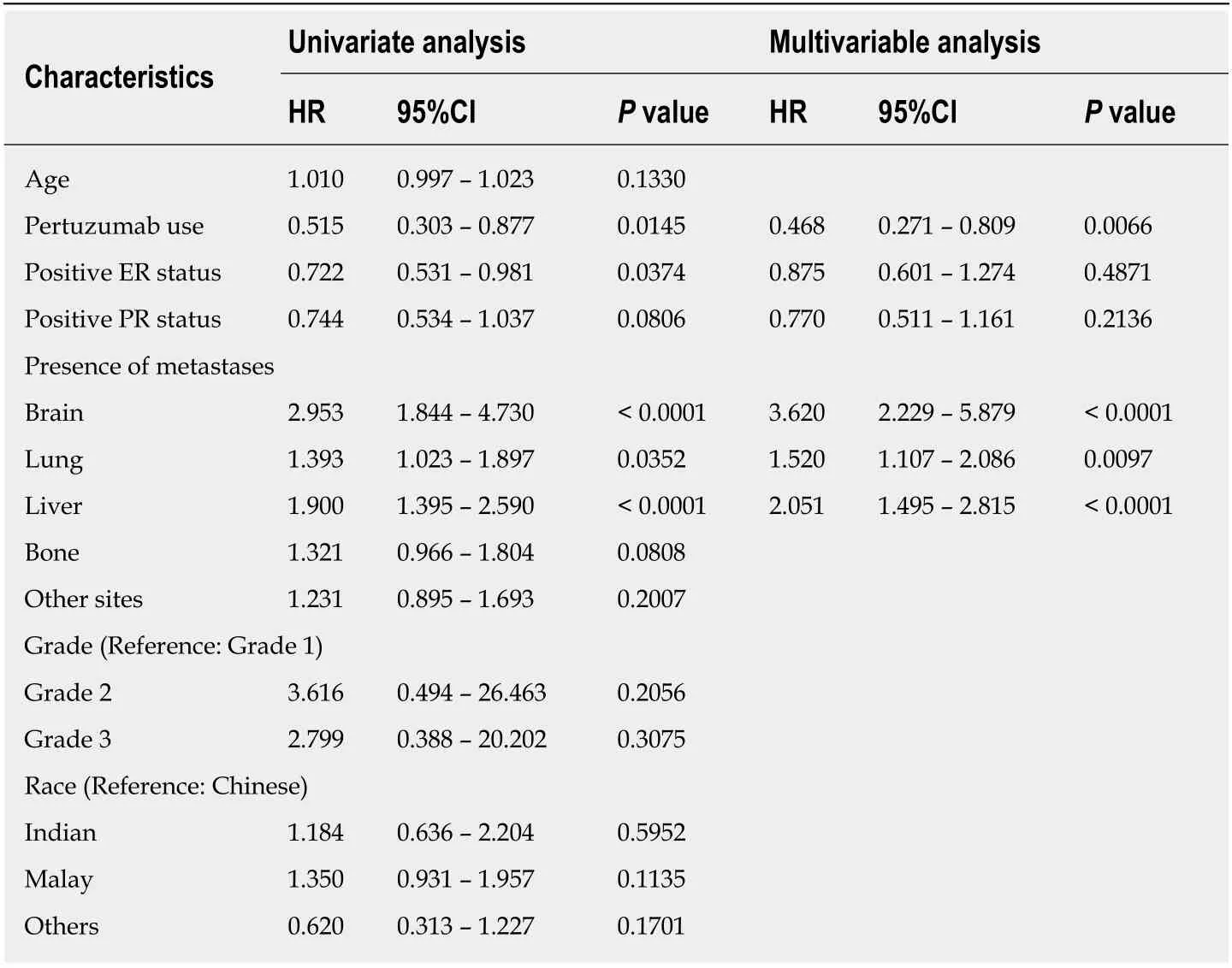
Table 2 Univariate and multivariate COX regression
Given the above conclusions and the finding that Pertuzumab accounted for a median of 50.3% of total chemotherapy costs or a median cost of SGD 170875 over 21.5 mo in our study group, policymakers should conduct further cost-effectiveness studies to review the costs and funding models for Pertuzumab, and weigh the costs against the survival benefit. Further quality of life studies would also be helpful to assess the value of Pertuzumab for HER2-positive MBC.
Limitations
This retrospective study is subjected to limitations of studies based on electronic health records, which may contain incomplete information. However, efforts to corroborate information with multiple data sources from the Joint Breast Cancer Registry (JBCR) to databases containing chemotherapy prescriptions as well as billing data helped to minimize missing or discrepant data. In addition, this study was conducted at a single tertiary care cancer centre which may limit the extrapolation of data to other healthcare settings.
Conclusion
This study shows that Pertuzumab use in the treatment of metastatic breast cancer is associated with a significant improvement in survival benefit, without significant serious adverse effects associated with anti-HER2 agents. However, the proportionate cost of Pertuzumab therapy remains high and further cost-effectiveness studies should be conducted.
ARTICLE HIGHLIGHTS
Research background
Pertuzumab is an anti-HER2 agent that has demonstrated promising clinical efficacy in Phase III trials such as the CLEOPATRA trial. Given the incidence of breast cancer and the proportion of Stage IV breast cancers at diagnosis in an Asian population, there is a need to evaluate its efficacy in an Asian population, and review its costs.
Research motivation
We aim to study the use of Pertuzumab in National Cancer Centre Singapore in a predominantly Asian population.
Research objectives
We attempted to elucidate the clinical efficacy of Peruzumab in HER2-positive Metastatic breast cancer, evaluate the incidence of Grade 3 cardiotoxicity, and the costs of treatment. In so doing,we hope to guide policy makers on the use of Pertuzumab as an important arm of therapy for metastatic HER2-positive breast cancer.
Research methods
We systematically selected the patients based on inclusion criteria further described in the manuscript, and retrieved relevant clinical variables such as billing records, treatment history,patient demographics, response and side effects. Statistical analyses were conducted using SAS Institute Inc 2013.
Research results
This study demonstrated statistically significant difference in median overall survival favouring the Pertuzumab group, with low incidence of Grade 3 Cardiotoxicity. However, costs in the pertuzumab group remain significantly higher than the non-Pertuzumab group.
Research conclusions
We found that Pertuzumab had statistically significant survival benefit in an Asian population in Singapore. This study proposes that Pertuzumab should be adopted as first line therapy for HER2-positive metastatic breast cancer. To summarize the current knowledge, it supports the findings of CLEOPATRA trial, in an Asian population in Singapore. No similar study has been done in an Asian population in Singapore. The implications of this study is that further costeffectiveness studies should be conducted on the usage of Pertuzumab.
Research perspective
This study demonstrated the clinical efficacy of Pertuzumab in an Asian population in Singapore, and serves as an impetus for future research on costs.
 World Journal of Clinical Oncology2020年3期
World Journal of Clinical Oncology2020年3期
- World Journal of Clinical Oncology的其它文章
- Glycoconjugation: An approach to cancer therapeutics
- Assessment methods and services for older people with cancer in the United Kingdom
- What factors influence patient experience in orthopedic oncology office visits?
- Thrombocytopenia with multiple splenic lesions - histiocytic sarcoma of the spleen without splenomegaly: A case report
- Roles of cell fusion, hybridization and polyploid cell formation in cancer metastasis
