Chrysoeriol ameliorates hyperglycemia by regulating the carbohydrate metabolic enzymes in streptozotocin-induced diabetic rats
Bskrn Krishnn, Airmi Rmu Gnesn, Rvinrn Blsurmni,Dinh Du Nguyen, Soon Woong Chng, Shoyun Wng, Jino Xio,Blmurlikrishnn Blsurmnin
aDepartment of Biochemistry, Sree Narayana Guru College, Coimbatore, 641105, Tamil Nadu, India
bDepartment of Food Science and Home Economics, School of Applied Sciences, College of Engineering, Science and Technology, Fiji National University, Fiji Islands
cDepartment of Environmental Energy and Engineering, Kyonggi University, Youngtong-Gu, Suwon, Gyeonggi-Do, 16227, South Korea
dCollege of Biological Science and Technology, Fuzhou University, Fuzhou 350108, China
eInternational Research Center for Food Nutrition and Safety, Jiangsu University, Zhenjiang 212013, China
fDepartment of Food Science and Biotechnology, College of Life Sciences, Sejong University, Seoul, 05006, South Korea
ABSTRACT
The present study aimed to evaluate the effects of chrysoeriol from Cardiospermum halicacabum in streptozotocin induced Wistar rats. Thirty rats were categorized as control, diabetic control supplemented with 0, 20 mg/kg chrysoeriol and 600 μg/kg BW of glibenclamide for 45-day trial period. Our results indicated that the inclusion of chrysoeriol (20 mg/kg) showed a significant reduction in plasma glucose, hemoglobin and glycosylated hemoglobin level with a rising of plasma insulin sensitivity. Further,downregulated enzymes including glucose 6-phosphatase, fructose 1,6-bisphosphatase, and glycogen phosphorylase as well upregulated enzymes such as hexokinase, glucose-6-phosphate dehydrogenase,pyruvate kinase, and hepatic glycogen content. There was a diminish action found in liver glycogen synthase of tested rat with a rise in gamma-glutamyl transpeptidase, towards normal levels upon treatment with chrysoeriol. The histopathological study con firmed that renewal of the beta cells of pancreatic of chrysoeriol and glibenclamide treated rats. In addition, the molecular docking of chrysoeriol against glycolytic enzymes including hexokinase, glucose-6-phosphate dehydrogenase, pyruvate kinase, using Argus software shows chrysoeriol had greatest ligand binding energy as equivalent to glibenclamide, as a standard drug. Thus, chrysoeriol found to be non-toxic with potential regulation on glycemic control and upregulation of the carbohydrate metabolic enzymes.
Keywords:
Cardiospermum halicacabum
Chrysoeriol
Anti-hyperglycemic
Carbohydrate metabolizing enzymes
1. Introduction
Hyperglycemia triggered by impaired glucose homeostasis and ineffective action of insulin are the primary characteristic of diabetes. An estimation in 2012 by the IDF’s Diabetes Atlas predicted more than 45% increase in the population affected by diabetes by the year 2030. This prediction favors India to be the diabetic capital of the world as the present affected cases with diabetes is around 63 million [1]. With the increasing side effects of using drugs from synthetic sources for the treatment of diabetes, plant and plant derived products are now gaining interest [2]. Management of diabetes has now shifted to scientifically validate various herbal preparations and/or con firm the potential antidiabetic effect in many herbal preparations [3,4].Presently pharmaceutical trials for plant derived products with the scope of antidiabetic potential have been proved to treat or target hypoglycemic threat [5–8]. Most of the plant derived bio-active compounds are targeted for therapeutic purpose.On these lines, preliminary research was conducted previously and con firmed that chrysoeriol (5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl) chromen-4-one; Fig. 1), as a primary compound found in Cardiospermum halicacabum, presents anti-inflammatory,anti-osteoporotic, anti-cancer [9–15] and antioxidant activity [16].Another encouraging observation has been found as no harmful side effects on oral administration of leaf extract of C. halicacabum[9] and showed that the potential effects of chrysoeriol on oxidative stress and hepatic marker enzymes in streptozotocin (STZ)induced rats [16]. This leads to exploration of chrysoeriol functional therapeutic action against diabetes induced in rats with detailed carbohydrate metabolic enzymatic action. Therefore, the main aim of the present study was to examine the carbohydrate metabolic enzymes and functional property of chrysoeriol on molecular action in STZ-induced rats.
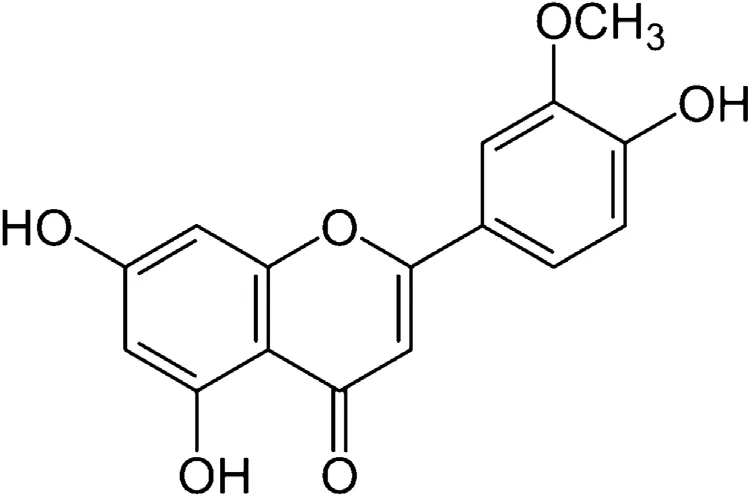
Fig. 1. Structure of chrysoeriol.
2. Materials and methods
2.1. Plant material and chemicals
C. halicacabum was obtained from the Department of Agriculture, Annamalai University, Tamil Nadu, India as a whole plant during March-April, 2018. The collected plant sample was identified and verified by Department of Botany from Annamalai University, with a voucher specimen deposited for future reference. The C. halicacabum leaves were shade dried and pulverized to fine powder. The chemical used for the proliferation of diabetes was streptozotocin (Sigma-Aldrich, MO, USA). All other chemicals mentioned in this study were analytical grade obtained from Hi Media (Mumbai, India). Chrysoeriol was isolated from C. halicacabum leaves, the method was followed as per previous study [16].
2.2. Ethical issue, experimental animals and housing
Adult male albino Wistar rats (9 weeks old; 180–200 g body weight [BW]) were procured from Animal Housing Unit, Department of Experimental Medicine, Rajah Muthiah Medical College and Hospital at Annamalai University. The animals were maintainedat(25±1)oC with a 12h light/dark cycle with feed and water ad libitum. This research protocol was approved by the Institutional Animal Ethics Committee of Rajah Muthiah Medical College and Hospital, Annamalai University (Reg. No. 160/1999/CPCHEA,Proposal No: 539).
2.3. Induction of experimental DM
The STZ at a concentration of 40 mg/kg BW was dissolved in 0.1 mol/L citrate buffer with pH 4.5, given at a single dose intraperitoneal. Before giving STZ, the experimental rats were fasted overnight and ensured for destruction of pancreas through plasma glucose levels (250 mg/dL) after 72 h STZ injection. which showed that rats with high glucose as type 2 DM. In order to overcome drug-induced hypoglycemic mortality, about 20% of glucose solution was administered for an initial 24 h after STZ treatment.
2.4. Animal experimental design
2.4.1. Preliminary study for dose fixation
A preliminary study was carried out to determine ED50of chrysoeriol on plasma glucose in STZ-induced diabetic rats. For this trial, control and diabetic control groups with 0.5% dimethyl sulfoxide (DMSO) and chrysoeriol at a concentration of 5, 10, 20 mg/kg BW was given to diabetic rats, which dissolved in 0.5% DMSO as a vehicle solution and fed by intubation. Followed by, blood samples were withdrawn through retro-orbital puncture at 0 and 2 h after chrysoeriol dosage. The glucose was estimated using Trinder with a reagent kit. This shows that the reduction of glucose level at three dosages of chrysoeriol with significant values. The higher concentration shows better reduction; thus 20 mg/kg BW was fixed as the optimum dosage for further work.
2.4.2. Long term study (45 days)
Control group was given with 0.5% DMSO (Group I), chrysoeriol 20 mg/kg BW (Group II), STZ-induced diabetic control with 0.5% DMSO (Group III), STZ-induced diabetic control with chrysoeriol 20 mg/kg BW (Group IV), and Group V was given glibenclamide 600 μg/kg BW and each group consist of six animals. The chrysoeriol and glibenclamide were given as a single dosage orally by intubation early morning for each day of experimental period. The body weight was measured at regular intervals throughout the trial,as per our earlier study [16].
2.5. Sample collection
At the end of experiment, the animals were fasted overnight and then blood samples through the retro-orbital plexus method under ketamin hydrochloride (50 mg/kg, i.m.) anesthesia. To estimate the plasma glucose and insulin, the anticoagulant tube consists of potassium oxalate and sodium fluoride (3:1) mixture was used. The liver tissues samples were homogenized (10%, m/V) by using Trisbuffer (0.1 mol/L Tris–HCl, pH 7.4). Followed by, centrifugation at 3000 × g for 10 min to collect the enzymes for biochemical analysis.For the histological investigations, the whole pancreas tissue was present in 10% impartial buffered formalin and then dehydrated in ethanol (50%–100%), followed by cleaning in xylene, and fixation in paraffin wax. Sections were cut at 4 μm thick in a rotary microtome, and changes occurred in the pathological lesion in the tissue were noted using microscopically after getting stained with haematoxylin and eosin (H & E).
2.6. Determination of biochemical analysis
The oral glucose tolerance test (OGTT) was conducted according to the earlier described method [17]. The blood samples were collected through cardiac puncture at the end of the study period.Followed by, glucose solution was given through intra-gastric tube, and then blood samples were collected at 30 min intervals (30–120 min) with the tube coated with C2K2O4and NaF, to collect the plasma glucose for further evaluation of biochemical analysis, which test using commercial kits (Sigma Diagnostic, Baroda, India) [18]. Insulin concentration was measured using ELISA kit method (Boeheringer-Manneheim Kit, Germany). The whole blood was used for the evaluation of total hemoglobin (Hb) and glycosylated hemoglobin (HbA1c), using diagnostic kits (Agappe Diagnostic, India).
The carbohydrate metabolic enzymes such as hepatic hexokinase, glucose 6-phosphate dehydrogenase, pyruvate kinase,glucose 6-phosphatase, and fructose 1,6-bisphosphatase activities were measured by the previously described methods [19–23]. The liver functional enzymes gamma-glutamyl transpeptidase (GGT)were estimated as per previous methods [24]. The glycogen content in the liver, and enzymes such as glycogen synthetase and glycogen phosphorylase were measured using previously described methods[25–27].
2.7. Molecular docking: protein structure and chemicals screened
The 3-diminsional structure of the focused on diabetic protein chrysoeriol (PDB:ID:IT02) was recovered from www.rCHb.org/pdb for protein information. The structural and active site investigations of molecular changes was carried out using Computed Atlas of Surface Topography of Proteins by Python visualization software, 2006.Further examination of 5-H donor like OH and NH, 10-H acceptors like N and O were screened through Lipinski’s standard using MW 500 g/mol, while logP under 5 and rotatable obligations under 10 was taken as standard molecule [28].
2.8. Statistical analysis
All the data were measured for statistical significance using oneway analysis of variance by Duncan’s Multiple Range Test and table values presented as mean and standard deviation. A probability value of P ≤ 0.05 was considered to be statistically significant in all test.
3. Results
3.1. Body weight, blood glucose and OGTT
From the study for dose fixation, the blood glucose concentration was measured at regular intervals in OGTT showed an elevation in contrast with normal control rats. However, the tested natural chrysoeriol showed that the potential action against oral glucose which found to be equivalent to the hypoglycemic drug to diabetic rats (Fig. 2). Further, the changes in BW, plasma glucose concentration for the experimental groups were shown in Table 1. There found a decline in the BW in STZ-induced diabetic rats ((141.65 ± 4.15) g) significantly (P < 0.05) compared to normal control rats ((207.11 ± 4.68) g) at 45th day, whereas, incline in plasma glucose concentration was seen in diabetic rats (group III(246.17 ± 8.05) mg/dL) treatment with chrysoeriol (242.38 mg/dl at 0 day and 124.45 mg/dl at 45th day). Besides, the diabetic rats treated with chrysoeriol and glibenclamide showed that variation in the BW ((205.45 ± 4.62) g) and plasma glucose ((99.78 ± 8.56)mg/dL) (P < 0.05) were 2-fold decreased – compared with diabetic control group rats.
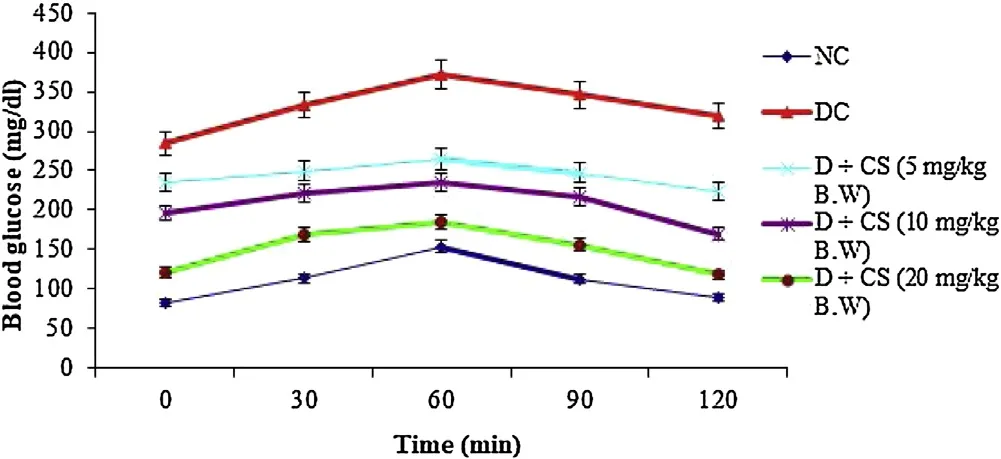
Fig. 2. Effect of various doses of chrysoeriol after 2 h on plasma glucose levels in STZ-induced diabetic rats.Values are given as means ± S.D. for six rats in each group.Values not sharing a common superscript differ significantly at P < 0.05 (DMRT).
3.2. Biochemistry
Table 2 represents the plasma insulin, Hb and HbA1c for all tested rats. Results showed that significant (P < 0.05) rise in the HbA1c ((1.24 ± 0.06) mg/g of Hb), but plasma insulin ((5.45 ± 0.46)μU/mL), Hb level ((6.05 ± 0.39) g/dL) were declined in the STZ induced-diabetic rats. After oral dosage of chrysoeriol to diabetic rats there was a modification found in HbA1C ((0.58 ± 0.04) mg/g of Hb), similar to that of glibenclamide drug with an upregulation of plasma insulin (11.87 ± 1.14) μU/mL and Hb levels (12.61 ± 0.93)g/dL. Figs. 3–7 showed that the impact of chrysoeriol administration on the carbohydrate metabolism and crucial enzymes production from liver cell of all experimental rats. The functional reaction of pyruvate kinase and glucose-6-phosphate dehydro-genase ((116.21 ± 7.52) and (3.64 ± 0.31) μmol of Pi/mg protein,respectively) were declined (P < 0.05) compared to the glibenclamide treated groups but increased in contrast with untreated diabetic control. Similarly, an elevation of glucose 6-phosphatase and fructose 1,6-bisphosphatase ((0.28 ± 0.03) and (0.47 ± 0.03)μmol of Pi/mg protein, respectively) were found in chrysoeriol treated diabetic rats in comparision with diabetic control rats. Conversely, after 45 days treatment of chrysoeriol on diabetic rats inverted the action of hepatic enzymes equivalent to glibenclamide treated rats but this change is not statistically significant.
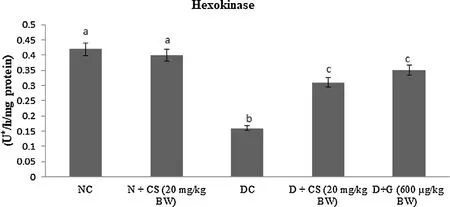
Fig. 3. Effect of chrysoeriol on hexokinase in the liver of STZ-diabetic rats.Values are given as means ± S.D. for six rats in each group.Values not sharing a common superscript differ significantly at P ≤ 0.05 (DMRT).
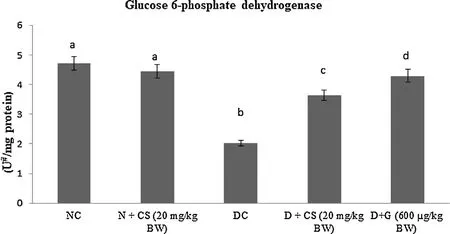
Fig. 4. Effect of chrysoeriol on glucose 6- phosphate dehydrogenase in the liver of STZ-diabetic rats.Values are given as means ± S.D. for six rats in each group.Values not sharing a common superscript differ significantly at P ≤ 0.05 (DMRT).
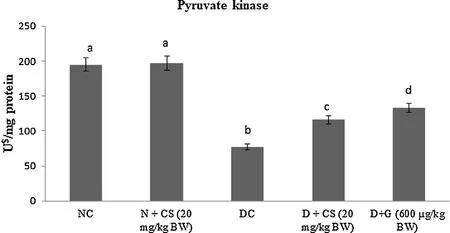
Fig. 5. Effect of chrysoeriol on pyruvate kinase in the liver of STZ-diabetic rats.Values are given as means ± S.D. for six rats in each group.Values not sharing a common superscript differ significantly at P ≤ 0.05 (DMRT).
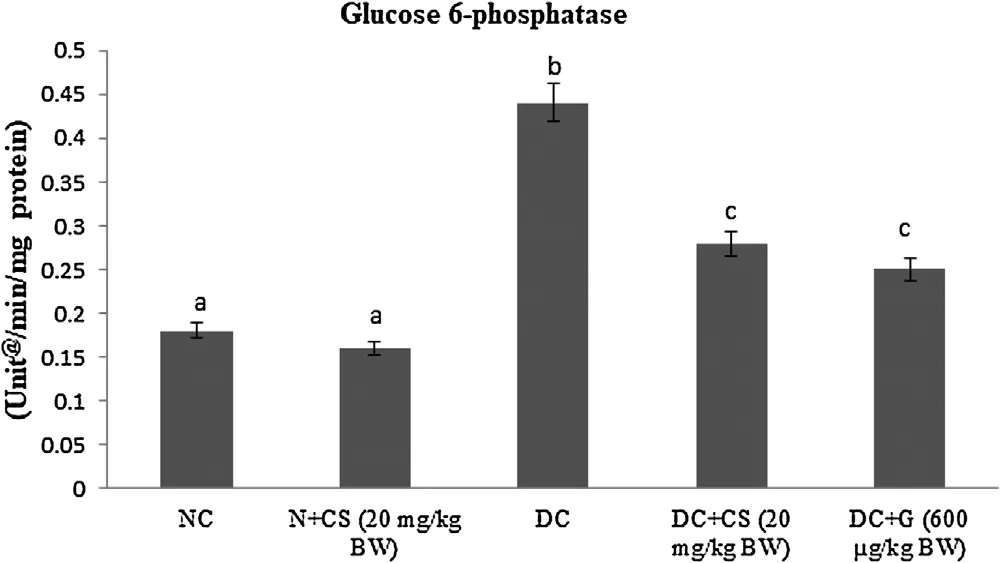
Fig. 6. Effect of chrysoeriol on glucose 6-phosphatase in the liver of STZ-diabetic rats.Values are given as means ± S.D. for six rats in each group.Values not sharing a common superscript differ significantly at P ≤ 0.05 (DMRT).
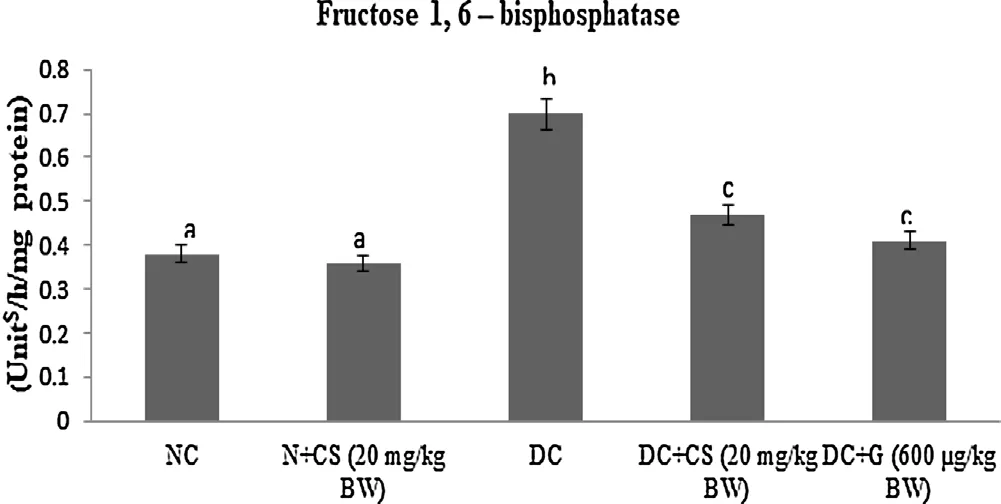
Fig. 7. Effect of chrysoeriol on fructose 1, 6-bisphosphatase in the liver of STZ-diabetic rats.Values are given as means ± S.D. for six rats in each group.Values not sharing a common superscript differ significantly at P ≤ 0.05 (DMRT).
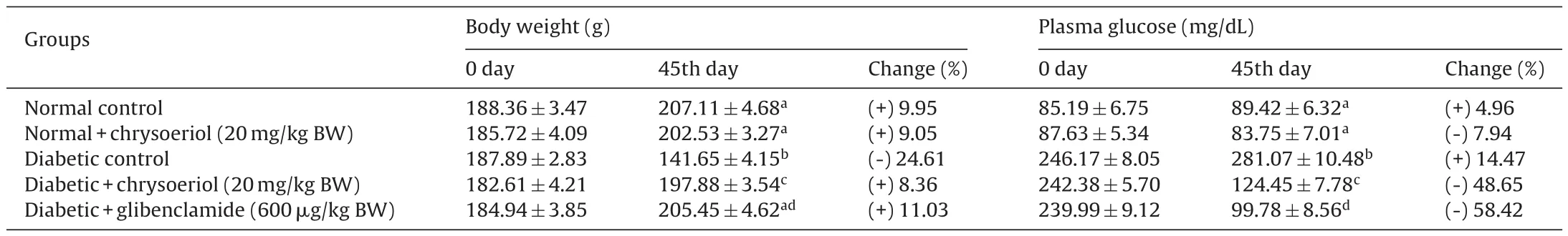
Table 1Effect of chrysoeriol on body weight and plasma glucose in STZ-induced diabetic rats.
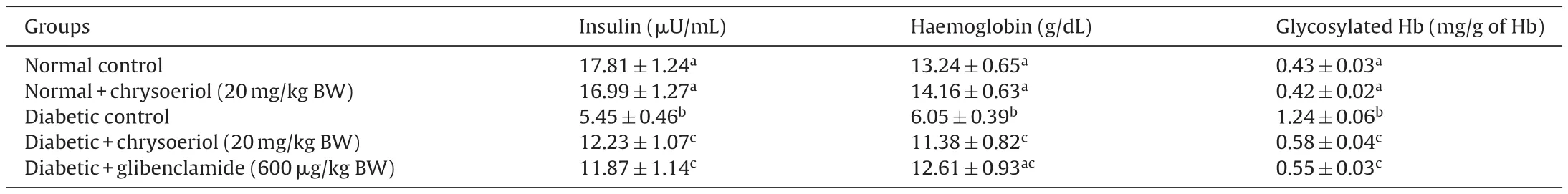
Table 2Effect of chrysoeriol on plasma insulin, haemoglobin and glycosylated haemoglobin in STZ-induced diabetic rats.
3.3. Liver glycogen and glycogen synthase and phosphorylase action
The results of stored glycogen in the liver in both diabetic and control groups, were presented in Table 3. There found a decline in glycogen storage ((20.87 ± 2.18) g tissue) and downregulation of glycogen synthase ((490.82 ± 9.19) mmol of UDP formed/h/mg protein), upregulation of glycogen phosphorylase((971.44 ± 24.84) mmol Pi liberated/h/mg protein) in the liver of diabetic rats in contrast with normal control rats (Table 3). However, chrysoeriol showed that significant (P < 0.05) variation in the above action which reverse the enzyme pathway inversely such as restored glycogen ((42.56 ± 3.76) mg/100 g tissue), improves glycogen synthase ((597.64 ± 11.31) mmol of UDP formed/h/mg protein) and glycogen phosphorylase ((785.20 ± 13.76) mmol Pi liberated/h/mg protein). These outcomes found to be that equivalent of glibenclamide drug in diabetic rats (group V).
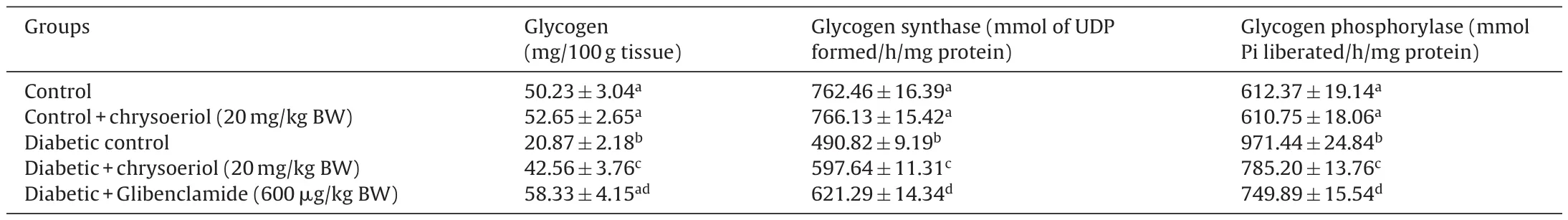
Table 3Effect of chrysoeriol on glycogen and activities of glycogen synthase and phosphorylase in the liver of STZ-diabetic rats.
3.4. Hepatic marker enzyme action
Liver functional enzyme GGT was tested in diabetic rats of all five groups, and the results were shown in Fig. 8. The results showed that the improved liver functional markers enzyme GGTafter the administration of chrysoeriol in STZ-induced diabetic rats.The GGT ((23.45 ± 2.02) IU/L) of all diabetic rats were significantly decreased (P < 0.05) after treatment of chrysoeriol, which found down-regulation of enzymes as compared to diabetic control rats.On the other side, the normal control rats given by chrysoeriol showed that controlled release of enzyme GGT ((16.43 ± 0.99) IU/L),which shows that chrysoeriol directly acts on the defense mechanism of hepatic cells.
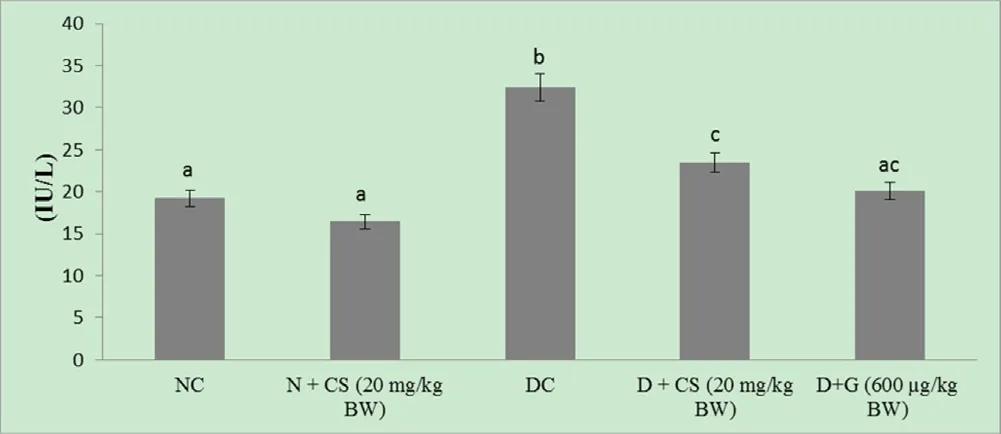
Fig. 8. Effect of chrysoeriol on gamma-glutamyl transferase in the serum of STZ-diabetic rats.Values are given as means ± S.D. for six rats in each group.Values not sharing a common superscript differ significantly at P ≤ 0.05 (DMRT).
3.5. Histopathological study for pancreas
The histopathology analysis of pancreas such as (A) Control: pancreas shows regular pancreatic islet cells, (B) Control + chrysoeriol: pancreas displays standard islet cells, (C)Diabetic: pancreas shows shrinkage of islet cells with fatty in filtration, (D) Diabetic rats with chrysoeriol; pancreas shows reduced fatty in filtration and regular islet cells, (E) Diabetic rats with glibenclamide: pancreas displays no fatty changes and normal pancreatic islets (Fig. 9).
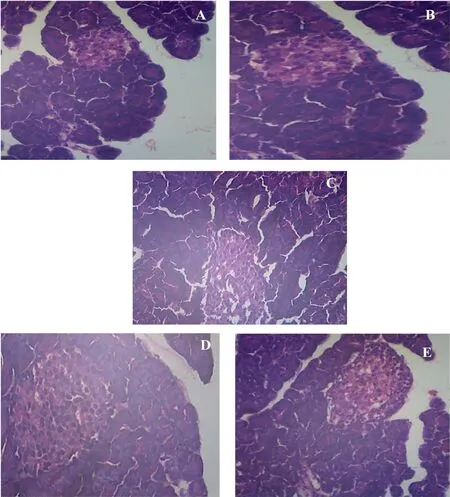
Fig. 9. Histomorphology images of pancreases in control and experimental rats. Original magnification 40×.
3.6. Molecular docking
To analyses the obtained results at the molecular level, Pubchem database was used and interpreted with specific chemical specification by Marvin Sketch Software. Molecular docking was evaluated using Argus lab. The inhibitor and target protein were geometrically upgraded and docked using docking software Argus lab.Also, the sub-atomic docking of chrysoeriol against carbohydrate using proteins engaged with glycolytic pathways, for example,hexokinase (-9.64165 kcal/mol), glucose-6-phosphate dehydrogenase (-8.63819 kcal/mol), pyruvate kinase (-8.49668 kcal/mol) used Argus software and chrysoeriol has shown best ligand binding energy (Figs. 10–12 and Tables 4 and 5).

Fig. 10. Docking study (a) protein (Hexokinase) – ligand (chrysoeriol) docking structure, (b) neighbor residues of ligand molecules.
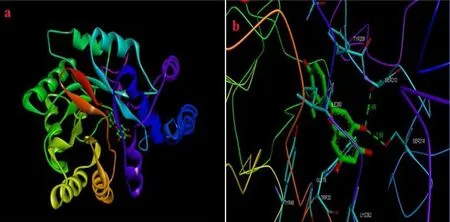
Fig. 11. Docking study (a) protein (Glucose-6-phosphate dehydrogenase) – ligand (chrysoeriol) docking structure, (b) neighbour residues of ligand molecules.
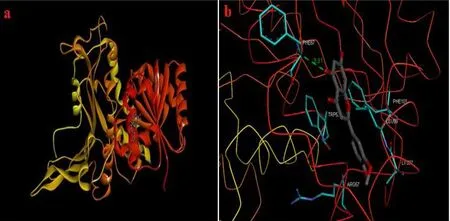
Fig. 12. Docking study (a) protein (Pyruvate kinase) – ligand (chrysoeriol) docking structure, (b) neighbour residues of ligand molecules.
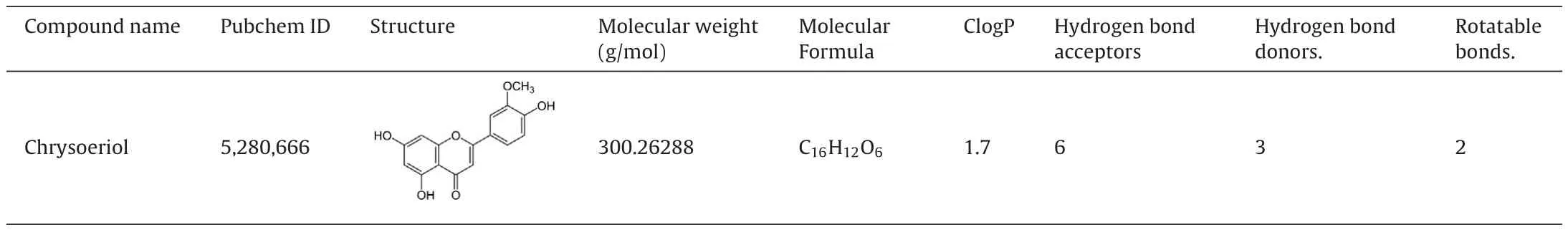
Table 4Docking results of chrysoeriol.
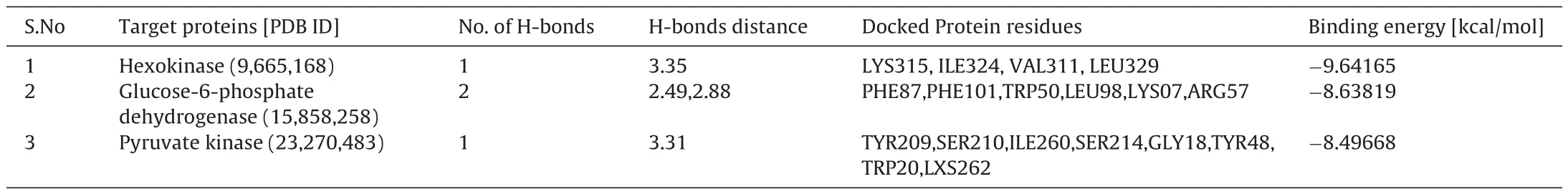
Table 5Docking results of chrysoeriol against hexokinase, glucose-6-phosphate dehydrogenase and pyruvate kinase protein.
4. Discussion
The STZ is broadly utilized to induce diabetes mellitus in the animal model and reported model to test diabetes. The diminished BW in STZ-induced diabetic rodents contrasted with control group rats demonstrates the extreme breakdown or loss of tissue proteins because of changes in the carbohydrate enzymes [29]. Previously, a study stated that lack of insulin in the body prompts the low formation of ATP molecules which decline protein stores in the tissue [30].In the present study, the changes of BW and plasma glucose in the regular dosage of chrysoeriol and glibenclamide of diabetic rats, this represents that the tissue protein was preserved from breakdown due to hypoglycemic nature of chrysoeriol. Further, the diabetic group rats showed a low concentration of Hb and increased HbA1C during the study period. The mechanism behind this result, due to insulin had anabolic effects on protein digestion which prevents the degradation of protein [31,32] and therefore, diminishing further production of Hb. Expanded glycation of protein has been seen as an outcome of diabetic intricacies. Thus, chrysoeriol significantly acts on the HbA1C to modify the level to normal which improves the iron-binding-protein (Hb).
It has been shown that the diabetic type and its intensity of β-cell destruction modify chrysoeriol action on diabetic rats.Thus, the articulation and production of insulin decreased and prompting hyperglycemia, a clinical sign of diabetes. According to the present outcomes, it is clear that the diabetic rats had a high accumulation of glucose levels than control rats. A few substances have demonstrated that the anti-diabetic impacts by affecting β-cells to invigorate insulin discharge due to the restoration of insulin sensitivity [33]. Further in the case of diabetes, it was reported that insulin inadequacy creating hyperglycemia with molecular changes in glucose pathway. In the present investigation, a critical diminished in blood glucose concentration with expansion of plasma insulin levels were seen in diabetic rats treated with chrysoeriol. Chrysoeriol may achieve glucose downregulation by promoting the enduring β-islet cells to discharge more insulin. Chrysoeriol possibly bringing down the blood glucose level due to pancreatic discharge of insulin from regeneration of Islets of Langerhans, or its activity to discharge bound insulin from recovered β-cells by restraining ATP delicate K+channels like glibenclamide. A past report demonstrated that the iso- flavnoid restrains ATP delicate K+channels and controls blood glucose level in STZ-diabetic rats [34]. The previous study recommended that plasma insulin is to keep-up the blood glucose homeostasis by upgrading the glycolysis and glycogen metabolism, with the attending decline of glycogenolysis in liver [35]. The outcome of histopathological images of pancreas emphasized the pancreatic islets with β-cells recapture and refurbishment of diabetic pancreas. Our results conformed that the destruction of the islets in STZ-induced rat which found to be irreparable if not treated.
Diminished glycolysis obstructed glycogenesis and expanded gluconeogenesis are a portion of progressions of glucose digestion in diabetic liver [36]. The STZ promptly drains β cells and accordingly decreases insulin discharge. In un-treated diabetes, enzymes for glucose pathway are clearly evolving. Persistent hyperglycemia is a significant supplier to such metabolic modifications that lead to the pathogenesis of diabetic difficulties, specifically neuropathy and micro-vascular infections [37]. Pyruvate kinase, a major glycolytic catalyst that catalyzes the conversion of phosphoenolpyruvate to pyruvate with production of ATP which was measured from rate of control and terminal compound. The movement of pyruvate kinase was reduced in a diabetic state and standardized by the organization of insulin to diabetic rodents[38]. Changes of the pyruvate kinase action has been observed during diabetes could prompt reduced glucose and ATP generation.Accordingly, the decreased movement of pyruvate kinase in the liver of diabetic case seems liable for the diminished glycolysis and enhanced gluconeogenesis [39]. The treatment given by chrysoeriol on diabetic rats indicated a noteworthy increment in plasma insulin that initiates restricts ATP synthesis, allosteric inhibitor of pyruvate kinase, in this manner expanding pyruvate kinase action to normal.Liver had critical function in keeping up blood glucose homeostasis through destruction in stored glycogen [40]. Further, the liver gluconeogenic enzymes including glucose-6-phosphatase and fructose-1,6-bisphosphatase were promptly enlarged in diabetic[41]. Additionally, the glucose-6-phosphatase found to be an essential gluconeogenic enzyme and necessary protein in the lumen of the endoplasmic reticulum of hepatocytes which catalyzes the de-phosphorylation of glucose-6-phosphatase to glucose and phosphate. Besides, the fructose-1,6-bisphosphatase was significant catalyst in the glyconeogenic pathway and its action advances the movement of hepatic glucose under diabetic condition [42].
Action of these catalysts resulting in high production of enzymes adding to the expanded glucose discharged from the liver [43,44].Therefore, our results corresponded with past reports that the expanded gluconeogenic compounds were demonstrated to be diminished after treatment with different triterpenoids such as ursolic acid, 1-glycyrrhetinic acid [45,46]. Diabetic rats treated with chrysoeriol and glibenclamide controlled the production of these enzymes through regulation of metabolic enactment and restraint of glycolysis and gluconeogenesis. Glycogen, the essential intracellular storable type of glucose and its vary in different tissues, particularly in liver shows an immediate impression of insulin movement since it controls glycogen by revitalizing glycogen production and restraining glycogen phosphorylase. Since the STZ causes damage of β-cells of islets of Langerhans bringing about a lessening in insulin levels, it could be anticipated that glycogen levels in tissues decline as glucose in the liver are inhibited without insulin and then recovered after treatment [47,48]. Decreased hepatic glycogen content in diabetic rats has been ascribed to diminished action of glycogen synthase and expanded movement of glycogen phosphorylase. Additionally, glycogen blend relies upon insulin because of its stimulatory impact. However, less production of insulin and resistant in the secretion of insulin in diabetic patients leads to low storage of glycogen [49–52]. Our outcomes showed that the chrysoeriol treatment makes more insulin from pancreatic β-cells and augmentation of glycogen content in the liver among diabetic rats by extending the activity of glycogen synthase and hinders glycogen phosphorylase.
Liver GGT proteins are considered as a marker enzyme of liver function. An expansion in the production of GGT showed that the diabetes may actuate dysfunction of liver cells. Thus, high GGT level in the liver tissue indicate defects in the glucose pathway. The insulin inadequate state prompts breakdown of protein, in this way upgrading amino acid catabolism which giving substrates to gluconeogenesis. Accordingly, high ALT indicates the type 2 diabetes and recommends a potential function of the liver in the pathogenesis of type 2 diabetes [53]. In this study, treatment with chrysoeriol down-regulates the hypersecretion of GGT in STZ-induced diabetic rats, this proves that chrysoeriol had a tendency to protect liver damage and restore the damaged liver cells, which are in agreement with our earlier report on ALT and AST hepatic marker enzymes[16]. Likewise, bioinformatics investigation offers an advantageous technique for effective in silico primer examination of new drug especially in case of natural products. The optimization technique found effective understanding of the action of chrysoeriol at molecular level. Chrysoeriol as an inhibitor docked against the target site was visualize through Argus lab.
5. Conclusion
The present investigation shows noteworthy action of chrysoeriol against STZ-induced type 2 diabetes, which restores the liver damage and control the degradation of β-cells in Islets of Langerhans by modulating carbohydrate metabolic enzymes. Furthermore, weakened insulin sensitivity and histopathologic study warrants the effectiveness of chrysoeriol against diabetic signs.However, the future planned utilization of chrysoeriol in the treatment of type 2 diabetes warrants thorough clinical examinations to examine the detailed action of active compounds in human pancreatic cell as an alternative natural medicine.
Funding information
The authors extend their sincere appreciations to the Indian Council of Medical Research, New Delhi (Grant no.59/4/2008/BMS/TRM) India for funding the research project. The authors also thankful to the Sejong University, Republic of Korea for their support.
CRediT authorship contribution statement
Baskaran Krishnan: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing, Project administration. Abirami Ramu Ganesan: Writing - original draft.Ravindran Balasubramani: Methodology, Formal analysis, Writing - review & editing. Dinh Duc Nguyen: Writing - review &editing. Soon Woong Chang: Writing - review & editing. Shaoyun Wang: . Jianbo Xiao: Writing - review & editing. Balamuralikrishnan Balasubramanian: Conceptualization, Writing - review &editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
- 食品科學(xué)與人類健康(英文)的其它文章
- Oxidation combined with Maillard reaction induced free and protein-bound Nε-carboxymethyllysine and Nε-carboxyethyllysine formation during braised chicken processing
- Determination of the total antioxidant and oxidant status of some galactagogue and herbal teas
- Rapid analysis of fifteen sulfonamide residues in pork and fish samples by automated on-line solid phase extraction coupled to liquid chromatography–tandem mass spectrometry
- Effect of AAPH oxidation on digestion characteristics of seed watermelon (Citrullus lanatus var) kernels protein isolates
- beneficial effects of high-pressure homogenization on the dispersion stability of aqueous hydrolysate from Mytilus edulis
- Effects of carbon sources and temperature on the formation and structural characteristics of food-related Staphylococcus epidermidis biofilms

