Oxidation combined with Maillard reaction induced free and protein-bound Nε-carboxymethyllysine and Nε-carboxyethyllysine formation during braised chicken processing
Zongshui Zhu, Rui Fng, Ming Hung,b,?, Yunji Wei, Gunghong Zhou
aCollege of Food Science and Technology, Nanjing Agricultural University, Nanjing, Jiangsu 210095, China
bNanjing Huang jiaoshou Food Science and Technology Co., Ltd, National R & D Center For Poultry Processing Technology, Nanjing, 210095, China
cHuai’an Customs, Nanjing, 210095, China
ABSTRACT
The objective of this work was to investigate the effects of oxidation and Maillard reaction on free and protein-bound Nε-carboxymethyllysine (CML) and Nε-carboxyethyllysine (CEL) formation during braised chicken processing. It was found that a positive correlation was observed between carbonyl, fat oxidation,Maillard reaction, CML and CEL (P < 0.05). The sulfhydryl groups could be used as potential indicators to evaluate the compounds’ interaction levels between Maillard reaction and protein oxidation. Frying promoted the formation of lysine (Lys), glyoxal (GO) and methylglyoxal (MGO) (P < 0.05); boiling enhanced the formation of GO and MGO (P < 0.05) while inhibited the levels of Lys (P < 0.05); sterilizing blocked the formation of MGO and Lys (P < 0.01) but improved GO levels (P < 0.05). Finally, a perspective was concluded that the Maillard reaction combined with oxidation is one of the main reasons for the formation of free and protein-bound CML and CEL during braised chicken processing.
Keywords:
Oxidation
Maillard reaction
Nε-carboxymethyllysine
Nε-carboxyethyllysine
Braised chicken
1. Introduction
Advanced glycation end products (AGEs) are chemicals formed at the advanced stage of Maillard reaction [1]. Nεcarboxymethyllysine (CML) and Nε-carboxyethyllysine (CEL) are two typical AGEs that existed in thermally processed meat products [2,3]. According to the AGEs forms in meat, CML and CEL can be divided into two categories: free form (existing on the surface of meat and meat products) and protein-buond form (covalently bonded with proteins and peptides) [4,5]. As one of the most famous traditional Chinese meat products, braised chicken is processed by typical thermal processing including deep-frying (160–180°C),boiling (90–95°C) and second sterilization [6,7]. However, reports about the levels of AGEs in the thermal processed braised chicken are limited [8]. Furthermore, the formation mechanism of free and protein-bound CML and CEL during thermal processing is also unclear.
It is reported that the CML and CEL are mainly formed via Maillard reaction, reducing sugar auto-oxidation, lipid peroxidation and polyol degradation [9]. According to the traditional point of view, excessive Maillard reaction is the internal mechanism of AGEs formation. The AGEs formation by Maillard reaction pathway firstly is Schiff’s base and Amadori rearrangement product(ARP) reaction, which could further promote the formation of dicarbonyl compounds [10,11]. Then, different AGEs are generated by the dicarbonyl compounds in combined with lysine or arginine[12]. Besides, CML and CEL not only could be generated via the complex Maillard reaction but also by lipid oxidation [13]. High temperatures during processing would promote lipid oxidation and a large number of reactive oxygen radicals generation. Further, these radicals induce more active α-dicarbonyl compounds formation in Maillard reaction, such as methylglyoxal (MGO) and glyoxal (GO) [14,15]. However, the effect of protein oxidation on the AGEs formation is still unclear. More importantly, Maillard reaction and protein oxidation could take place concomitantly during meat processing, both reactions are closely interconnected, and each reaction pathway may affect the reaction product formation[16].
So, this study aimed to investigate the levels of AGEs at different stage of braised chicken thermal processing (frying, boiling,and second sterilization) as well as provide a point of view about the co-induction effects of oxidation and Maillard reaction on free and protein-bound CML and CEL formation during braised chicken processing. The data and viewpoints of this study can guide the formation mechanism of AGEs of Chinese traditional thermal processed meat products represented by braised chicken processing.
2. Materials and methods
2.1. Materials and reagents
Fifty-seven 40-days-old white feather broilers were purchased at Weigang market in Nanjing, Jiangsu province of China. Soybean oil, caramel, and spices were purchased from Nanjing Suguo supermarket (Jiangsu, China). 5,5′-dithiobis,(99% DTNB), 2,4-dinitrophenylhydrazine (DNPH), trichloroacetic acid (TCA), thiobarbituric acid (TBA), guanidine hydrochloride,1,1,3,3-tetrathoxypropane, sodium tetraborate decahydrate and ethylenediaminetetraacetic acid disodium salt (EDTA-Na2) are all analytically grade and chicken CML/CEL double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) kit is purchased from Nanjing Maibo Reagent Co., Ltd. The solid-phase extraction (SPE)columns Oasis MCX cartridge (60 mg/3 mL, 30 μm) are obtained from Waters Corporation (Milford, MA, USA).
2.2. Preparation of braised chicken
A total of 57 live Arbor Acres broilers (40-days-old, mixed-sex,3.0–3.5 kg) were randomly divided into 3 treatment groups: (1) frying group; (2) boiling group; (3) sterilization group. Each treatment group consisted of 3 replicates. Feed was removed from the broilers at about 12 h before slaughter. All broilers were transported in a truck for 25 min from the holding area to the abattoir. After resting for an hour, the broilers were slaughtered according to the commercial slaughter process. All birds were bled by severing the jugular vein and carotid artery using a hand-held knife on one side of the neck to allow bleeding for 150 s. After that, the feathers and internal organs were removed and the bodies were put into ice and then transferred to the refrigerator (4°C) for the whole night. All procedures were approved by the Animal Care and Use Committee(Technical standard for broiler welfare in slaughtering, DB37/ 2828-2016) of the College of Food Science and Technology of Nanjing Agricultural University [17].
The soybean oil was put into the fryer, caramel water was spread evenly into the body before frying (sugar:water = 4:6, m/V). Frying conditions are 160–180°C, 1–3 min. After frying, the chicken was put into a boiling pot. Chicken and marinade (70% reused marinade+ 30% water) at a ratio of 1:2 was put into the pot. Boiling conditions are 90–95°C, 5–60 min. Then the chicken was vacuumed and sterilized at 100°C in a water bath for 5–30 min.
2.3. Preparation of marinade
Addition per 1 L of water: fennel 0.24 g; amomum villosum 0.2 g;tangerine peel 0.24 g; clove 0.2 g; nutmeg 0.2 g; galangal 0.12 g;amomum tsao-ko: 0.32 g; pepper: 0.4 g; cinnamon 0.12 g; angelica root 0.4 g; octagonal 0.32 g; ginger 0.6 g; sugar 10 g; soy sauce 4 g;salt 20 g.
2.4. Determination of free and protein-bound CML and CEL
The samples of free and protein-bound CML and CEL were separated and prepared according to the method of Sun et al.and Niu et al. with slight modifications [5,18]. Brie fly, the main preparation method of free CML and CEL is as follows: 1 g of braised chicken breast was accurately weighed and put into 10 mL polyethylene (PE) tube with 5% pre-cooled TCA, homogenized twice at 10000 r/min (IKA-Ultra-Turrax T-25 mixer, Braun), and then centrifuged at 8000×g for 5 min (Allegra 64R high-speed frozen centrifuge, Beckman USA). The supernatant was shaken for 1 min with 10 mL of n-hexane and this process was repeated three times.The upper layer of fat was discarded and 5 mL of the liquid was aspirated into the MCX solid-phase extraction cartridge for further purification.
The main preparation method of the protein-bound CML and CEL is as follows: braised chicken breast meat (0.4 g), sodium borate buffer solution and sodium borohydride were mixed for reduction reaction overnight. Then, TCA (5 mL, 20%) and n-hexane were added and centrifuged at 10000 × g for 30 min. The lower precipitate layer was placed into a pressure-resistant bottle, 3 mL of concentrated hydrochloric acid (12 mol/L) was added, and then mixtures were acidified at 110°C for 24 h. After that, the acid solution was diluted to 8 and 3 mL of the liquid was aspirated into MCX solid-phase extraction cartridge for further separating and purification.
Levels of free and protein-bound CML and CEL Chicken were determined by using the ELISA kit [8]. Samples with high concentration were diluted to fall within the linear range of the standard curve (10–320 ng/mL CML, R2= 0.9994; 0.25–8 ng/mL CEL,R2= 0.9998).
2.5. Measurement of A294 nmand A420 nm
The absorbance at 294 and 420 nm are usually used to indicate the number of reaction products formed at the early and late stages of the Maillard reaction [19]. One gram minced meat of chicken breast mixed skin and 9 mL of phosphate buffer solution (PBS, pH 7.2) were accurately weighed and added into a PE tube. The supernatant was obtained by homogenizing twice at 80000 r/min and centrifuging at 5000 × g for 5 min. 200 μL supernatant was taken and absorbance at 294 and 420 nm were measured respectively by a microplate reader (M2e IKA, Germany).
2.6. Determination of carbonyl content
For the levels of carbonyl measurement, the method of Liu et al.was conducted with slight modification [20]. One gram minced meat of chicken breast mixed skin and 9 mL of PBS were added into a polyethylene (PE) tube and homogenized at 5000 r/min for 30 s. Next, a 50 μL DNPH (10 mmol/L) solution contained 2 mol/L hydrochloric acid was added. After reacting for 50 min at room temperature, 2 mL of 20% TCA was added and shacked uniformly.The supernatant was discarded and washed three times with 5 mL of ethyl acetate/ethanol (V/V, 1:1). The precipitate was dissolved into 6 mol/L guanidine hydrochloride and kept at room temperature for 30 min. Then samples were centrifuged at 10000 × g for 10 min. Finally, the supernatant was collected and the absorbance value at 370 nm was measured using a microplate reader (M2e IKA, Germany). The final carbonyl value was expressed as nmol/mg protein.
2.7. Determination of sulfhydryl groups and TBARs values
The sulfhydryl groups were determined by the method of Xue et al. [21] with slight modification. One gram minced meat of chicken breast mixed skin and 9 mL of PBS were added and homogenized at 5000 r/min for 30 s (4°C). The active sulfhydryl determination procedure: supernatant (0.5 mL) plus 4.5 mL of PBS and 20 μL of DTNB (10 mmol/L DTNB dissolved in 20 mmol/L KH2PO4) were added into a tube and mixed evenly. The main procedure of the total sulfhydryl determination: supernatant (0.5 mL)plus 4.5 mL PBS and 0.5 mL DTNB (dissolved in 8 mol/L urea) were added into a tube and mixed evenly. Both samples were kept at room temperature for 1 h and then the absorbance at 412 nm was measured using a microplate reader (M2e IKA, Germany), respectively. The protein concentration of samples was determined with a PierceTMBCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL, USA) based on bicinchoninic (BCA) for the colorimetric detection and quantitation of total protein. The final results were determined as μmol/mg protein using a molar extinction coefficient of 1.36 × 104L/mol·cm.
The determination of fat oxidation was carried out according to the method of Ultrera et al. [22] with minor modifications.Minced meat of chicken breast mixed skin (4 g) and 26 mL 7.5% TCA were added, homogenated (9000 r/min) and centrifuged for 10 min(10000 × g, 4°C). 1 mL of supernatant was taken and 0.02 mol/L TBA was added and mixed. The solution was placed in a water bath at 100°C for 60 min. After cooling to room temperature, the absorbance was measured at 532 nm. The standard curve was made with 1,1,3,3-tetraethoxypropane and the results were expressed as mg MDA/kg of meat.
2.8. Determination of GO, MGO, and Lys
The determination of GO and MGO was based on the method of Sawicki and Gilbert et al. [23,24] with minor modifications. One gram minced meat of chicken breast mixed skin and 9 mL of PBS were mixed and homogenized at 5000 r/min for 30 s (4°C). The absorbance at 233 nm was used to measure the levels of GO. Sample solution (0.5 mL), sodium acetate solution (1 mL, 1.5 g/L) and hydroxylamine hydrochloride (2 mL, 2 g/L) were added into a tube and mixed. The absorbance value was measured at 233 nm after half one hour incubation (60°C). The MGO was measured by 2,4-DNPH spectrophotometric determination. Sample solution (0.5 mL)and 1 mL 2,4-DNPH (0.02 mol/L, dissolved in HCL-ethanol solution)were added into a PE tube with mixed. Then, the tube was incubated at 42°C for 40 min, allowed to cool for 5 min, and the absorbance at 432 nm was measured by a microplate reader (M2e IKA, Germany).
The levels of Lys were measured using the method described by Church et al. and Guan et al. [25,26] and with slight modification.Lys standard (0.146 g) was mixed with A solution (0.004 g OPA +1 mL methanol + 3 mL distilled water) and B solution (100 mmol sodium borate + 20% SDS + 100 μ L mercaptoethanol) to a concentration of 0.25–2 mmol/L standard solution. The absorbance was measured at 340 nm and a standard curve was prepared. The concentration of Lys in the sample solution was calculated by the standard curve, and the final results were expressed as mg/g of meat.
2.9. Statistics analysis
All experiments were determined with three repetitions (n = 3)and expressed as mean ± standard deviation (SD). SAS analysis software (SAS software research institute, USA, version 8.1) was used for statistical analysis of the data, One-way ANOVA method was used for the analysis of variance and Duncan’s multiple range test was used to compare the differences between mean values. P < 0.05 indicated a significant difference in the results. SPSS analysis software (Version 16; IBM Corp. Armonk, NY) was used for Pearson’s correlation.
3. Results and discussion
3.1. Levels of free and protein-bound CML, CEL, A294 nm, and A420 nm
After thermal processing (frying, boiling, and sterilization) of the braised chicken, the level changes of free and protein-bound CML,CEL and Maillard reaction were shown in Table 1. In general, the levels of protein-bound CML and CEL were much higher than those of free. For raw meat, the levels of protein-bound CML were about 24.5 times higher than that of free, and the levels of protein-bound CEL were about 168 times higher than that of free. After frying,the levels of protein-bound CML were 10.5–12.8 times higher than the free CML. The levels of protein-bound CEL were about 12.5–22.0 times higher than free CEL. After boiling, the levels of protein-bound CML were 10.4–12.8 times higher than the free form. The levels of protein-bound CEL were about 11.8–15.8 times higher than that of free. After sterilization, the levels of protein-bound CML content were 10.3–10.7 times higher than free CML, whereas, the levels of protein-bound CEL were about 11.1–14.7 times higher than free CEL. The above results indicated that different thermal treatments during braised chicken processing had different effects on free and protein-bound CML and CEL formation. In summary, protein-bound AGEs were the main form during braised chicken processing, which was consistent with our previous report [8].
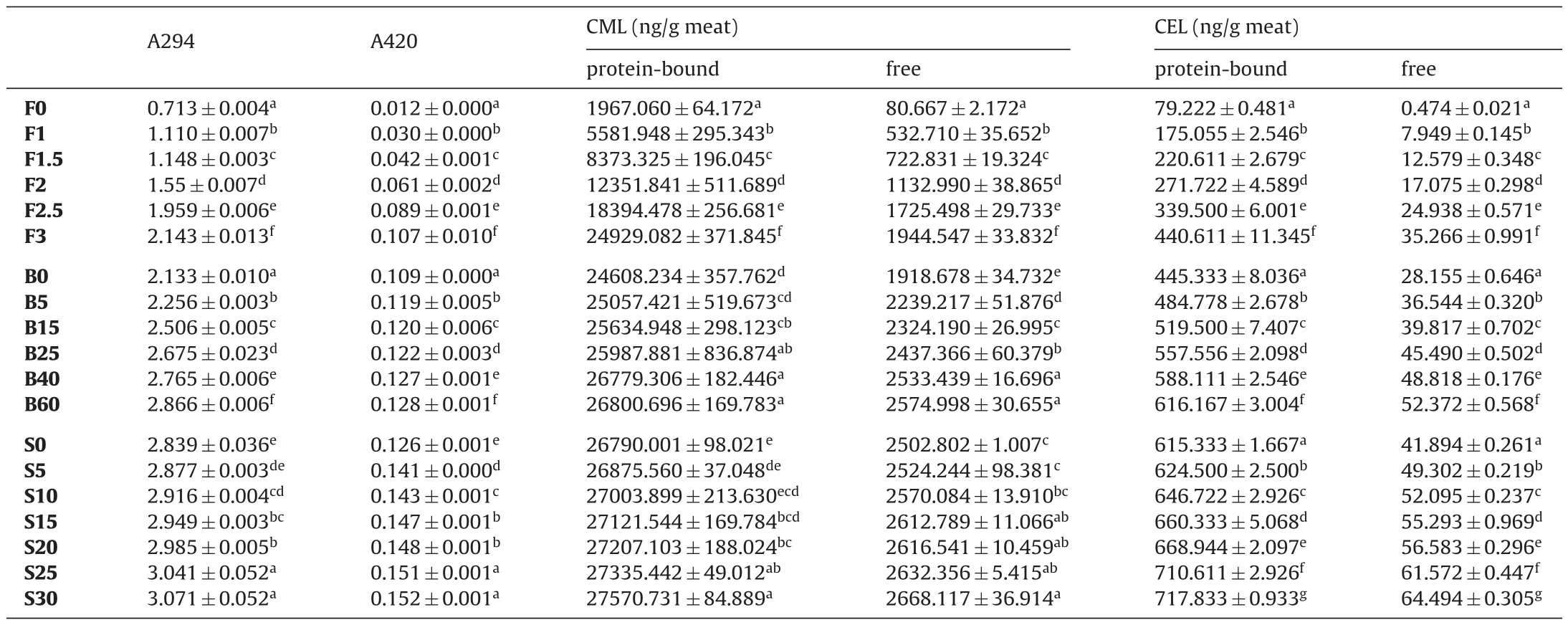
Table 1Effect of fried, boiled, sterilized treatments on the levels of AGEs and Maillard reaction product.
As shown in Table 1, with the increase in frying time, the levels of free and protein-bound CML and CEL also increased. Frying 3 min compared to 0 min, the levels of protein-bound CML increased by 12.7 times, and levels of free CML increased by 24.1 times, whereas,the levels of protein-bound CEL increased by 5.61 times, and the levels of free CEL increased by 74.9 times. Similarly, the levels of free and protein-bound CML and CEL continued to increase with the boiling time. Boiling 60 min compared to 0 min, the levels of protein-bound CML increased by 1.08 times, and the levels of free CML increased by 1.34 times, whereas, the levels of protein-bound CEL increased by 1.38 times, and the levels of free CEL increased by 1.86 times. During high-temperature sterilization, the levels of free and protein-bound CML and CEL also continued to increase with the sterilization time. Sterilizing 60 min compared to 0 min, the levels of protein-bound CML increased by 1.03 times, and the levels of free CML increased by 1.06 times, whereas, the levels of protein-bound CEL increased by 1.16 times, and the levels of free CEL increased by 1.54 times. Based on the results above, it was summarized that the formation of AGEs during frying was faster than boiling and sterilization processes.
Furthermore, A294nmand A420nmalso increased with frying time. To be specific, frying 3 min compared with 0 min, A294nmincreased by 3.01 times and A420nmincreased by 8.91 times. For boiling, A294nmand A420nmalso continued to increase with the boiling time. After boiling 3 min compared with 0 min, A294nmincreased by 1.34 times and A420nmincreased by 1.20 times. Moreover, sterilizing 30 min compared with 0 min, A294nmincreased by 1.08 times while A420nmincreased by 1.27 times.
For raw meat, the ratio of A294nm/A420nmwas 57.7. After frying,the ratio of A294nm/A420nmwas 20.0–36.5. After boiling, the ratio of A294nm/A420nmwas 19.47–22.22. After sterilization, the ratio of A294nm/A420nmchanged to 19.9–20.4. The above results indicated that the early stage of Maillard reaction was extremely Influenced a lot during braised chicken processing, the reason for this phenomenon was probably attributed to the formation of di-carbonyl compounds at the early stage of Maillard reaction [12]. It was found that thermal processing accelerated the Maillard reaction during braised chicken frying, boiling, and sterilization. Furthermore, the Maillard reaction showed a higher activity during frying, whereas,lower activity was exhibited at boiling and high-temperature sterilization processes.
3.2. Effects of deep-frying, boiling and sterilization on oxidation of fat and protein
The effects of deep-frying, boiling and sterilization processing on protein carbonyl and fat oxidation of braised chicken were shown in Fig. 1. The results of Figs. 1A, 1B and 1C exhibited that the protein carbonyl and TBARs value continued going up with the increase in frying, boiling and sterilization time. After frying 3 min compared with 0 min, the carbonyl value increased by 1.83 nmol/g protein, and the TBARs value increased by 2.69 mg MDA/kg meat.After boiling 60 min compared with 0 min, the protein carbonyl increased by 1.14 nmol/g protein, and the TBARs value increased by 1.16 mg MDA/kg meat. After sterilization 30 min compared with 0 min, the protein carbonyl increased by 1.27 nmol/g protein as well as the TBARs value increased by 1.18 mg MDA/kg meat. The results of Fig. 1 were consistent with the changes of free and protein-bound CML and CEL, A294nm, and A420nmin Table 1.
The protein sulfhydryl groups include total, active and concealed sulfhydryl. Protein oxidation would destroy the ‘SH-HS’ types of covalent bonds in the sulfhydryl groups and result in the content of sulfhydryl decrease [27]. The changes in active and total sulfhydryl levels during frying, boiling and sterilization processing of braised chicken were shown in Fig. 2. The results exhibited from Figs. 2A and 2B indicated that with the frying and boiling time increase, the active and total sulfhydryl values declined first and then went up. This phenomenon indicated that during the initial period (0–1.5 min) of frying and boiling (0–25 min), a severe protein oxidation reaction in the braised chicken may occur, and the reduced rate of active sulfhydryl was quicker than the rate of disulfide bond formation by concealed sulfhydryl. As the disulfide bond was destroyed, the total sulfhydryl group was also reduced. After 1.5 min frying or 25 min boiling, the protein was over-oxidized, and the concealed sulfhydryl groups were completely released. For this phenomenon, it could be explained that a large number of free radicals in the early period of frying were formed by Maillard reaction and the broken disulfide bond was recombined by free-radical grafting [28]. Finally, the active sulfhydryl level increased slowly.
The results in Fig. 2C showed that with the sterilization time increase, the active and total sulfhydryl increasingly went up and then become stable. This phenomenon was probably due to the muscle fibers were severely destructed after frying and boiling,protein was denatured and the spatial structure was unfolded [29].Then it could be also illustrated that a large amount of free radicals accumulated by oxidation and Maillard reaction interacted with these loosely structured proteins further during frying and boiling and then the active and total sulfhydryl groups were slowly recovered and eventually got balance.
From the variation of total and active sulfhydryl, it could be inferred that there was the interaction of complicated compound between protein oxidation and Maillard reaction. Protein oxidation provided a large number of free radicals for Maillard reaction so that the sulfhydryl was reduced, and Maillard reaction could further induce the protein oxidation. Therefore, the level changes of sulfhydryl groups could be used as one important indicator for compounds interaction between Maillard reaction and protein oxidation.
3.3. Effects of deep-frying, boiling and sterilization on levels of GO, MGO, and Lys
GO, MGO and Lys are three typical precursor substances playing an important role during the formation of CML and CEL [9]. The level changes of GO and MGO during braised chicken processing was shown in Fig. 3. It could be concluded in Figs. 3A and 3B that the levels of GO and MGO gradually went up with the increase in frying and boiling time, and the increasing trend of GO and MGO during frying processing exhibited an exponential growth, and the fitting function of GO growth model was y = 0.0718 e0.4148x, R2= 0.9947; MGO growth model fitting function was y = 0.0158 e0.7223x, R2= 0.9322.The increasing trend of GO and MGO during boiling accorded with a logarithmic growth model. The fitting function of GO growth model was y = 0.8262 ln (x) + 0.6518, R2= 0.9201; the fitting function of MGO growth model was y = 0.8262 ln (x) + 0.6518, R2= 0.9201.It could be concluded in Fig. 3C that with the sterilization time increase, the GO contents increased but the MGO trends were in reverse. This phenomenon was probably due to the oxidation combined the Maillard reaction was mild occurred with the increase in sterilization time. However, after frying and boiling treatments,large amounts of GO and MGO were produced, and AGEs were mainly formed by the combination of Lys and di-carbonyl compounds [30]. For sterilization, the reason why levels of MGO decline was due to CEL could also be formed by the combination of MGO and Lys residue [31]. Besides, GO had been combined with many Lys residues during frying and boiling, so the CML formation rate was less than the GO decomposition rate during sterilization.
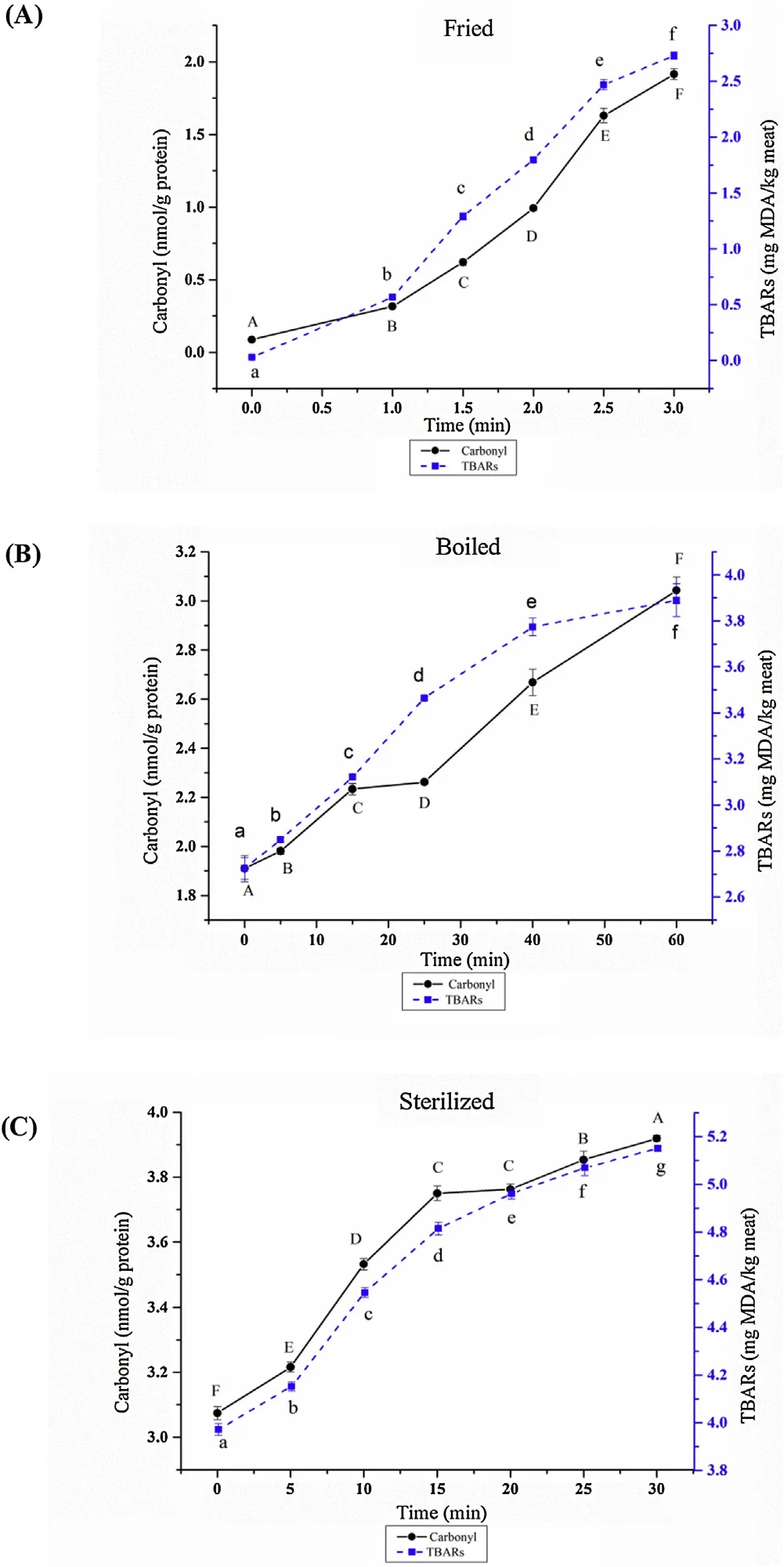
Fig. 1. Effect of different braised chicken processing stage on levels of carbonyl and TBARs. Different letters in the same treatment indicated significant differences, n = 3, P <0.05.

Fig. 2. Effect of different braised chicken processing stage on levels of sulfhydryl groups. Different letters in the same treatment indicated significant differences, n = 3, P <0.05.
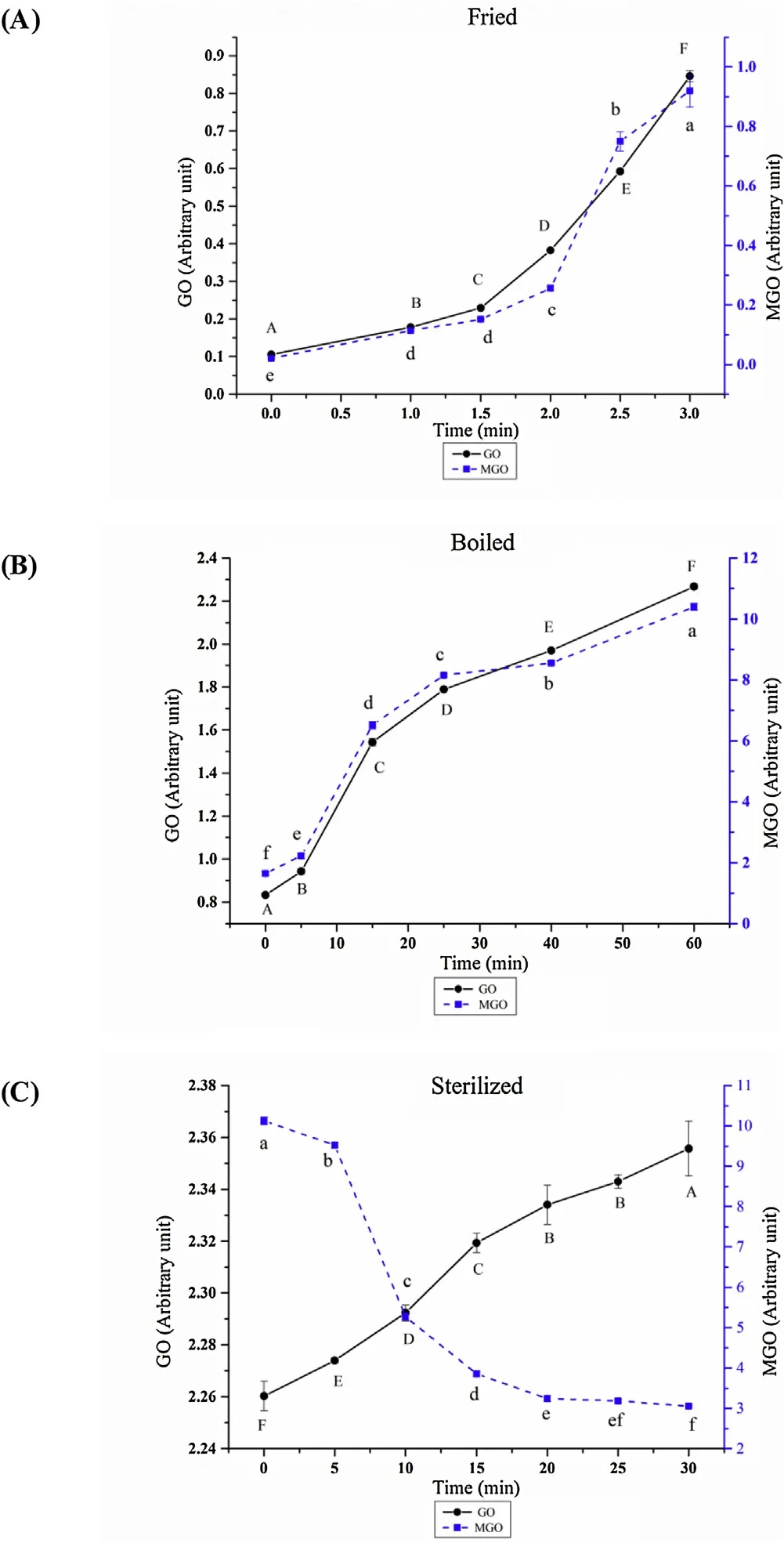
Fig. 3. Effect of different braised chicken processing stage on levels of GO and MGO. Different letters in the same treatment indicated significant differences, n = 3, P < 0.05.
The level changes of Lys during braised chicken processing was shown in Fig. 4. The results in Fig. 4A showed that with the increase in frying time, the levels of Lys gradually increase,which the reason was that frying promoted the Maillard reaction activity and the protein-bound Lys was gradually released and exposed due to the thermal degradation of the protein. The results in Figs. 4B and 4C showed that the levels of Lys gradually decrease with an increase in boiling and sterilization time. This phenomenon could be attributed to the combination of Lys and dicarbonyls, which was one of the main reactions for CML and CEL formation.
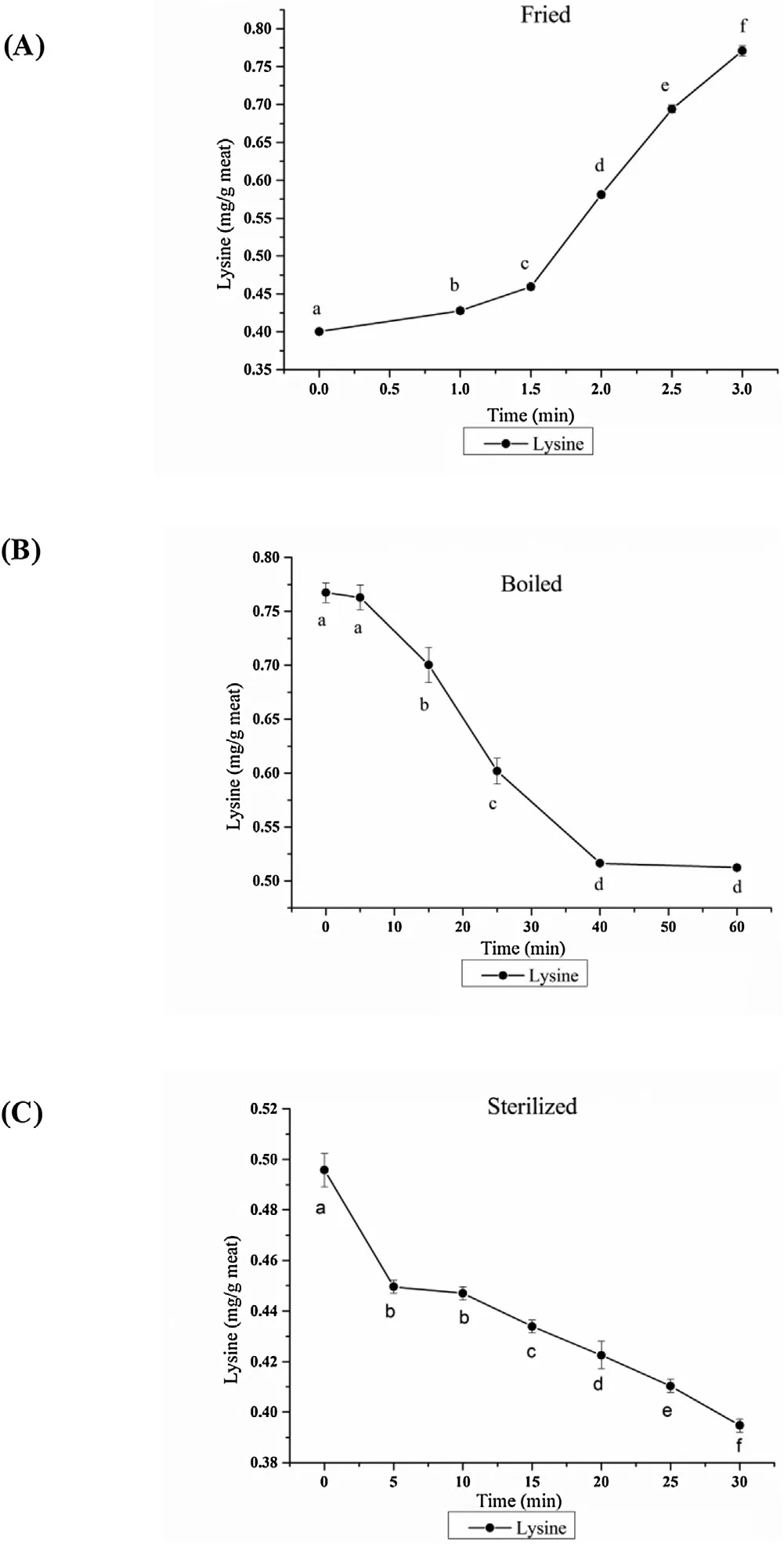
Fig. 4. Effect of different braised chicken processing stage on levels of Lys. Different letters in the same treatment indicated significant differences, n = 3, P < 0.05.
3.4. Correlation analysis
The above studies proved that different heat treatments during braised chicken processing took an important role in the formation of AGEs, oxidation compounds, di-carbonyls, and Lys. However, it was unclear about the relationship between oxidation and Maillard reaction during braised chicken processing as well as correlation result in free and protein-bound CML and CEL formation. So, we conducted Pearson’s correlation analysis of all indexes. The results were shown in Fig. 5.

Fig. 5. Correlation analysis of CML, CEL, Maillard reaction, precursors and oxidation during braised chicken processing. A. fried; B. boiled; C. sterilized. The color changes from blue to red indicating the correlation is changed from weak to strong.
It was observed that a significant positive correlation was found between A294nm, A420nm,protein carbonyl and fat oxidation during frying, boiling and sterilization (P < 0.05). Moreover, A294nm,A420nm, protein carbonyl, fat oxidation, and free and protein-bound CML and CEL also had a significant positive correlation with each other (P < 0.05). These results indicated that there was a strong correlation among Maillard reaction, protein oxidation and fat oxidation during frying, boiling and sterilization processing. These reactions jointly affected the free and protein-bound CML and CEL formation. Specifically, frying was a primary stage that free and protein-bound CML and CEL largely formed because of the high activity of Maillard reaction and oxidation, while during boiling and sterilization, the free and protein-bound CML and CEL accumulated slowly because of a lower reaction activity compared with frying.
Besides, the free and protein-bound CML and CEL were significantly positive correlated with Lys during frying (P < 0.01).The free protein-bound CML and CEL were significantly negative correlated with Lys during boiling processing (P < 0.01), but the two forms of AGEs were significantly positive correlated with GO and MGO during frying and boiling (P < 0.01). The phenomenon above indicated that precursor substances of AGEs were promoted by the interaction between oxidation and the Maillard reaction at frying and boiling processing. During sterilization, free proteinbound CML and CEL were significantly positive correlated with GO(P < 0.01), and significantly negative correlated with MGO and Lys
(P < 0.05). This phenomenon indicated that the sterilization process had little effect on compounds’ interaction between oxidation and Maillard reaction for the formation of free and protein-bound CML and CEL. It could be illustrated that the reaction between dicarbonyl compounds and lysine was spontaneous and induced by protein auto-oxidation under the high-temperature condition [14].
Thus, a point of view on the formation of free and proteinbound CML and CEL in braised chicken processing was concluded that compounds interaction between Maillard reaction and oxidation was one of the main reason. Firstly, the levels of free and protein-bound CML and CEL varied with different processing,protein-bound AGEs are the main form of AGEs formed in every processing stage. Especially, the levels of compounds’ interaction between Maillard reaction and protein oxidation could be predicted by the changes of sulfhydryl groups. Maillard reaction combined with oxidation may result in changes in grafting between free radi-cals and proteins. These free radicals would attack myosin, causing changes in spatial structure and active site generation [2]. The Pearson’s correlation analysis indicated that the frying processing was the main stage affecting the formation of free and protein-bound CML and CEL, which was because of combination between the complex Maillard reaction and oxidation under high temperature.While during boiling and sterilization, the free and protein-bound CML and CEL were accumulated slowly. The reason for these differences may be due to the difference in oxidation environment at different thermal processing conditions [2]. For example, the chicken was exposed to high fat oxidation and high-temperature environment for frying, which provided a suitable oxidation environment for the whole AGEs large formation. However, when the chicken was boiled in the marinade, the natural spices contained in marinade rich in flavonoids and polyphenol antioxidants may exhibit potential suppression effects on AGEs formation [32]. That would be another reason why the whole AGEs in chicken increased faster during boiling than frying. Besides, when the chicken was sterilization, AGEs reaction kinetic conditions may be changed after fried and boiled treatments [5]. So the interaction of the compounds between Maillard reaction and oxidation would be more complicated, which should be investigated further.
Secondly, Maillard reaction and oxidation in braised chicken processing had different effects on the formation of GO, MGO and Lys. Various processing methods showed different correlations on sulfhydryl groups, GO, MGO, and Lys. The compounds’ interaction between oxidation and the Maillard reaction during frying and boiling promoted the precursors of AGEs formation and showed a negative correlation with the changes of sulfhydryl level. The oxidation combined with Maillard reaction during sterilization had little effect on AGEs formation because of the di-carbonyl compounds reacted with lysine was spontaneous under the high-temperature conditions. So the AGEs precursor formation under different heat processing in braised chicken would affect the interaction between oxidation and Maillard reaction, and in turn, Influence the AGEs formation.
4. Conclusions
In conclusion, protein-bound CML and CEL are the main forms of AGEs during the processing of braised chicken. Besides, the levels of CML and CEL are not only Influenced by oxidation and Maillard reaction conditions, but also the levels of precursors. Overall, oxidation combined with Maillard reaction is one of the main reasons for AGEs formation under braised chicken thermal processing. However, the braised chicken processing conditions are so complicated that it is necessary to establish a simple model for AGEs formation mechanism investigation in the future.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by China Agriculture Research System(CARS-41-Z06), National Key R&D Program (2016YFD040040303),Key R&D Program (Modern Agriculture) of Jiangsu Province(BE2019308) and Nanjing Customs Scientific Research Project (No.2020KJ24).
- 食品科學(xué)與人類健康(英文)的其它文章
- Determination of the total antioxidant and oxidant status of some galactagogue and herbal teas
- Rapid analysis of fifteen sulfonamide residues in pork and fish samples by automated on-line solid phase extraction coupled to liquid chromatography–tandem mass spectrometry
- Effect of AAPH oxidation on digestion characteristics of seed watermelon (Citrullus lanatus var) kernels protein isolates
- beneficial effects of high-pressure homogenization on the dispersion stability of aqueous hydrolysate from Mytilus edulis
- Effects of carbon sources and temperature on the formation and structural characteristics of food-related Staphylococcus epidermidis biofilms
- Isolation and identification of Starmerella davenportii strain Do18 and its application in black tea beverage fermentation

