Secondary metabolites from mangrove fungus Aspergillus sp. and their biological activities*
, , ,
(1. School of Chemistry, Sun Yat-sen University, Guangzhou 510275, China; 2. Guangdong Key Laboratory for New Pharmaceutical Dosage Forms, Guangdong Pharmaceutical University, Guangzhou 510006, China)
Abstract:Seventeen compounds (1-17) were isolated from the marine fungus Aspergillus sp. Their structures were determined by nuclear magnetic resonance (NMR) spectroscopy and mass spectrum (MS). Among them, compound 1 was first found as a naturally occurring compound and the known compounds 2-17 were confirmed via spectrum and literatures. The bioactivities of selected compounds were preliminarily evaluated. The compound 2 exhibited moderate inhibitory activities toward three cancer cells, RPMI8226, OCI-MY5, and MCF-7 cells, and compound 10 had significant protective effects on prolonging the survival life of C. elegans under heat stress. In addition, the compound 9 and 7 showed slight inhibition effects against Streptococcus pyogenes and meanwhile the compound 17 possessed slightly inhibitory activity on Streptococcus pneumoniae.
Key words:marine fungus; natural products; cytotoxicity; neuroprotective effects; antibacterial effects
Due to the unique transitional land-marine ecosystem formed by air and periodic tide, mangrove is widely regarded as a dynamic community ecotone with a rich diversity of microbial communities. Mangrove is the second largest ecological group of marine derived fungus which has produced the huge potential of active metabolites[1]. This research to a strain of the South China Sea mangrove fungusAspergillussp. focused on the enlarged fermenting and cultivating.Aspergillusfungi are widely distributed in mangrove of South China Sea. In recent years, studies have shown that a variety ofAspergillusfungi from marine sources, including seawater, seabed sediment, marine plants and animals[2], produce more than 120 kinds of bioactive secondary metabolites which have good antibacterial, antitumor, antioxidant, anti-inflammatory and anticoagulant diverse biological activities[3]. Take it into account, it is of great significance to develop the more bioactive compounds with novel structure and novel mechanism of action. In this study, we reported the isolation, purification and biological activities of 17 compounds from mangrove fungusAspergillussp.
1 Experimental section
1.1 Reagents and materials
TheC.elegansstrain N2 (wild type) nematodes were used in this study which were purchased from CGC (Caenorhabditis Genetic Center, Minneapolis, MN, USA). Agar (MBCHEM), tryptone (OXOID), sodium chloride (Guangzhou Chemical Reagent Factory, China), the reagents for the nematode growth medium were agented by WHIGA (Guangzhou, China). RPMI-8226, OCI-MY5, MCF-7, A549, KB and Hela cancer cell lines were received from the American Type Culture Collection (ATCC, Rockville, MD). The microorganisms namelyEscherichiacoliATCC8739,EnterobactercloacaeATCC13047,PseudomonasaeruginosaATCC9027,StaphylococcusaureusATCC6538,StreptococcuspneumoniaeATCC49619,StreptococcuspyogenesATCC19615 were obtained from Guangdong Culture Collection Center. Other reagents utilized such as methanol, dichloromethane, petroleum ether, sodium chloride and so on were purchased from Guangzhou Chemical Reagents Company, Guangzhou, China unless otherwise specified.
1.2 General methods
Melting point (Tm) was detected on Fisher-Johns hot-stage apparatus, uncorrected. Optical rotation was measured on a Schmidt + Haensch Polartronic HH W5 polarimeter and was uncorrected. NMR data were recorded in acetone, using TMS as internal reference on a Varian Inova 500 MB NMR spectrometer (1H, 500 MHz;13C, 125 MHz), Bruker Avance 400 MB NMR spectrometer (1H, 400 MHz;13C, 101 MHz) and Bruker Avance 300 MB NMR spectrometer (1H, 300 MHz;13C, 75 MHz). HREIMS were measured on a Thermo MAT95XP High Resolution mass spectrometry. EI were recorded on a Thermo DSQ EI-mass spectrometer, ESI were on TSQ Quantum Ultra LC-MS and FAB-MS were on ZAB-HS double focussing mass spectrometer. Column chromatography was carried out on silica gel (200-300 mesh, Qingdao Haiyang Chemical Co. Ltd, China) and sephadex-LH20 (GE Healthcare, Pharmacia, Sweden).
1.3 Fungus strain
The strain of mangrove endophytic fungusAspergillussp. was isolated from a branch ofB.gymnoihizaSavigny, growing in the coastal salt marsh of the South China Sea in Guangxi province. The specimen was stored at the School of Chemistry, Sun Yat-sen University, Guangzhou, China.
1.4 Fermentation, extraction and isolation
Starter cultures were maintained on cornmeal seawater agar. Plugs of agar supporting mycelia growth were cut and transferred aseptically into a 500 mL Erlenmeyer flask containing 200 mL of liquid medium, and incubated at 28 ℃ on a rotary shaker for 5-7 days. The mycelium was aseptically transferred into 1000 mL Erlenmeyer flasks containing 300 mL PDB medium and incubated at (28 ± 1) ℃ for 30 days under stationary conditions. The cultures (40 L) were filtered through cheesecloth. The filtrate was concentrated to about 5 L below 55 ℃, and extracted three times with an equal volume of ethyl acetate. The mycelium was air-dried first, and then extracted three times with methanol (5 L×3). After concentrated in vacuo, the combined extract (30.5 g) was chromatographed on silica gel CC using gradient elution with petroleum ether (PE) and ethyl acetate (EA) mixture or dichloromethane (DCM) and methanol mixture to give compounds1-17.
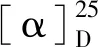
Isoaustione (2): White crystal;Tm: 293-296 ℃;1H NMR (400 MHz, CDCl3)δ: 6.58 (1H, d,J= 9.9 Hz), 6.03 (1H, d,J= 9.9 Hz), 5.27 (1H, s), 5.24 (1H, s), 4.28 (1H, q,J= 6.5 Hz,), 2.87 (1H, d,J= 14.2 Hz,), 2.71 (1H, s), 2.57 (1H, td,J= 13.4, 4.9 Hz), 2.28 (1H, dd,J= 14.2, 1.5 Hz), 1.83 (1H, dt,J= 13.8, 3.7 Hz), 1.63-1.60 (1H, m), 1.56 (3H, d,J= 1.5 Hz), 1.43 (3H, s), 1.37 (3H, s), 1.30 (3H, d,J= 2.5 Hz), 1.28 (3H, s), 1.25 (3H, d, J =.7 Hz).13C NMR (101 MHz, CDCl3)δ: 212.53,172.22, 164.22, 147.11, 146.47, 136.17,129.92, 119.41, 108.22, 90.83, 86.05, 76.42, 66.31, 55.42, 45.83, 42.67, 41.08, 27.18, 26.15, 25.90, 23.25, 22.39, 15.41, 15.29, 12.74; EI-MSm/z: 426. The X-ray crystallographic analysis of compound2was shown in the Fig.1 and Table 1.
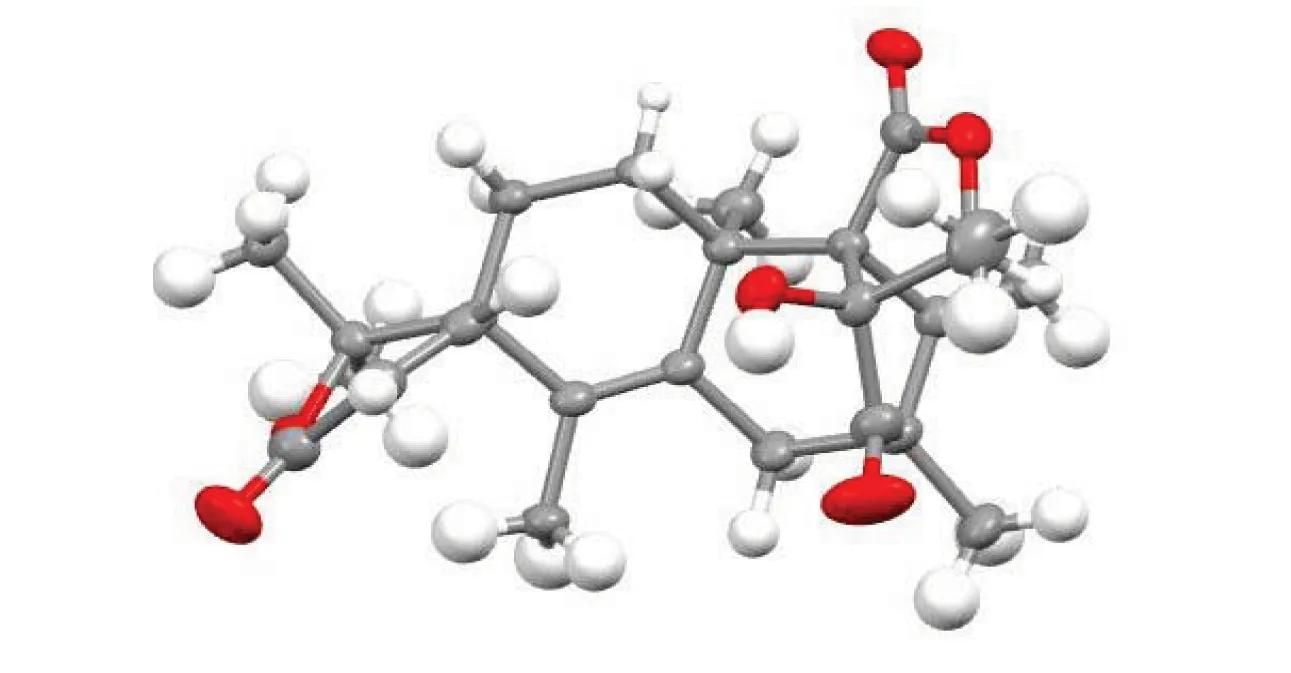
Fig.1 Single crystal structure of compound 2
Table 1 Crystal parameters of compound 2
CCDC database identifier: WAQTOZ, deposition number: 773039
Helvolic acid (3): White crystal;Tm: 255-258 ℃;1H NMR (500 MHz, CDCl3)δ: 7.30 (1H, d,J= 10.1 Hz), 5.89 (1H, d,J= 8.8 Hz), 5.86 (1H, d,J= 10.1 Hz), 5.24 (1H, s), 5.11 (1H, t,J= 8.8 Hz), 2.80-2.76 (1H, m), 2.65-2.62 (1H, m), 2.60-2.56 (1H, m), 2.50-2.46 (2H, m), 2.44-2.40 (1H, m), 2.27 (1H, d,J= 12.5 Hz), 2.23 (1H, dd,J= 14.7, 8.8 Hz), 2.16-2.13 (1H, m), 2.12 (3H, s), 2.14-2.08 (1H, m), 1.98 (1H, d,J= 14.7 Hz), 1.95 (3H, s), 1.90 (1H, d), 1.84-1.81 (1H, m), 1.70 (3H, s), 1.61 (3H, s), 1.58-1.56 (1H, m), 1.45 (3H, s), 1.28 (3H, d,J= 6.8 Hz), 1.18 (3H, s), 0.93 (3H, s).13C NMR (125 MHz, CDCl3)δ: 208.75, 201.37, 174.07, 170.20, 168.90, 157.23, 147.71, 132.92, 130.39, 127.85, 122.78, 73.79, 73.47, 52.67, 49.44, 47.22, 46.59, 41.73, 40.67, 40.41, 38.17, 28.59, 28.35, 27.54, 25.94, 25.74, 23.93, 20.74, 20.50, 18.33, 17.95, 17.77, 13.11; ESI-MSm/z: 567 [M-H]-.
Fumitremorgin C (4): White solid;1H NMR (500 MHz, CDCl3)δ: 7.92 (1H, s), 7.43 (1H, d,J= 8.6 Hz), 6.85 (1H, d,J= 2.0 Hz), 6.82 (1H, dd,J= 8.6, 2.0 Hz), 5.98 (1H, d,J= 9.5 Hz), 4.92 (1H, d,J= 9.5 Hz), 4.18 (1H, dd,J= 11.5, 4.8 Hz), 4.10 (1H, t,J= 8.1 Hz), 3.83 (3H, s), 3.66-3.61 (2H, m), 3.51 (1H, dd,J= 15.9, 5.0 Hz), 3.10 (1H, dd,J= 15.8, 11.7 Hz), 2.44-2.38 (1H, m), 2.28-2.21 (1H, m), 2.09-2.04 (1H, m), 1.97 (3H, s), 1.98-1.94 (1H, m), 1.65 (3H, s).13C NMR (125 MHz, CDCl3)δ: 169.58, 165.78, 156.53, 137.05, 134.03, 132.21, 124.17, 120.76, 118.89, 109.51, 106.24, 95.32, 59.25, 56.81, 55.79, 51.03, 45.43, 28.61, 25.73, 23.07, 21.95, 18.11; ESI-MSm/z: 380 [M+H]+, 378 [M-H]-; EI-MSm/z: 379.
Pseurotin A (5): White crystal;Tm: 157-158 ℃;1H NMR (500 MHz, CDCl3)δ: 8.47 (1H, s), 8.32 (2H, d,J= 7.6 Hz), 7.64 (1H, t,J= 7.4 Hz), 7.49 (2H, t,J= 7.8 Hz), 5.58-5.55 (1H, m), 5.25 (1H, t,J= 9.9 Hz), 4.75 (1H, s), 4.71 (1H, d,J= 11.4 Hz), 4.59 (1H, d,J= 3.8 Hz), 4.23(1H, d,J= 11.7 Hz), 3.45 (3H, s), 2.18-2.14 (1H, m), 2.13-2.06 (1H, m), 1.67 (3H, s), 0.98 (3H, t,J= 7.5 Hz).13C NMR (125 MHz, CDCl3)δ: 196.39, 195.47, 186.02, 166.76, 136.57, 134.78, 132.44, 130.81, 128.70, 126.54, 113.19, 92.89, 90.51, 73.02, 71.10, 70.68, 51.75, 21.38, 14.09, 6.13; ESI-MSm/z: 430 [M-H]-.(3S,8aS)-Hexahydro-3-[[6-methoxy-2-(3-methyl-1-oxo-2-buten-1-yl)-1H-indol-3-yl]methyl]pyrrolo[1,2-a]pyrazine-1,4-dione (6): Yellow solid;1H NMR (500 MHz, CDCl3)δ: 8.71 (1H, s), 7.66 (1H, d,J= 8.9 Hz),7.00 (1H,s),6.85 (1H,dd,J= 8.9,2.1 Hz),6.81 (1H,d,J= 2.1 Hz),6.57 (1H,s),4.37 (1H,dd,J= 9.1,3.4 Hz),4.03 (1H,t,J= 7.5 Hz),3.95-3.91 (1H,m),3.87 (3H,s),3.65-3.60 (1H,m),3.59-3.54 (1H,m),3.46 (1H,dd,J= 14.6,9.1 Hz),2.31-2.28 (1H,m),2.23 (3H,s),2.19-2.18 (1H,m),2.02 (3H,s),1.98-1.95 (1H,m),1.86-1.84 (1H,m).13C NMR (125 MHz,CDCl3)δ: 183.78,169.42,165.55,159.91,158.12,137.29,133.59,122.68,122.10,121.56,118.51,112.76,93.69,59.15,56.79,55.58,45.43,28.27,28.09,25.77,22.63,21.46; ESI m/z: 396 [M+H]+; ESI-MSm/z: 394 [M-H]-; EI-MSm/z: 395.
Demethoxyfumitremorgin C (7): White solid;1H NMR (500 MHz,CDCl3)δ: 7.79 (1H,s),7.58 (1H,d,J= 7.6 Hz),7.37 (1H,d,J= 7.8 Hz),7.22-7.18 (1H,m),7.17-7.14 (1H,m),6.03 (1H,d,J= 9.5 Hz),4.93 (1H,d,J= 9.5Hz),4.22 (1H,dd,J= 11.6,4.9 Hz),4.13 (1H,t,J= 8.4 Hz),3.68-3.64 (2H,m),3.59 (1H,dd,J= 15.9,5.0 Hz),3.17-3.11 (1H,m),2.45-2.40 (1H,m),2.30-2.21 (1H,m),2.07 (1H,m),2.01 (3H,d,J= 1.1 Hz),1.99-1.92 (1H,m),1.65 (3H,d,J = 1.1 Hz).13C NMR (125 MHz,CDCl3)δ: 169.55,165.70,136.18,134.33,133.48,126.30,124.02,122.26,120.12,118.38,111.17,106.54,59.27,56.86,51.02,45.44,28.62,25.72,23.09,21.91,18.17; ESI-MSm/z: 348 [M-H]-; EI-MSm/z: 349.
Chaetominine (8): White solid;1H NMR (500 MHz,CDCl3)δ: 8.24 (1H,d,J= 8.0 Hz),7.93 (1H,s),7.81-7.75 (1H,m),7.71 (1H,d,J= 8.0 Hz),7.63 (1H,d,J= 8.0 Hz),7.52-7.50 (1H,m),7.49-7.47 (1H,m),7.44-7.39 (1H,m),7.24 (1H,t,J= 7.5 Hz),7.05 (1H,s),5.82-5.78 (1H,m),5.54 (1H,s),4.45 (1H,q,J= 6.9 Hz),3.02-2.99 (1H,m),2.67 (1H,d,J= 11.6 Hz),1.76 (3H,dJ= 6.9 Hz).13C NMR (125 MHz,CDCl3)δ: 171.57,165.22,160.97,147.90,147.34,139.14,137.23,134.75,130.97,127.63,127.37,126.99,126.05,124.29,121.84,115.67,83.19,77.50,60.32,50.71,38.94,14.52; ESI-MSm/z: 401 [M-H]-; EI-MSm/z: 402.
Emodin-8-methyl ether (9): White solid;1H NMR (500 MHz,Acetone-d6)δ: 13.21 (2H,s),7.40-7.36 (1H,m),7.24 (1H,d,J= 2.3 Hz),7.00-6.97 (1H,m),6.84 (1H,d,J= 2.3 Hz),3.87 (3H,s),2.32 (3H,s).13C NMR (126 MHz,Acetone-d6)δ: 186.95,182.35,164.19,163.93,162.61,146.74,137.56,132.61,124.09,119.04,114.79,113.74,106.82,104.84,55.89,20.92; ESI-MSm/z: 283 [M-H]-; EI-MSm/z: 284.
Monomethylsulochrin (10): White solid;1H NMR (500 MHz,CDCl3)δ: 13.00 (1H,s),7.05 (1H,d,J= 2.2 Hz),6.64 (1H,d,J= 2.2 Hz),6.49 (1H,s),6.09 (1H,s),3.74 (3H,s),3.71 (3H,s),3.40 (3H,s),2.32 (3H,s).13C NMR (125 MHz,CDCl3)δ: 199.45,166.10,164.31,160.91,157.14,156.11,147.98,128.48,128.24,111.04,110.44,107.84,103.14,102.90,56.24,55.68,52.25; ESI-MSm/z: 345 [M-H]-; EI-MSm/z: 346.
Fusarubin (11): Yellow solid;1H NMR (300 MHz,CDCl3)δ: 13.01 (1H,s),12.63 (1H,s),6.16 (1H,s),4.83 (2H,dd,J= 18.4,18.4 Hz),3.87 (3H,s),3.01 (1H,d,J= 18.1 Hz),2.63 (1H,d,J= 18.0 Hz),1.43 (3H,s).13C NMR (75 MHz,CDCl3)δ: 182.5,177.4,161.4,159.9,158.0,133.0,122.7,110.9,109.9,108.0,94.7,63.0,56.7,20.2; ESI-MSm/z: 307 [M+H]+; EI-MSm/z: 306.
Dihydrocitrinone (12): White solid;1H NMR (300 MHz,DMSO-d6)δ: 16.21 (1H,s),15.96 (2H,s),4.41(1H,dq,J= 13.8,6.6 Hz),2.94 (3H,dq,J= 13.8,4.8 Hz),1.18 (3H,d,J= 4.8 Hz),1.13 (3H,d,J= 6.6 Hz).13C NMR (75 MHz,DMSO-d6)δ: 174.95,165.69,164.07,145.54,110.39,101.74,99.66,76.26,34.34,19.61,18.78,9.52; ESI-MSm/z: 267 [M+H]+; EI-MSm/z: 266.
Altechromone A (13): White solid;1H NMR (300 MHz,Acetone-d6)δ: 6.66 (2H,s),5.9 (1H,s),2.70 (3H,s),2.28 (3H,s).13C NMR (75 MHz,Acetone-d6)δ: 178.52,163.69,160.86,159.79,142.34,116.49,115.29,111.09,100.88,22.26,19.19; ESI-MSm/z: 191 [M+H]+; EI-MSm/z: 190.
Djalonensone (14): White solid;1H NMR (500 MHz,DMSO-d6)δ: 11.79 (1H,s),10.28 (1H,s),7.20 (1H,d,J= 2.5 Hz),6.71 (1H,d,J= 2.0 Hz),6.63 (1H,d,J= 2.0 Hz),6.59 (1H,d,J= 2.5 Hz),3.90 (3H,s),2.72 (3H,s).13C NMR (125 MHz,DMSO-d6)δ: 166.13,164.63,164.08,158.52,152.59,138.39,137.75,117.55,108.77,103.35,101.58,99.13,98.44,55.78,24.95; ESI-MSm/z: 273 [M+H]+; EI-MSm/z: 272.
2-methylquinizarin (15): Yellow solid;1H NMR (300 MHz,CDCl3)δ: 12.1 (1H,s),12.00 (1H,s),7.83 (1H,dd,J= 0.9,7.5 Hz,),7.65 (2H,m),7.29 (1H,dd,J= 1.2,8.4 Hz,),7.10 (1H,s),2.48 (3H,s).13C NMR (75 MHz,CDCl3)δ: 192.4,181.8,162.6,162.3,149.2,136.8,133.6,133.2,124.5,124.3,121.3,119.9,115.8113.7,22.4; EI-MSm/z: 254.
Pyrrolopiperazine-2,5-dione (16): Yellow solid;1H NMR (500 MHz,CD3OD)δ: 4.22 (1H,t,J= 1.95,7.95 Hz),4.10 (1H,dd,J= 1.1,16.8 Hz),3.74 (1H,d,J= 16.8 Hz),3.57 (1H,m),3.51 (1H,m),2.32 (1H,m),2.01 (1H,m),1.97(2H,m).13C NMR (125 MHz,CD3OD)δ: 171.96,166.44,59.86,47.65,46.33,29.38,23.31; FAB-MSm/z: 155 [M+1]+.
Ergochrome EE (17): White solid;1H NMR (500 MHz,Acetone-d6)δ: 7.43 (2H,d,J= 8.5 Hz),6.62 (2H,d,J= 8.5 Hz),3.80 (2H,d,J= 11.0 Hz),2.65 (2H,dd,J= 6.0,6.0 Hz),2.30,2.44 (4H,m),1.03 (6H,d,J= 6.5 Hz),3.59 (6H,s),11.57 (2H,s),2.05 (2H,s),13.56 (2H,s).13C NMR (125 MHz,Acetone-d6)δ: 158.6,117.5,140.3,107.7,159.0,75.5,29.9,35.9,178.3,101.8,186.7,106.4,85.3,17.9,170.2,52.9; EI-MSm/z: 638.
1.5 Thermotolerance assay
Model organisms, such asC.elegans, zebrafish, yeast and drosophila melanogaster, are powerful tools in drug screening and signaling research because of their short life cycles, availability and low costs[4].This experiment aimed to explore the protective effects of compounds onC.elegansduring heat treatment. Nematodes were cultured at 20 ℃ on the nematode growth medium (NGM) plates using livingEscherichiacolistrain OP50 as food. For synchronization, adult worms were shifted to another NGM plate for a few hours, and then the worms were lysed by hypochlorite treatment to collect synchronized eggs. The hatched L1 larvae were transferred to fresh NGM plates supplemented with DMSO or specified compound which had been previously dissolved inE.coliOP50. The final concentration of DMSO was 0.1%. In thermotolerance assay, at least 50 synchronized worms were randomly selected and shifted from 20 ℃ to 37 ℃. Worms were 4 d old after synchronized and treated with each compounds when they were at L1 stage. The viability and mortality of the worms were counted every hour. Death was defined as nonresponsiveness to gentle mechanical touch or a halt of food intake. All compounds were dissolved in dimethyl sulfoxide (DMSO) and stored at 4 ℃ until use.
1.6 Cytotoxic assay
RPMI-8226 cells, OCI-MY5 cells, hela cells, MCF-7 cells, A549 cells and KB cells were cultured in DMEM, i.e., contained 10% FBS and antibiotics (100 U/mL penicillin and 100 g/mL streptomycin), at 37 ℃ in a humidified atmosphere containing 5% CO2. The RPMI-8226 and OCI-MY8 cells were utilized to evaluate the anticancer activity of the compounds with MTT assay. Briefly, the cells were seeded into 96-well plates at 1×104cells per well in 200 μL of culture medium. Incubating 24 hours, 100 μL medium were removed and replaced with 100 μL of new medium which contain serial dilutions of compound from 0.01 to 50 μmol/L for 72 h. Control cells were treated with DMSO at a final concentration (less than 0.1%) as the highest concentration of compound. Twenty microliters of MTS solution per well was added 4 h before culture termination. Absorbance was read at 490 nm with a 96-well plate reader. The MCF-7, A549, Hela and KB cells were used to measure the cell viability with MTT assay. Analogously, 2×104cells in 100 μL were exposed to various concentrations of a seris of compounds for 24 h. The cells with Epirubicin were used to positive control. After grown for 48 h, 20 μL pf 5 mg/mL MTT assay stock solution in PBS was added to each well and then incubated for 4 h, the medium containing unreacted MTT was carefully removed. Then, the obtained blue formanzan crystals were dissolved in 200 μL/well DMSO, and measuring the absorbance by BioTek Synergy H4 hybrid reader at wavelength of 490 nm. The IC50values of the compound was determined from the plots of the cell viability percentage versus the compound concentration.
1.7 Antibacterial activities assay
According to the reported literatures and the content of remaining samples, this study was also done to evaluate the effectiveness of the compounds1,9-17by determining how effective they are in inactivate the most commonly encountered gram-negative bacteria (Escherichiacoli,Enterobactercloacae,Pseudomonasaeruginosa) and gram-positive bacteria (Staphylococcusaureus,Streptococcuspneumoniae,Streptococcuspyogenes). Bacteria colonies were maintained on Mueller-Hinton Agar medium. Twenty-four hours old pure cultures were prepared for use each time. A standardized filter paper disc-agar diffusion procedure (known as Kirby-Bauer method) frequently used to determine the drug susceptibility of microorganism isolated from an infectious person. This method allows for the rapid determination of the efficacy of a drug by measuring the diameter of the zone of inhibition that results from the diffusion of the agent into the medium surrounding the disc. In this procedure, sterile Petri dish capacity of uniform sizes were impregnated with 1 mg/mL concentration of different samples and then placed on the surface of Muller-Hinton agar plate that has been seeded with the organism to be tested and the plates were incubated at 25 ℃ for 24 or 48 hours. After incubation the plates were examined for the zone of inhibition which was indicated in millimeter.
1.8 Statistics
Statistical analysis was done using SPSS Statistics 21 software. Survival curves were analyzed by the life table method and evaluation of the effects of compounds on the mean survival time were done by Wilcoxon rank sum test. All the curves and column diagrams were performed using Graphpad Prism 7.0 software.
2 Results and discussion
2.1 Secondary metabolites from mangrove fungus Aspergillus sp.
As part of our ongoing investigation of natural products from marine invertebrate-derived fungi in the South China Sea, the fermentation broth and mycelia of anAspergillussp. Fungus (Fig.2) were extracted by EtOAc and MeOH. TheAspergillussp. Fungus was isolated from a branch of B. gymnoihiza Savigny, growing in the coastal salt marsh of the South China Sea in Guangxi province. The metabolites from it have showed a variety of biological activities, including cytotoxicities, antibacterial effects and antioxidations.
Fig.2 The fermentation of Aspergillus sp. fungus
By a series of separation methods (column chromatography, sephadex LH-20, and semi-preparative HPLC), secondary metabolite1was first founded as a naturally occurring compound and together with the known compounds2-17(Fig.3) were separated and purificated. In 1992, it was reported the isolation and structure elucidation of sphingofunginA,B,CandD(Fig.4)[5], a new family of antifungal metabolites produced byAspergillusfumigatusATCC 20857. After the discovery of sphingofungins’ family, the synthesis of those compounds was focused by researchers. During the synthesis of sphingofunginA,BandD[6], there was generally forming an intermediate product (1) and further could convert into sphingofungins. This time we found1from marine fungusAspergillussp. and it could be proved that there existed a close biogenic relationship between1and sphingofungin.
Compared with the literature, compounds2-17were elucidated by NMR and mass spectrometry as isoaustione (2)[7], helvolic acid (3)[8], fumitremorgin C (4)[9], pseurotin A (5)[10], (3S,8aS)-Hexahydro-3-[[6-methoxy-2-(3-methyl-1-oxo-2-buten-1-yl)-1H-indol-3-yl]methyl]pyrrolo[1,2-a]pyrazine-1,4-dione (6)[11], demethoxyfumitremorgin C (7)[12], chaetominine (8)[13], emodin-8-methyl ether (9)[14], monomethylsulochrin (10)[15],fusarubin (11)[16], dihydrocitrinone (12)[17], altechromone A (13)[18], djalonensone (14)[19], 2-methylquinizarin (15)[19], pyrrolopiperazine-2,5-dione (16)[20], and ergochrome EE (17)[21]. Compound2was characterized by spectral and X-ray Crystallographic Analysis. The diversified skeleton with multifunctional groups were separated from the fungusAspergillussp., including alkaloids (4,5,6,7and8), peptide (16), terpenoid (1), phenolic and ketone (2,3,9,10,11,12,13,14,15and17). Therefore, it is interesting to further explore the bioactivities of the varied structures of those separated compounds.
2.2 Cytotoxic assay
Compounds1-4and10were selected to evaluate for inhibitory activities against multiple myeloma cell lines (RPMI8226 and OCI-MY5) (Table 2). The compound2showed moderate inhibitory activities. Therefore,2was further assessed inhibitory activities on other four cancer cells lines including MCF-7, A549, KB and Hela cells lines, and epirubicin was used as a positive control (Table 3).The results showed that2(isoaustinone) had inhibitory activities toward MCF-7, A549 and Hela cancer cell lines, especially against MCF-7 cell line. Compound2has a special structural skeleton, so further studies was aimed at developing its anticancer mechanism in our laboratory.
2.3 Thermotolerance assay
Compounds3-6,9and10were selected to evaluate their effects on prolonging lifespan ofC.elegansunder heat stress. The survival time ratios of different compounds were shown in Fig.5. The results showed that all selected compounds could prolong the survival life ofC.elegansunder heat stress, and compound10showed most significant protective action. These compounds may be promising candidates for the treatment of diseases of the central nervous system.

Fig.3 Structures of compounds 1-17
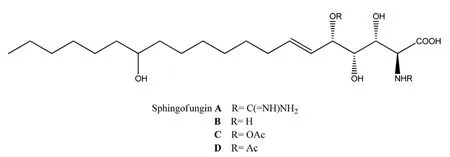
Fig.4 Structures of sphingofungin A, B, C and D
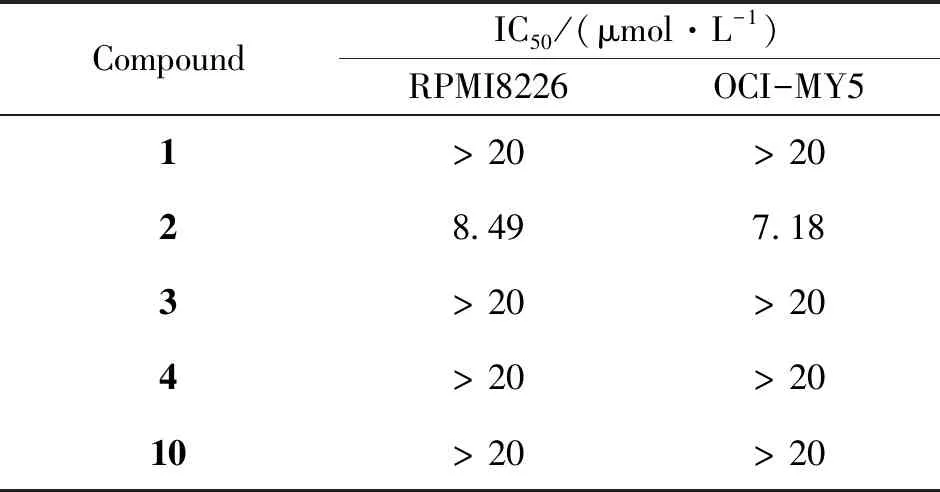
Table 2 In vitro cytotoxic activity of compound 1-4 and 10
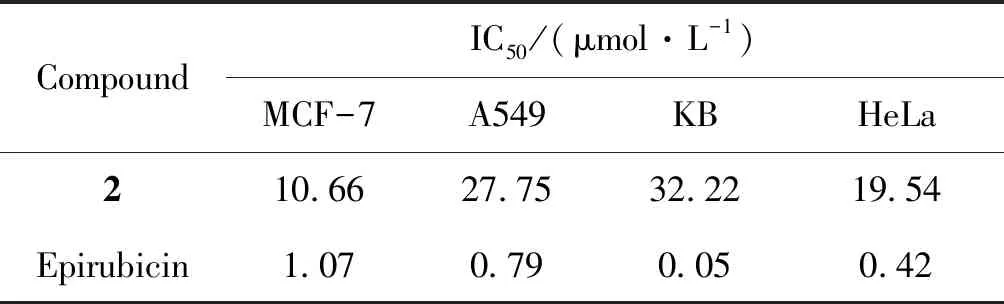
Table 3 In vitro cytotoxic activity of compound 2
2.4 Antibacterial activities assay
The diffusion tests were applied to six microorganism including Gram-positive and Gram-negative bacteria. The antimicrobial capacity was obtained by measuring the diameters of zones of inhibition in millimeters. Compounds1, and9-17were selected to evaluate the effectiveness against the most commonly encountered bacteria. The results were summarized in Table 4. The result showed that major compounds had no actions on bacteria except compound9,13, and15. The scale for evaluating the antibacterial activity was given by reference[22], it had classified the diameters of inhibition zones (D) of the bacterial growth in four classes: highly inhibitory:D≥ 28 mm; inhibitory moderately: 16 mm ≤D< 28 mm; inhibitory slightly: 10 ≤D< 16 mm; not inhibitory:D< 10 mm. The compound9and16have a slight inhibition againstStreptococcuspyogenes; compound12showed the slight inhibition onEscherichiacoliand meanwhile the compound17possessed slightly inhibitory activity onStreptococcuspneumoniae. The sensitivity may be improved by increasing the concentration referring to the diameter of the positive standard, Gentamicin.
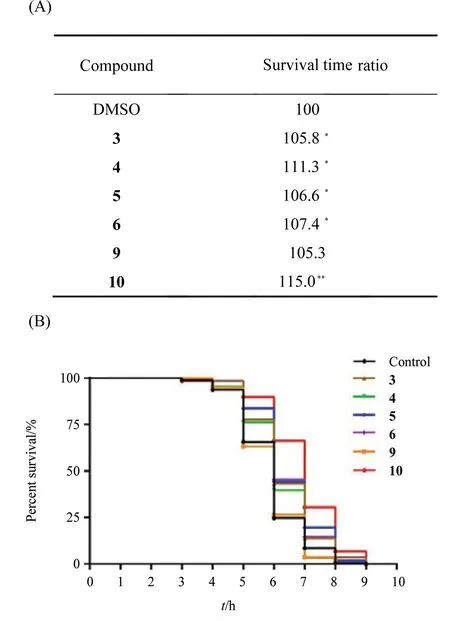
Fig.5 Protective effects of compounds 3~6,9,10 against heat stress(A) Survival time ratio of compounds 3~6,9,10. * P <0.05, **P <0.001 (relative to the blank DMSO); (B) Survival curves of 6 compounds with most potent protective effects in heat shock experiment
3 Conclusions
In summary, 17 compounds with various structures including alkaloids, ketones, terpenoid and peptide, were separated and purified from the mangrove fungusAspergillussp. which was widely distributed and rich in South China Sea. As the diversity of compounds, preliminary bioactive assays including cell viability, thermotolerance assay ofC.elegansand antibacterial assay were carried on. The cell lines RPMI8226, OCI-MY5, and MCF-7 exhibited moderate inhibition with compound2. It is amazing thatC.elegansunder heat stress pretreated with compound10could be observably protected. Furthermore, the compound9and16have a slight inhibition againstStreptococcuspyogenes; compound12showed the slight inhibition onEscherichiacoliand meanwhile the compound17possessed slightly inhibitory activity onStreptococcuspneumoniae. The results suggested that the separated secondary metabolites from abysmal sea have a promising way to explore new lead compounds of medicine and are valuable for further research.
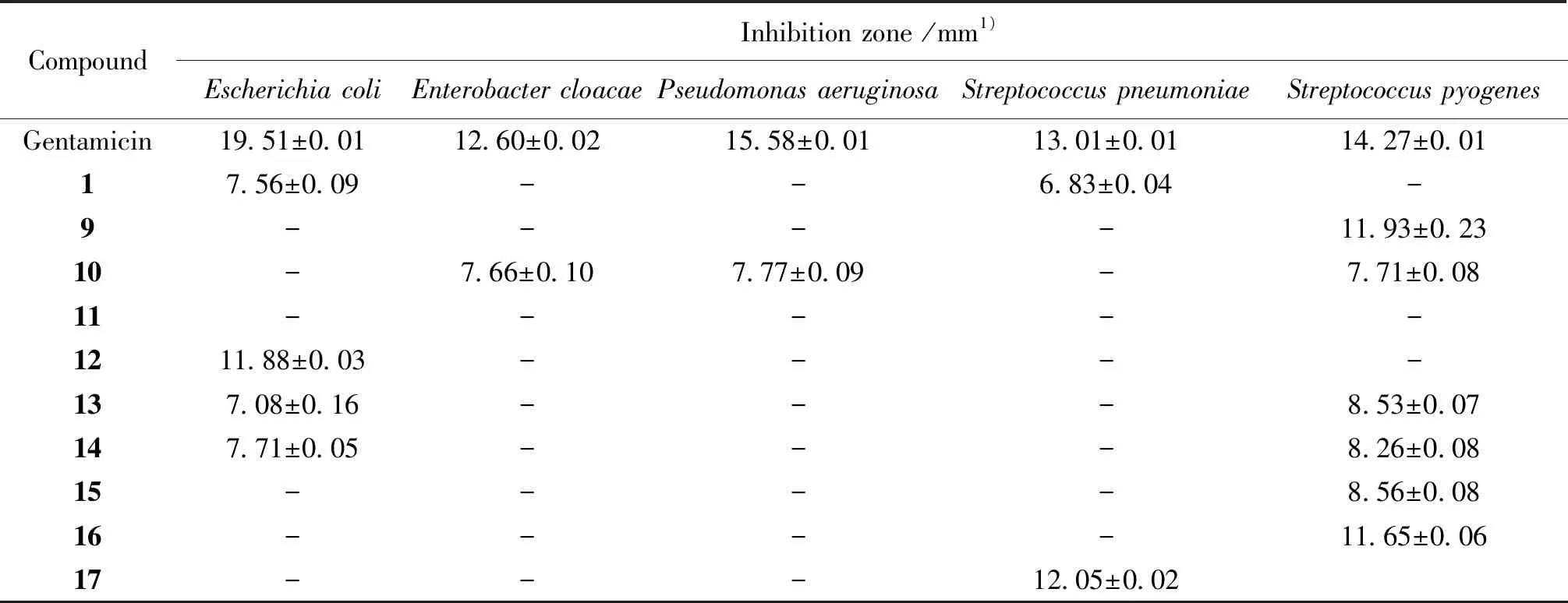
Table 4 Values diameters (mm) of inhibition zones on five bacteria
1) Vales are means ± SD of three separate experiments done in triplicate; -: lecture impossible
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21172271), the Natural Science Foundation of Guangdong Province, China (Grant No. S2011020001231 and 2017A030313064) and Major Scientific and Technological Special Project of Administration of Ocean and Fisheries of Guangdong Province (GDME-2018C013).

