有機相化學鍍鋁法制備Al/石墨烯復合材料粉末
段 政 徐明英 高建峰*, 郝 敏 劉 艷 馬 潔 白培康
(1中北大學理學院,太原 030051)
(2中北大學材料科學與工程學院,太原 030051)
0 Introduction
In recent years,graphene has been a promising application prospect in the field of composite materials due to its excellent mechanical/functional properties and its unique physical/chemical properties[1-2].Compared with graphene reinforced polymer matrix composites[1],graphene reinforced metal matrix composites(GNP-MMC),especially graphene reinforced aluminum matrix composites (Gr/Al),have not been studied in depth.The special atomic structure and properties of graphene make it an excellent additive for the preparation of reinforced and toughened composites.As distinct from other carbon allotropes such as carbon nanotubes (CNTs)and graphite,graphene has the characteristic 2D structure,which makes its specific surface area larger than those of CNTs or graphite[3],thus providing more area to interact with the matrix material.Therefore,graphene is an ideal reinforcement for the preparation of high-performance composites of any kind of matrix(whether polymer[4-6],ceramic[7-8],or metal[9-11]),and is expected to improve the mechanical and thermal properties of the aluminum matrix greatly when added to the aluminum matrix.
At present,the methods for preparing Gr/Al are casting[12-14],ball-milling[15-17],powder metallurgy[18]and friction stir processing (FSP)[19].The nanosized(or microsized)Gr/Al can be prepared by casting and ball-milling methods;GNP-MMC prepared by powder metallurgy technology have good physical and chemical properties;Gr/Al prepared by the FSP method hasfew impurities.However,theabove methods have a common problem that the complicated structure Gr/Al prepared by the above methods is longtime,high cost and even difficult to form the shape;moreover,the graphene is easily agglomerated in the matrix and has poor bonding property with the matrix interface.Thiscannotmeetthe material requirements of selective laser melting (SLM).The metal powder for SLM forming need high purity,good sphericity,small particle size and narrow particle size distribution (15 ~45 μm).Metal powder properties directly affect the stability of the SLM-forming process and the structural properties of the part.Because of the large difference in density between graphene and the aluminum matrix,poor wettability,and difficulty in uniform dispersion in the aluminum matrix,the preparation of Gr/Al powder has become a difficult problem in the application of SLM forming of additive manufacturing.
As far as we konw,there is no report on the synthesis of Al/graphene composites by the electroless plating method.The purpose of this study is to prepare the graphene/aluminum composite powder by uniformlyplatingmetalaluminum atomson the surface of graphene by the electroless plating method.Then,it can be used to prepare Gr/Al with metal aluminum or aluminum alloy,which is expected to solve the problem of easy agglomeration of graphene in the aluminum matrix,improve the interface between graphene and aluminum matrix,and meet the requirements of SLM for materials.
1 Experimental
1.1 Materials and reagents
The graphene/aluminum composite powder was prepared with aluminum powder(AR,purity 99.0%,purchased from Beijing Chemical Reagent Factory)and graphene (average layer 6 layers,Tangshan Jianhua Technology Development Co.,Ltd.).Anhydrous aluminum chloride (purchased from Tianjin Chemical Reagent Factory)and iodine(purchased from Zhengzhou Chemical Reagent Factory)were sealed and stored in a brown bottle as an initiator.Furthermore,commercial NaH(purity 60%,purchased from Aladdin)was stored under a N2atmosphere to prevent oxygen/moisture exposure.Ethyl bromide(EtBr)(AR,purchased from Damao Chemical Reagent Factory)was distilled over anhydrous calcium chloride under N2atmosphere to remove water.Toluene and tetrahydrofuran(THF)(AR,purchased from Damao ChemicalReagent Factory)were distilled on sodium metal under N2atmosphere to remove water.H2(purity 99.99%,purchased from Taiyuan Industrial Gas Factory)as shielding gas was dried using a drying device.
1.2 Activation of aluminum powder
All reactions were carried out in an anhydrous and anaerobic environment (hydrogen atmosphere).After repeated replacement with H2,3 g of aluminum powder,traces of anhydrous aluminum chloride and iodine (about 0.015 g),and 20 mL of freshly sealed ethidium bromide (EtBr)were added to a 250 mL three-necked flask.The mixture was heated to 39℃and refluxed under a stream of hydrogen with stirring.After about 30 min,the reaction started,and the white smoke appeared,and then the mixture turned slightly black.As a result,the aluminum powder began to be activated and gradually generated an alkyl aluminum sesquihalide and activated aluminum.After 90 min,15 mL of THF (as a complexing agent)was added to the reaction system,and the reaction was continued for 10 min.The mixture was quickly centrifuged,and the resulting supernatant (i.e.,alkylaluminum sesquihalide)was sealed and stored.
1.3 Plating
All reactions were carried out in an anhydrous and anaerobic environment(hydrogen atmosphere).0.2 g of graphene was weighed and added into 30 mL of toluene and the alkyl aluminum sesquihalide solution prepared in 1.2.The mixture was dispered uniformity by ultrasonic for 30 min,and 5.0 g of NaH was subsequently added,and the mixture was stirred and refluxed at 110℃for 90 min.After the reaction was completed,the mixture was filtered,washed with absolute ethanol and distilled water successively,and then the filter residue was freeze-dried.Finally,the graphene/aluminum composite powder was obtained.
1.4 Characterization
Fourier transform infrared (FTIR)spectra of the samples (4 000~500 cm-1) were measured on an FTIR-8400S infrared spectrometer using the KBr compression method.The microstructure and elemental composition of the samples were analyzed by the field emission scanning electron microscopy (SEM)(INSPECTF-50)(the acceleration voltage was 20 kV),energy dispersive spectroscopy (EDS)and selected area electron diffraction(SAED).The structures of the graphene and Al/graphene composite powder were examined using a Rigaku Ultima TypeⅣ X-ray diffractometer equipped with a Cu Kα radiation (λ=0.154 056 nm)with 40 kV of the working voltage,30 mA of the working current,5°~80°of the scanning range and 0.01°of the step size.The Raman spectra of the samples were determined using an inVia Reflex-type microscopic confocal laser Raman spectrometer with incident laser light at a wavelength of 532 nm.The porous structure of the sample was characterized by an ASAP2020 automatic specific surface area and porosity analyzer,and the specific surface area of the sample was determined.
2 Results and discussion
2.1 Surface analysis
The graphene used in the experiment was a commercially available graphene,which was prepared by the redox method.Therefore,the oxygen-containing groups such as hydroxyl group,carbonyl group,and epoxy group were presented on the surface of the graphene.
Fig.1 presentsthecomparison ofthe FTIR spectra of graphene and Al/graphene composite powder.Characteristic peaks of graphene in the infrared spectrum were observed forthe -OH stretching vibration absorption peak at 3 452 cm-1,and the C-O stretching vibration absorption peak at 1 125 cm-1.In addition,the absorption peaks at 1 648,1 639 and 1 546 cm-1were produced from the C=C stretching vibration of the sp2structure in graphene[20].No absorption peak corresponding to C=O was observed in Fig.1,indicating that the graphene that had been used was not graphene oxide.For the Al/graphene composite powder,all the characteristic peaks of the graphenewere weakened,especially the oxygencontaining groups because that the graphene was reduced by the reduction system and the surface and interlayersofthe graphenewere deposited with aluminum during electroless plating[21].Therefore,FTIR spectroscopy provided convincing evidence that graphene was indeed reduced by the system to facilitate electroless plating.
Fig.2(a,b)show the SEM images of Al/graphene composite powder.It can be seen that the graphene was spatially curved after electroless aluminum plating to form pores of different sizes and irregularities.The formation of these pores could be attributed to the action of aluminum.Because the graphene is multilayered,the interlayer spacing was increased by ultrasound during the experiment.After the aluminum alkyl sesquihalide was reduced by NaH,the metal aluminum atomsweremorelikelytoenterthe interlayer voids,grow and extend,and interact with the defect regions in the graphene to generate a tensile force,which caused the graphene to bend and form ink-bottle-type pores.Fig.2(c,d)show the EDS spectra of spot 1 and spot 2 in Fig.2(a,b),respectively.The spot 1 was a point on the agglomerate of aluminum,which was very bright under electron microscopy.It can be seen from Fig.2(c)that the aluminum content was low,substantially existed in the form of alumina and contained a small amount of aluminum chloride,which indicated that the aluminum atom was staked and then oxidized.The spot 2 in Fig.2(b)was bright and the overall distribution was uniform.Fig.2(d)shows that the aluminum content was high and only a very small amount of aluminum was oxidized;furthermore,most of them still existed in an elemental form,indicating that the coating of graphene could protect the aluminum elementin the hole from being oxidized.There were a lot of spots the same as spot 2 after electroless plating.When Gr/Al is made of Al/graphene composite powder with aluminum or aluminum alloy,it can greatly improve the compatibility of graphene with metal and meet the requirements of the SLM for material.
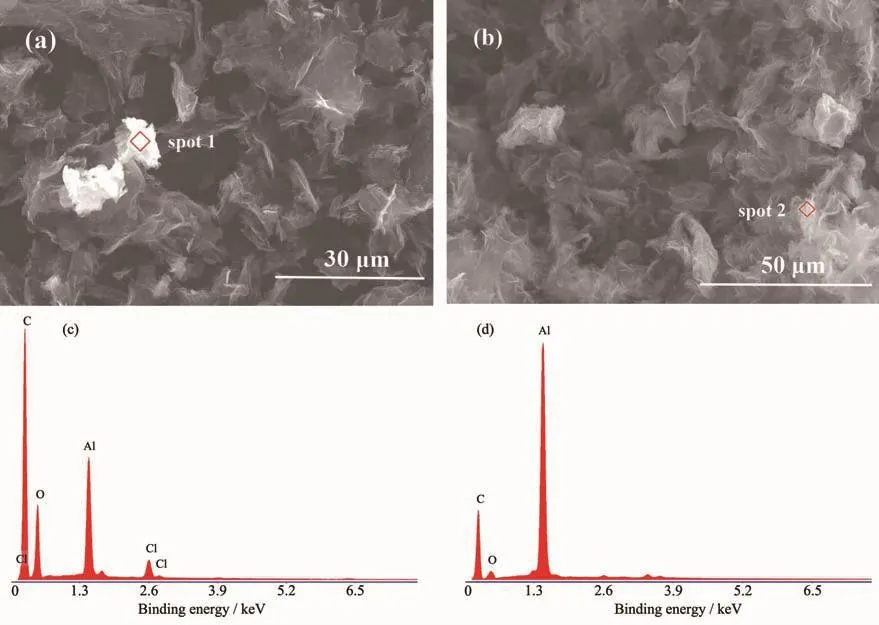
Fig.2 (a,b)SEM images of Al/graphene composite powder;(c)Composition analysis at spot 1;(d)Composition analysis at spot 2
2.2 Structural analysis
As shown in Fig.3(a),according to the classification method proposed by the International Union of Pure and Applied Chemistry,the adsorption isotherm curve of the Al/graphene composite powder presented typical typeⅣadsorption behaviors at-196℃and the H3-type hysteresis loop.The adsorption of N2molecules was single-layer and multi-layer adsorption in the micropores and mesopores,respectively,and the adsorption curve grew with a high slope in the relative pressure range of0 <P/P0<0.45,which indicated the large number of micropores and mesopores.In addition,these pores provided enough space to adsorb N2molecules.At the relative pressure range of 0.45<P/P0<1,the adsorption curve rised sharply,and unsaturation occured at the end of the curve.This situation indicated that a certain number of macropores were present in the sample,and the N2molecules caused capillary condensation in these pores,resulting in a significant increase in the amount of adsorption.Furthermore,as seen in Fig.3,there was a clear hysteresis loop,which proved the presence of open (parallel plate holes with four open sides)and the ink-bottle micro-and mesopores in the sample.The swelling of micropores and mesopores resulted in irreversible absorption of N2molecules in these pores.Besides, the irreversible chemical interactions between N2and the surface of the sample may also cause a hysteresis loop in the lower relative pressure range.Because of the presence of open(parallel plate holes with four open sides)and ink-bottle macro-pores and mesopores in the sample,the N2molecules showed capillary condensation, resulting in a significanthysteresisloop in thehigherrelative pressure range.
As shown in Fig.3(b),the pore size distribution curveshowsthatthemesoporesand micropores accounted for the majority,and the maximum pore diameter was 1.6~3.7 nm.However,the curve was wide,and the pore diameter was not uniform.The Brunauer-Emmett-Teller specific surface area of the Al/graphene composite powder was 91 m2·g-1,which was much lower than the theoretical specific surface area of single-layer graphene.On the one hand,the graphene that has been used was multi-layered.On the other hand,graphene underwent spatial bending deformation to form a cladding structure after aluminum plating,so a large amount of surface area was not available for nitrogen adsorption.And this part of the surface area that could not be used for N2adsorption was combined with aluminum.So when Al/graphene composite powder is finally used for the preparation of Gr/Al,it can greatly improve the compatibility of graphene with the metal matrix.

Fig.3 (a)N2adsorption-disorption isotherms of the Al/graphene composite powder;(b)Pore size distribution curve from the desorption branch

Fig.4 Raman spectra of graphene and Al/graphene composite powder
Fig.4 presents the Raman spectra of graphene and Al/graphene composite powder.The raw graphene exhibited a D band and G band at 1 332 and 1 566 cm-1in Fig.4(a),respectively,and the appearance of the D band indicated that the graphene was defective.The form of defects can be expressed as oxygencontaining groups, carbon-carbon double bonds,carbon five-membered rings,and carbon sevenmembered rings,which indirectly verify the correctness of the infrared spectra of Fig.1.The position of the G band appeared at 1 566 cm-1,which is lower than the literature[22](1 580 cm-1).The shape of the 2D band appearing at 2 677 cm-1was asymmetrical,indicating that the graphene used was a multilayered structure because the G band had red-shift with the increase of the number of graphene layers.Fig.4(b)shows that the peaks at 1 353 and 1 583 cm-1belong to D band and G band of the Al/graphene composite powder,respectively.Obviously,the Al/graphene composite powder had a blue-shift relative to the raw material graphene,and the D band and G band positions were almost identical to the single-layer graphene.The reason is that it could cause the pitch of the graphene layer to increase when the aluminum atoms entered into the interlayer of the graphene,exhibiting some of the properties of the single-layer graphene.
It can be found that ID/IG(the intensity ratio of the D peak to the G peak)changed from 0.704 to 1.013,indicating that the degree and disorder of graphene defectswereincreased afterelectroless plating.
Fig.5 presents the XRD patterns of the graphene and Al/graphene composite powder.The very weak diffraction peak at 2θ=10.9°corresponded to the(002)plane of graphene oxide,and the weak diffraction peak of the graphite(100)plane was located at 2θ=42.9°.Moreover,there was a broad but insufficiently strong diffraction peak at 2θ=24.0°,corresponding to the(002)plane of the expanded graphite;however,the 2θ angle of this diffraction peak was smaller than the theoretical value,and the interplanar spacing d=0.368 4 nm was calculated from the Bragg′s law.In summary,the graphene with low crystallinity was obtained by a redox method for graphite,and the oxygen-containing groups existed on the surface.

Fig.5 XRD patterns of graphene and Al/graphene composite powder
The characteristic peaks of Al were clearly discernible (PDF No.04-0787)in the XRD pattern of the Al/graphene composite powder.It can be seen from Fig.5 that there were four strong diffraction peaks for 2θ at 38.5°,44.7°,65.1°and 78.2°,corresponding to(111),(200),(220)and(311)plane,respectively.In addition,the diffraction peak of the(002)plane of the expanded graphite was located at 2θ=24.0°and the weak diffraction peak of the graphite(100)plane was located at 2θ=42.3°.However,the diffraction peaks corresponding to Al4C3were not detected.Briefly,the bonding between the graphene after aluminum plating and the aluminum is not based on chemical bonds but physical interactions.
2.3 Microscopic surface analysis
To analyze the adsorption of aluminum on the surface and layers of graphene,the following model assumptions were made:
(1)The system is a constant temperature and pressure system that is not influenced by external factors;
(2)There are α-phase (graphene phase)and βphase(metal aluminum phase),and the phase interface is SS,as shown in Fig.6;
Fig.6 Schematic diagram of the surface phase
(3)The phase between the AA surface and the BB surface in Fig.6 is the surface phase(including by-products such as sodium bromide and alkane of the reactions in the solution).The properties at AA surface are the same asthe α-phase and the properties at BB surface are identical with the βphase.Thus,all changes that occur at the interface are included in the surface phase.
The volumes of the two phases from the bulk phases α and β to the SS face are represented by Vαand Vβ,respectively.Assuming that the concentrations are uniform in Vαand Vβ,the total amount of substance of component i in the whole system is ci,αVα+ci,βVβ,ci,αand ci,βare the concentrations of component i in the α-phase and β-phase,respectively.However,because the concentrations in the surface phase is not uniform,the value is different from the actual amount of substance(ni),and the difference is represented by ni,σ,then:

This difference is the surface excess.The surface excessperunitarea,orthesurface excessof component i is:

where A is the area of SS.
The two-component systems have the following formula:

where G is the free energy;μ is a chemical potential.
For the surface phase,the surface energy σA also contributes to Gσ,so

where Gσis the free energy of the surface phase,σ is the surface tension, μ1,σ, μ2,σare the chemical potential of component 1 and 2 in the surface phase,respectively.Because the system is equilibrated at a certain temperature and pressure,the chemical potentials μ1and μ2of the respective components are constant in each phase and interface.Under constant temperature and pressure conditions,if an infinitesimal change occurs in the system,according to formula(4),then:
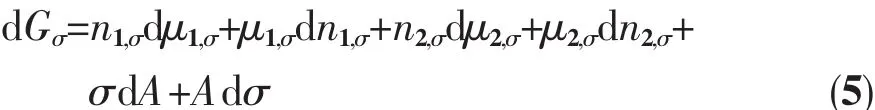
If there is only a slight change in the interface area in the system under constant temperature and pressure,the amount of substance of component 1 and 2 on the interface changes,so that the surface excesses n1,σand n2,σalso change accordingly.Minor changes in surface free energy should be:
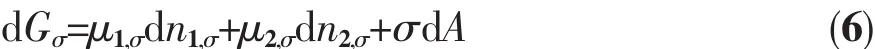
It can be derived from equations(5)and(6):

Divide both ends by A and contact equation(2):

Assuming that the position of the surface is selected as being where the surface excess of the solvent(component 1)is zero,which means Γ1=0,then equation(8)can be re-written as follow:

At equilibrium,aluminum(component 2)has the same chemical potential in the surface phase and the bulk phase,ie., μ2,σ=μ2(in the bulk phase).In the bulk phase,-dμ=RTlnα2,brought into equation(9),

where Γ2is the surface excess of aluminum, α2is the activity of aluminum in solution,σ is the surface tension of the solution,R is gas constant;T is thermodynamic temperature.The surface excess is much greater than the concentration of aluminum in the surface phase,at which point the surface excess can be approximated as the surface concentration.The concentration of aluminum(c)can be used instead of the activity α2,so the subscript is omitted under constant temperature conditions,and the formula(10)can be written as:

where Γ is the adsorbing capacity,c is the concentration of solution.Because the aluminum obtained by the reduction is a solid and the concentration is constant (assuming 1),the formula (11)can be simplified as:

where K is a positive constant.That is,the surface excess of aluminum in the graphene surface and interlayer is related to the rate of change of solvent surface tension.If the surface tension becomes smaller(dσ<0),the Γ is positive,which means that the concentration of aluminum in the surface layer is greaterthan in the solution,which ispositive adsorption;if the surface tension becomes larger,the Γ is negative,which means the concentration of aluminum in the surface layer is less than in the solution,which is negative adsorption.

Fig.7 SEM images of the Al/graphene composite powder corresponding to the added amount of NaH
For the experimental system,the addition or not of NaH and the amount of addition have a great influence on the change of the surface tension of the solution.When NaH was absent from the system,the active alkyl aluminum sesquihalide decomposed at high temperature,and molecular bromine was formed.Then,the molecular bromine reacted with a trace amount of water in the system to form a mineral acid.The presence of this acid increased the surface tension of the solution.As shown in Fig.7(a),graphene exhibited negative adsorption on aluminum,that is,it did notadsorb aluminum,so aluminum itself agglomerated.With the addition of NaH,the alkyl aluminum sesquihalide was reduced and decomposed,and some by-products were changed from molecular bromine to inorganic salt sodium bromide,so that the amount of inorganic acid formed was reduced,and the change of surface tension of the solution was smaller than before.The degree of agglomeration of aluminum wasweakened,and graphene begins to adsorb aluminum,as shown in Fig.7(b,c).When the added amount of NaH is slightly larger than the theoretical value,the reductive decomposition ofthe alkyl aluminum sesquihalide did notproducethe byproduct molecular bromine,which means the system did not contain a mineral acid.However,the presence of another by-product alkane reduced the surface tension ofthe solution,so thataluminum was adsorbed substantially on the surface and interlayers of the graphene without self-agglomeration,as shown in Fig.7(d).This situation is consistent with the ideal system assumption.As shown in Fig.7(e,f), with continuing to increase the amount of NaH,the extra NaH reduced the oxygen-containing groups of the graphene,so that the interlayer spacing becomes smaller,the force become larger,and the formed graphene/Al new phase was agglomerated.
3 Conclusions
Novel Al/graphene composite powder was prepared by a new organic phase electroless aluminum plating method in the current study.It is not necessary to perform any pretreatment(sensitization or activation)on graphene.At high temperature,the alkyl aluminum sesquihalide was decomposed and reduced to aluminum atoms by NaH,and the aluminum atoms adsorbed and deposited on the defects of the graphene.Ultrasound increased the spacing of the graphene layer,and the interaction of the aluminum atoms with the defect region caused the graphene sheet to be bent and formed a cladding structure.Microscopic surface analysis shows that the adsorption of aluminum by graphene is related to the rate of change of the surface tension of the solution.The situation that the surface tension of the solution become smaller that could facilitate the adsorption.Theaddition ofNaH can notonlyreducethe activation energy ofaluminum produced by the decomposition of the alkyl aluminum sesquihalide,but also indirectly reduce the surface tension of the solution.When the added amount of NaH was slightly larger than the theoretical value,the aluminum plating effect was the best,and no agglomeration occured.Consequently,the advantages of a relatively uniform distribution of Al on the surface and interlayers of graphene as well as special porous structure and high specific surface area may provide some information for solving the problems of the further preparation of Gr/Al,such as easy agglomeration of graphene in the aluminum matrix,and not being firmly bonded on the interface between the graphene and the matrix.
———理學院

