Cr3+和Ni2+復(fù)合摻雜的尖晶石氧化物用于提高鉆孔熱電池放電性能
宋恒旭 牛永強(qiáng) 趙宇宏 連冬曉 任敬霞 張妍妍 侯 華
(中北大學(xué)材料科學(xué)與工程學(xué)院,太原 030051)
0 Introduction
Thermal batteries have been widely used in the military since they were invented during World War II[1].The thermal battery as a reserve battery can be stored for decades because its electrolyte is a poor ionic and electronic solid salt at ambient temperature.The battery function only when the solid salt electrolyte becomes molten,so it is suitable for working in high temperature environments.Recently,a considerable amount of work has been done to apply the principle of thermal batteries to geothermal exploration and oil/gas drilling.As we know,the typical electrolyte of thermal battery such as LiCl-KCl eutectic has a melting point as high as 352℃[2],while the environments of geothermal and oil/gas boreholes have a temperature usually below 300℃[3].It is necessary to modify the thermal battery system to adapt to lower temperature applications. In recent years, there has been increasing activity using the nitrate electrolyte system based on its lower melting point,for example,the composition with 33.21%(w/w)LiNO3-66.79%(w/w)KNO3nitrate salt melts at 124.5℃[4].
Since the conventional sulfide cathode materials are not compatible with molten nitrates,a considerable amount of work has been done to screen suitable candidate cathode materials and oxides are considered suitable alternatives[5].Ag2CrO4as a cathode material has been reported with Li-Al anode and nitrate electrolyte between 160 to 215℃by Giwa[6].After that,Guidotti et al.examined Ag2CrO4[7],LiMn2O4[8],MnO2[3,8],CrO2,and LiCoO2with lithium alloy anode and KNO3-LiNO3eutectic electrolyte over a wider temperature range.More recently manganese oxides have been increasing attention because they exhibit not only large specific capacity but also environment-friendly.Niu et al.studied the electrochemical behavior of MnO2cathode material in various nitrate electrolyte systems over a temperature range of 150 to 300℃and current densities from 10 to 30 mA·cm-2[9-12].Meanwhile,Wang et al.examined the discharge performance of lithium manganese oxide and their derivatives by doped Ni[13]and Co[14]with lithium alloy anode and KNO3-LiNO3eutectic electrolyte.A more stable voltage plateau and a larger specific capacity can be obtained by doping in LiMn2O4,and we increased the specific capacity and improved its rate performance by doping chromium into LiMn2O4in previous work[15].
In order to enhance specific capacity and voltage stability of cathode material,Cr and Ni ions were introduced into spinel lithium manganate simultaneously.This material was used as a cathode material for rechargeable lithium-ion batteries at room temperature[16-17],but has not been reported at higher temperatures as far as we know.Here,we reported the material characteristics of chromium-nickel substituted spinel oxide materials and analyzed their discharge performance in single batteries over a temperature range of 200 to 300℃.
1 Experimental
1.1 Materials preparation
For the preparation of spinel LiCrxNiyMn2-x-yO4(0≤x≤0.3,0≤y≤0.3)material,stoichiometric amounts of LiOH·H2O (95.0%(w/w),Fengchuan,China),Cr2O3(99.0%(w/w),Sinopharm,China),NiO (99.0%(w/w),Aladdin,China)and MnO2(>97.5%(w/w),Sangon Biotech,China)powders were mixed and ground using a planetary ball-milling machine (Nanjing Nanda Instrument Plant,QM-3SP4,China).After this,the powders were precalcined at 470℃for 6 h,then heated to the temperature of 600℃for 6 h and 750℃ for 12 h in a resistance furnace in an air atmosphere.The precipitates were then natural cooling of the resistance furnace to room temperature and finally through grinding and sieving.
The separator mix consisted of 65%(w/w)LiNO3-KNO3eutectic electrolyte and 35%(w/w)MgO powder(>98%(w/w),BBI Life Sciences,China),where the MgO powder was used as a binding agent to resist electrolyte flow when it melted.The preparation method of separator mix refered to Guidotti′s report[7].The catholyte consisted of 70%(w/w)of the active cathode material,20%(w/w)LiNO3-KNO3electrolyte and 10%(w/w)graphite powder (>99%(w/w),Sangon Biotech,China).Graphite powder was added to improve the conductivity of the cathode material because the conductivity of the lithium manganate material was poor.The anolyte consisted of Li-Mg-B alloy (58%,4%and 38%(w/w),respectively).
1.2 Material characterization
The crystalline structures of the spinel LiCrxNiyMn2-x-yO4(0≤x≤0.3,0≤y≤0.3)materials were carried out on a X-ray diffractometer(XRD,Rigaku D/max-Rb,Japan)with Cu Kα1radiation(λ=0.154 08 nm)in a 2θ range of 15°~85°at the speed of 2°·min-1.The XRD instrument was operated at accelerating voltage of 40 kV and current of 100 mA.The morphologies of the samples were performed by a field emission scanning electron microscopy(FESEM;Zeiss merlin compact,Germany)operated at 10 kV.The thermal stabilities and compatibilities of the materials were analyzed by a simultaneous differential scanning calorimetry (DSC,Netzsch STA 449 C,Germany)and thermogravimetric analysis(TGA).The samples powder were sealed in Al2O3pans and were heated at a rate of 10 K·min-1from room temperature to 500℃in high-purity argon atmosphere.
1.3 Single-cell testing
The single cell was constructed with anolyte,separator mix,catholyte and current collectors.The anolyte pellet was punched into a diameter of 16 mm,thickness of 1 mm,and mass of 0.188 g wafer by a die.While the separator mix(0.2 g)and catholyte(0.2 g)powders were placed by layer in a die and pressing them to a pellet with a pressure of 14 MPa.Finally,304 stainless steel of current collectors and the two electrode pellets were combined into a single cell with sandwich structure.
The discharge performance of the single cell was measured by an electrochemical test instrument(CT-4008,Neware Technology Co.Ltd.,China).Steadystate currents of 19 to 57 mA (10 to 30 mA·cm-2,respectively)were loaded on the single cell at a temperature of 200 to 300℃.The discharge of the single cell was terminated when the voltage dropped below 1.00 V.It should be noted that above preparations,processing,and handling operations with materials and cells were conducted in a glovebox with an atmosphere of high-purity argon (The volume fraction of water and oxygen is less than 1×10-6).
2 Results and discussion
2.1 XRD analysis
Fig.1 shows the XRD patterns of the spinel LiCrxNiyMn2-x-yO4(0≤x≤0.3,0≤y≤0.3)samples.As can be seen from Fig.1 that the diffraction peaks of spinel samples with different doping amounts were wellconsistentwith the characteristic peaks of LiMn2O4(PDF No.70-3120)with an Fd3m space group.No additional diffraction peaks were observed except for lithium manganate,indicating a good purity of the spinel samples.It can be clearly observed that the diffraction peaks of the doped derivative were shifted to a large angle compared to LiMn2O4according to the partial enlargement of the (400)plane diffraction peak,which was caused by the decrease of lattice parameters due to doping.As we know,the average oxidation states of manganese ions in LiMn2O4is+3.5,that is,the amount of Mn3+and Mn4+ions exist in a ratio of 1∶1.X-ray photoelectron spectrometry(XPS)analysis show that the oxidation states of Cr and Ni in lithium manganate are+3 and+2,respectively[17],and the two ions have a radius of 0.061 5 and 0.070 nm,respectively.Both of Cr3+and Ni2+ions substitute the Mn3+ions in lithium manganate,thus the Mn3+ions(0.064 5 nm)shift to the smaller radius of the Mn4+ions(0.053 nm)with the addition of Ni2+ions in order to maintain electrical neutrality.The lattice parameters of the spinel sample calculated based on the XRD diffraction data are listed in Table 1,and this result is consistent with the above discussion.However,the sample with the most doped amount of Ni2+ions did not have the smallest interplanar spacing,which may be related to the loss of oxygen in the sample.
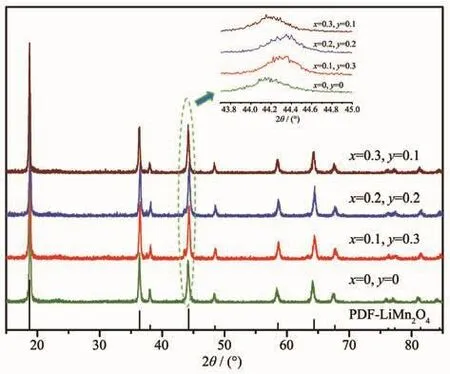
Fig.1 XRD patterns of the spinel LiCrxNiyMn2-x-yO4(0≤x≤0.3,0≤y≤0.3)samples
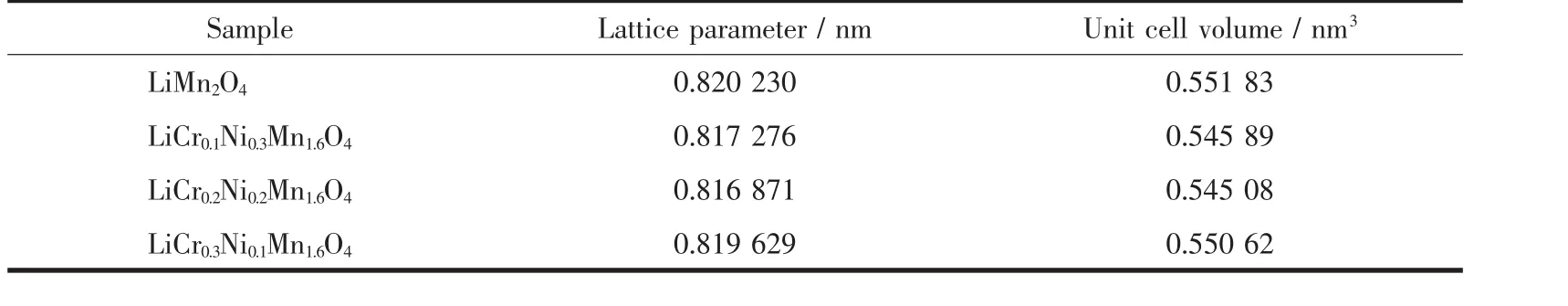
Table 1 Structure parameters of LiCrxNiyMn2-x-yO4(0≤x≤0.3,0≤y≤0.3)samples
2.2 SEM images
Fig.2 shows SEM images of the spinel LiCrxNiyMn2-x-yO4(0≤x≤0.3,0≤y≤0.3)with different dopants.As shown in Fig.2a,the undoped LiMn2O4powder had a relatively uniform particle size about 200 nm and an irregular granular shape of surface morphology.While the lithium manganate materials doped with chromium and nickel all exhibited as regular octahedralcrystalline particles,and the particle size became uneven compared to the undoped lithium manganate materials,that is,larger particles began to appear.It can be further observed that LiCr0.2Ni0.2Mn1.6O4sample showed the largest particle size non-uniformity from Fig.2c.According to report by Kang et al.[18],octahedral morphology with good crystallinity contributes to the improvement of the thermal stability of the materials,which is consistent well with the discharge performance of the single cell.
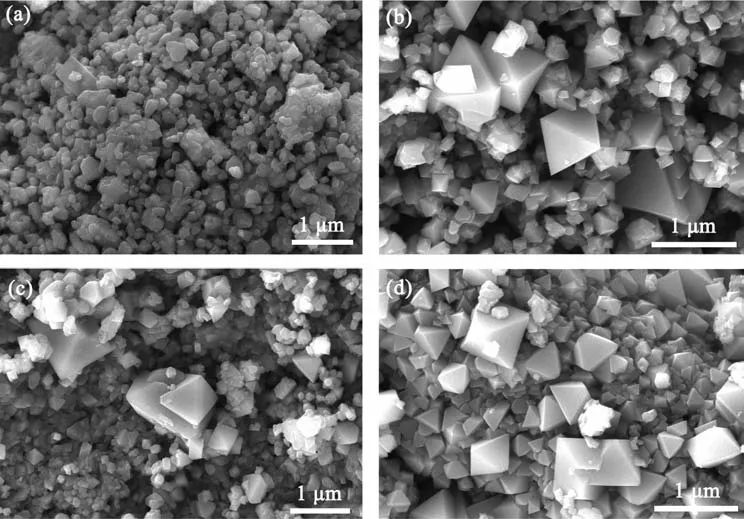
Fig.2 SEM images of(a)LiMn2O4,(b)LiCr0.1Ni0.3Mn1.6O4,(c)LiCr0.2Ni0.2Mn1.6O4and(d)LiCr0.3Ni0.1Mn1.6O4 powders prepared by solid state method
2.3 Thermal analyses
In order to characterize the thermal stability of the spinel samples,the LiCr0.2Ni0.2Mn1.6O4was analyzed by DSC and TG,and the result was shown in Fig.3.No obvious endothermic or exothermic peak was observed from the DSC curve from room temperature to 500℃.From the TG curve,no weight loss was observed during the heating process,except for the weight loss caused by the atmospheric fluctuation at the beginning.The result indicates that the prepared active cathode material has a good thermal stability.
To characterize the thermal compatibility between the spinel samples with the LiNO3-KNO3eutectic electrolyte and the thermal stability of the catholyte material,the catholyte material containing 70%(w/w)LiCr0.2Ni0.2Mn1.6O4,10%(w/w)graphite,and 20%(w/w)electrolyte were analyzed by DSC and TG and the result was shown in Fig.4.As can be seen from the TG curve in Fig.4,only one major endotherm at 130.9℃,which can be assigned to the melting of the LiNO3-KNO3electrolyte.In Guidotti′s report,the melting point of LiNO3-KNO3eutectic electrolyte is 124.5℃[19].This difference is mainly due to the fact that the test samples in this work are not pure LiNO3-KNO3eutectic electrolyte.From the TG curve,no obvious weight loss was observed from room temperature to 400℃,however,the weight loss began to apparent at temperatures higher than 400℃,which is caused bythethermaldecomposition ofnitrates electrolyte accompanied by the generation of O2[12].
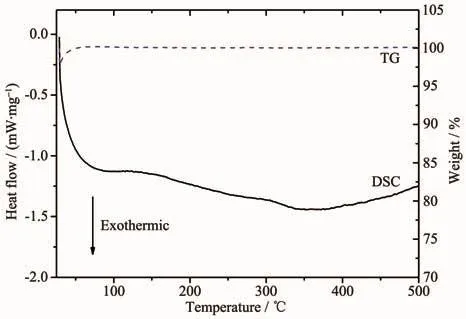
Fig.3 DSC and TG curves of spinel LiCr0.2Ni0.2Mn1.6O4 sample
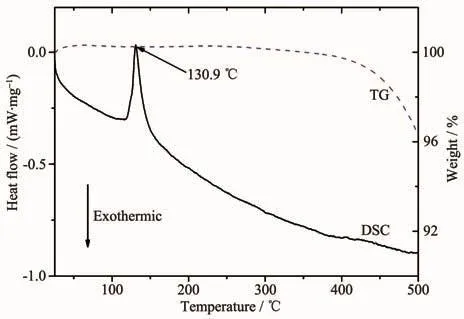
Fig.4 DSC and TG curves of the catholyte material containing 70%(w/w)LiCr0.2Ni0.2Mn1.6O4,10%(w/w)graphite,and 20%(w/w)LiNO3-KNO3 electrolyte
The thermal compatibility between the LiNO3-KNO3eutectic electrolyte and the Li-Mg-B anolyte has been reported by Niu et al[10].Here,three endothermic events on the DSC curve corresponded to the impure phase,the melting of LiNO3-KNO3electrolyte and the melting oflithium,respectively.In addition,no obvious exothermic event was observed from room temperature to 450℃.
Based on the above results,the safe operating temperature of this battery system can reach 400℃,which has met our needs of the battery operating temperature from 200 to 300℃.
2.4 Single-cell testing
Fig.5 shows the galvanostatic discharge behavior of the LiCrxNiyMn2-x-yO4/Li-Mg-B single battery with a constant current of 10 mA·cm-2at a temperature of 200℃.It can be seen from Fig.5 that there were three distinct discharge plateaus(2.75~2.70 V,2.30~2.10 V,1.70~1.50 V,respectively.)during discharge.It was noteworthy that the voltage of the undoped lithium manganate was slight higher than that of the doped lithium manganate at the initial discharge,however,the voltage of the undoped lithium manganate was lower than that of the doped lithium manganate at the second and third voltage plateaus about 0.2 and 0.3 V,respectively.Samples doped with chromium and nickel have almost the same discharge plateaus,and LiCr0.3Ni0.1Mn1.6O4had the largest discharge capacity of 585.14 mAh·g-1when the discharge voltage dropped to 1.0 V.
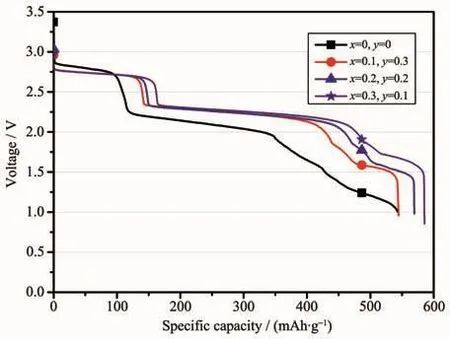
Fig.5 Galvanostatic discharge behavior of the LiCrxNiyMn2-x-yO4/Li-Mg-B single battery with a constant current of 10 mA·cm-2 at a temperature of 200℃
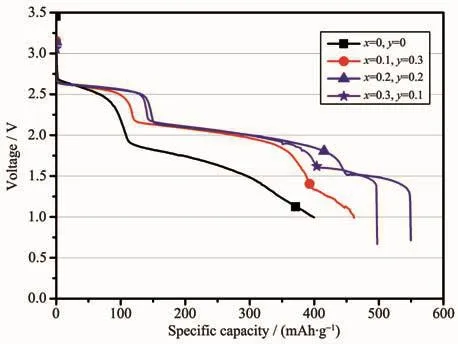
Fig.6 Galvanostatic discharge behavior of the LiCrxNiyMn2-x-yO4/Li-Mg-B single battery with a constant current of 30 mA·cm-2 at a temperature of 200℃
Fig.6 shows the galvanostatic discharge behavior of the LiCrxNiyMn2-x-yO4/Li-Mg-B single battery at a higher current density of 30 mA·cm-2at a temperature of 200℃.As shown in Fig.6,the galvanostatic discharge profiles were similar to the condition at 10 mA·cm-2,there were also three distinct discharge plateaus during discharge.The first and second voltage plateaus of the doped lithium manganate were at the ranges of 2.50~2.65 and 1.75~2.15 V respectively,while the third voltage plateau had a significant difference in doping levels.It is obvious that samples doped with chromium and nickel outperformed undoped lithium manganate in both voltage and capacity.The reasons for such excellent discharge performance of chromium and nickel co-doping materials are listed as follows:First,the binding energies of Cr-O and Ni-O in MO2are 1 142 and 1 029 kJ·mol-1,respectively,which is stronger than the binding energy of Mn-O of 946 kJ·mol-1[20-21].Therefore,the doping of Cr and Ni enhances the stability of lithium manganate spinel structure.Second,the partial substitution of Cr3+and Ni2+for Mn3+reduces the trivalent Mn3+ions in spinel,which alleviates the local distortion in MnO6octahedrons consisting of the spinel structure[22].Third,as we mentioned above in the SEM section,a good crystallinity octahedral structure obtained by doping Cr and Ni is more favorable for the thermal stability of the material.Compared with the condition at 10 mA·cm-2,the overall performance including voltage and capacity of the battery system decreased at 30 mA·cm-2,which could be attributed to the poor electrical conductivityofthespinelmaterial.Notably,the LiCr0.2Ni0.2Mn1.6O4had the largest discharge capacity of 549.57 mAh·g-1when the discharge voltage dropped to 1.0 V,which was higher than 37.6%of undoped original spinel oxide (399.5 mAh·g-1).This result indicates that partial substitution of chromium and nickel for manganese is more effective to improve the cell performance at higher currents.
In order to further investigate the discharge performance of the LiCrxNiyMn2-x-yO4/Li-Mg-B cell at a higher temperature,the single cell was designated to discharge at 300℃at a constant current density of 10 and 30 mA·cm-2,as shown in Fig.7 and 8,respectively.Compared with the condition at 200℃,the discharge curves also presented three voltage plateaus,the difference being that the second and third voltage platforms differ less,which was especially noticeable at lower current densities of 10 mA·cm-2(Fig.7).As shown in Fig.7,the initial discharge plateau was stable in the range of 2.60~2.75 V,while the subsequent voltage platform exhibited a large voltage difference with the doping amount.This result may be mainly cause by an increase in unexpected side reactions at high temperatures,resulting in a degradation of voltage stability.In terms of capacity,the battery system had an outstanding performance under this condition,and the LiCr0.1Ni0.3Mn1.6O4had the largest discharge capacity of713.29 mAh·g-1, even the undoped original spinel had a specific capacity of 629.07 mAh·g-1.This is due to the improvement of good electrolyte conductivity at high temperatures.The main reason for the increase of discharge capacity is attributed to the higher ionic conductivity ofthe electrolyte by enhanced kinetics at higher temperature.
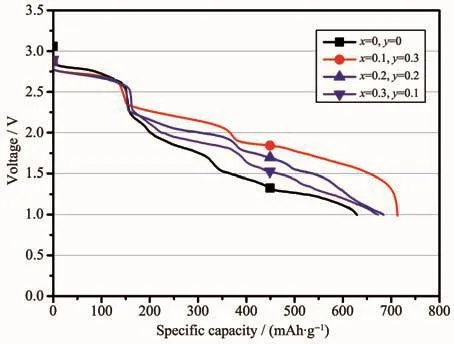
Fig.7 Galvanostatic discharge behavior of the LiCrxNiyMn2-x-yO4/Li-Mg-B single battery with a constant current of 10 mA·cm-2 at a temperature of 300℃
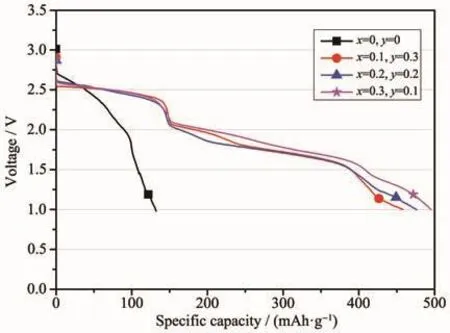
Fig.8 Galvanostatic discharge behavior of the LiCrxNiyMn2-x-yO4/Li-Mg-B single battery with a constant current of 30 mA·cm-2 at a temperature of 300℃
We found that the double ion doped spinel materials have better discharge performance than the single ion doped spinel materials under this condition.For example,the specific capacity of LiNi0.3Mn1.7O4[13]and LiCr0.1Mn1.9O4[15]spinel under the same conditions are 335.95 and 614.29 mAh·g-1respectively,which is much lower than that of LiCr0.1Ni0.3Mn1.6O4of 713.29 mAh·g-1in this work,and the main reason for this result may be the synergistic action of the two ions.
From Fig.8,it can be seen that the original LiMn2O4sample exhibited poor discharge performance at a higher current density of 30 mA·cm-2at a temperature of 300℃.After partial substitution of Mn in lithium manganate with Cr and Ni,the discharge specific capacity of the battery has been dramatically improved from the original 132.00 mAh·g-1to the highest 495.43 mAh·g-1(LiCr0.1Ni0.3Mn1.6O4).
3 Conclusions
Cr and Ni doped spinel LiCrxNiyMn2-x-yO4(0≤x≤0.3,0≤y≤0.3)cathode materials have been successfully synthesized through solid-state method and maintained the Fd3m spatial structure of the spinel which has been confirmed by XRD results.Partial substitution of Cr3+and Ni2+for Mn in spinel improves battery voltage and capacity at current densities from 10 to 30 mA·cm-2over a temperature range of 200 to 300℃.The LiCrxNiyMn2-x-yO4/Li-Mg-B battery system had the most outstanding discharge performance at a constant current density of 10 mA·cm-2at 300℃,and the LiCr0.1Ni0.3Mn1.6O4had the maximal specific capacity of 713.29 mAh·g-1.The dramatic improvement in discharge performance of the battery system is mainly because that the doping of chromium and nickel enhanced the structural stability of spinel lithium manganate oxides.Based on the stable structure of the material,the battery has a more significant improvement in discharge performance at higher current densities,in other words,doping makes the battery have better rate discharge performance.In addition,it should be noted that the discharge performance of the battery in different environments depends on the amount of dopant.
Based on the above results and discussion,the LiCrxNiyMn2-x-yO4/LiNO3-KNO3/Li-Mg-B battery system was evaluated suitable for using as a power supply for geothermal borehole applications.
Acknowledgements:This work was supported by the National Natural Science Foundation of China (Grants No.51774254, 51774253, 51701187, U1610123, 51674226,51574207,51574206,51804279,51801189)and the Science and Technology Major Project of Shanxi Province (Grant No.MC2016-06).

