Cryopreservation of strip spawned sperm using programmable freezing technique in the blue mussel Mytilus galloprovincialis*
LIU Yibing (劉一兵) , LIU Shiwen (劉詩(shī)文) , LIU Bingli (劉冰莉),QIN Jianguang (秦建光) , XU Tong (許通) , LI Xiaoxu (李孝緒)
1 Liaoning Ocean and Fisheries Science Research Institute, Dalian 116023, China
2 College of Science and Engineering, The Flinders University of South Australia, Adelaide, South Australia 5042, Australia
3 College of Fisheries and Life Science, Dalian Ocean University, Dalian 116023, China
4 South Australian Research and Development Institute, West Beach, South Australia 5024, Australia
Abstract In this study, a programmable freezing technique has been developed for strip spawned sperm in the blue mussel, Mytilus galloprovincialis. The optimized key parameters include cooling rate, endpoint temperature, thawing temperature, sugar addition and sperm to oocyte ratio. The sperm quality was assessed by the fertilization rate or the integrity of sperm component and organelle. The highest post-thaw sperm fertilization rate was 91%, which was produced with sperm cryopreserved in 8% dimethyl sulfoxide at the cooling rate of -4°C/min from 2°C to -30°C before being plunged into liquid nitrogen for at least 12 h,thawed in a 20°C seawater bath and fertilized at sperm to egg ratio of 50 000:1. The addition of glucose,sucrose or trehalose to 8% dimethyl sulfoxide could not further improve fertilization rates. The fluorescent assessments showed that the post-thaw sperm plasma membrane integrity and acrosome integrity were significantly damaged in comparison with fresh sperm.
Keyword: blue mussel; Mytilus galloprovincialis; strip spawning; sperm cryopreservation
1 INTRODUCTION
The blue mussel,Mytilusgalloprovincialisis one of the most important aquaculture species in the world(Liu et al., 2016a). In order to maintain its long-term sustainable development and competitive advantages,techniques that could assist all year-round hatchery production or genetic improvement programmes have become a major interest to the industry (Pettersen et al., 2010; Lazo and Pita, 2012). Sperm cryopreservation has been considered as an effective technique that would achieve these goals and has been largely investigated in oysters and abalone (Hassan et al.,2015; Liu et al., 2015a). InM.galloprovincialis,sperm cryopreservation has been investigated by Matteo et al. (2009) and Liu et al. (2016a). In the former, although approximately 65% fertilization rate was achieved with sperm spawned naturally, this method of sperm collection could compromise sperm quality due to potential contamination (such as mucus,seawater and feces), thus resulting in low post-thaw sperm quality (Paniagua-Chavez et al., 1998; Gwo et al., 2002; Dong et al., 2005). In the latter, on the other hand, although 90% fertilization rate has been achieved with sperm collected by strip spawned method, the non-programmable freezing technique is generally less reliable in comparison with the programmable freezing technique (Clulow et al.,2008). For example, in comparison with the nonprogrammable freezing technique, better post-thaw sperm qualities, such as motility and/or plasma membrane integrity, have achieved with programmable freezing technique in dog (Rota et al., 2005), human(Stanic et al., 2000), stallion (Clulow et al., 2008) and honey bee,Apimellifera(Hopkins and Herr, 2010).These advantages usually make the programmable freezing technique as the preferential selection for the application of cryopreservation in breeding programmes and semen cryobank establishments(Stanic et al., 2000; Clulow et al., 2008; Tiersch,2008). Nevertheless, the programmable freezing technique has not been evaluated to cryopreserve strip spawned sperm in theM.galloprovincialis.
Studies in livestock and aquatic species have demonstrated that the addition of sugar is an effective strategy to improve the quality of post-thaw sperm(Lyons et al., 2005; Purdy, 2006; Cabrita et al., 2010;Liu et al., 2014a, 2015a), and has been applied in rams (Jafaroghli et al., 2011), boars (Gómez-Fernández et al., 2012), black-lip pearl oysters,Pinctadamargaritifera(Lyons et al., 2005), farmed greenlip abalone,Haliotislaevigataand blacklip abalone,H.rubra(Liu et al., 2014a, 2015b).
The integrity of sperm components and organelles is of importance for sperm to perform their functions and can be evaluated under fluorescent microscopy using specific fluorescence dyes (Zhang et al., 2012;Liu et al., 2016a). This staining technique has been applied in various species to provide detailed information for sperm quality assessment in cryopreservation studies, such as in farmed greenlip abalone (Liu et al., 2014a) and blue mussel (Liu et al., 2016a).
In the present research, the sperm collected with the strip spawned method were used to investigate the parameters (cooling rate, thawing temperature,endpoint temperature and sperm to oocyte ratio) key to the development of programmable freezing technique inM.galloprovincialis. The supplementation of various types and concentrations of sugar was also evaluated for the purpose of improvement of postthaw sperm quality after cryopreservation.
2 MATERIAL AND MATHOD
2.1 Broodstock and gamete collection
The mature blue mussels (65–85 mm in length)were supplied by Kinkawooka Mussels in Port Lincoln, South Australia and delivered to the South Australian Research and Development Institute(SARDI), Adelaide with a chilled transportation.When the strip spawned method was applied,randomly selected animals were open and genders were determined by the gonad colour with the male being milky white. The male gonads were removed individually and transferred in a culture dish with prefilled 1 μm filtered seawater (FSW) at the volume of 30 mL. Sperm were scraped from the gonad and left undisturbed for 30 min. The sperm debris were removed with 90 μm and 25 μm sieves. The sperm motility was estimated under a light microscope using subsamples. In each experiment, sperm with motility>70% was mixed equally from five or more males and stored on ice. The sperm concentration was determined as described by Liu et al. (2016a) for the same species and standardized to 4× 108/mL in this study. The sperm were used within 2 h of collection.
Females were induced to spawn separately using the thermal shock method described by Liu et al.(2016a). Oocytes from one mussel were collected on a 35 μm sieve after through a 90-μm sieve to remove the debris. After rinse they were washed into a container. When no fertilized oocytes (with one or two polar bodies or dividing cells) were observed under a light microscope 15 min later, oocytes from three or more females were mixed and stored on ice(0°C). The oocyte density was determined (Liu et al.,2016a) and standardized to 1×105/mL in this study.The fresh oocyte was used within 2 h after spawning.
2.2 Chemical preparation and equipment
Dimethyl sulfoxide (DMSO), sucrose, glucose,trehalose and propidium iodide (PI) were all in the AR grade and purchased from Sigma-Aldrich Pty Ltd., St Louis, MO, USA. The live/dead sperm viability kit (L-7011) and the LysoTrack Green DND-26 (LYSO-G) packages (L-7526) were purchased from Invitrogen Australia. The former was used to evaluate sperm Plasma Membrane Integrity (PMI)whereas the latter to evaluate Acrosome Integrity(AI). The cryoprotective stock solutions and the working solutions for evaluating PMI and AI were prepared as described by Liu et al. (2016a).
A CL863 programmable freeze controller(Cryologic, Mulgrave, Victoria, Australia) was used.The straws (Minitube, Germany) were placed into a cryochamber (model: CC23F) and frozen by liquid nitrogen (LN). The procedures used in this study to regulate temperatures during freezing and establish the required water temperature in the thawing bath were the same as reported in our previous research on greenlip abalone sperm cryopreservation (Liu et al.,2016b).
2.3 Evaluation of sperm quality
In the present study, the sperm quality was evaluated by fertilization rate or sperm PMI and AI according to the methods described by Liu et al.(2016a) for the same species. SYBR14/PI and LYSO-G/PI were used for sperm PMI and AI assessments, respectively.
2.4 Experiment
2.4.1 Effects of cooling rates on post-thaw sperm fertilization rate
DMSO at a final concentration of 6% or 8% and an equilibration period of 10 min has been identified by Liu et al. (2016a) for the non-programmable cryopreservation method in this species and was applied in this and subsequent experiments. In this experiment, the effects of cooling rates at -3, -4, -5,-6, and -7°C/min on post-thaw sperm fertilization rate were evaluated. Pre-cold freshly strip spawned sperm were equilibrated on ice for 10 min in 6% and 8%DMSO (final concentrations). They were then moved into cryo-straws (0.25 mL) and placed into the programmable freezer. The straws were held at 2°C for 5 min and then frozen at different cooling rates to the endpoint temperature (-30°C) before being stored in LN. After at least 12 h in LN, the straws were thawed in a 60°C seawater bath until ice melted and recovered in an 18°C seawater bath. The fertilization rate was used to evaluate post-thaw sperm quality at a sperm to oocyte ratio of 10 000:1. The sperm to oocyte ratio has been determined according to the method described by Liu et al. (2016a). In this and subsequent experiments all treatments were repeated three times using different sperm pools.
2.4.2 Effects of thawing temperatures on post-thaw sperm fertilization rate
Sperm mixed with 8% DMSO and frozen at -4°C/min produced the highest post-thaw sperm fertilization rate in the previous experiment and these parameters were applied hereafter. In the present experiment, the following thawing temperatures were evaluated: 20,30, 40, 50, 60, 70 and 80°C. The other procedures were as described in Section 2.4.1.
2.4.3 Effects of endpoint temperatures on post-thaw sperm fertilization rate
Sperm thawed at 20°C produced the highest postthaw sperm fertilization rate and this temperature was used hereafter. In the current experiment, the following endpoint temperatures were assessed: -20,-30, -40, -50, -60, -70 and -80°C. The other procedures were as described in Section 2.4.2.
2.4.4 Assessment of sugar type and concentration on post-thaw sperm fertilization rate
The endpoint temperature at -30°C produced the highest post-thaw sperm fertilization rates and was used in this and subsequent experiments. In this experiment, 8% DMSO and its combination with glucose, sucrose and trehalose at a concentration of 0.5%, 1%, 2%, and 4% were evaluated (Liu et al.,2016a). The other procedures were the same as in Section 2.4.3.
2.4.5 Effects of different sperm to oocyte ratios on post-thaw sperm fertilization rate, PMI and AI
As no improvement in post-thaw sperm fertilization rate was shown by adding different types and concentrations of sugar in the previous experiment,8% DMSO was used to assess the effects of different sperm to oocyte ratios (10 000:1, 20 000:1, 30 000:1,40 000:1 and 50 000:1) in this experiment. The other procedures were the same as in Section 2.4.4, although PMI and AI were applied to assess sperm quality.
2.5 Statistical analysis
Results were presented as mean±standard deviation(SD) and data were arcsine transformed to comply with the ANOVA assumption of data normality and homoscedasticity before statistical analyses using SPSS 20 (SPSS, Chicago, IL, USA). At-test for independent samples was applied to compare the sperm PMI and AI in both cryopreserved and fresh sperm. Two-way analysis of variance (ANOVA) was applied in the Section 2.4.1 and one-way ANOVA was applied in the others. The least-significant difference (LSD) comparison test was applied when the significance was observed atP<0.05.
3 RESULT
3.1 Effects of cooling rates on post-thaw sperm fertilization rate
Significant interaction (P<0.05) between cooling rate and DMSO concentration was found. The postthaw sperm fertilization rates were higher at -4 and-5°C/min than other cooling rates in both 6% and 8%DMSO (Fig.1). No significant difference (P>0.05) was found between these two cooling rates in 6% DMSO(39.9%±4.7% and 42.8%±4.1% fertilization rates for -4 and -5°C/min, respectively), whereas significant difference was found in 8% DMSO achieving the highest post-thaw sperm fertilization rate of 50% (P<0.05).
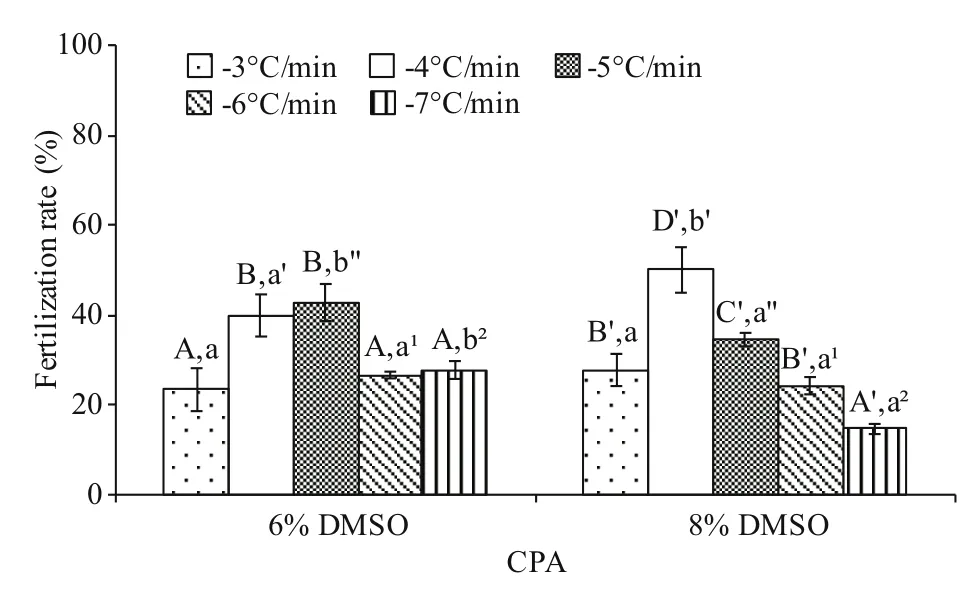
Fig.1 Post-thaw sperm fertilization rate (%) after freezing at different cooling rates in 6% and 8% DMSO ( n=3)of M. galloprovincialis
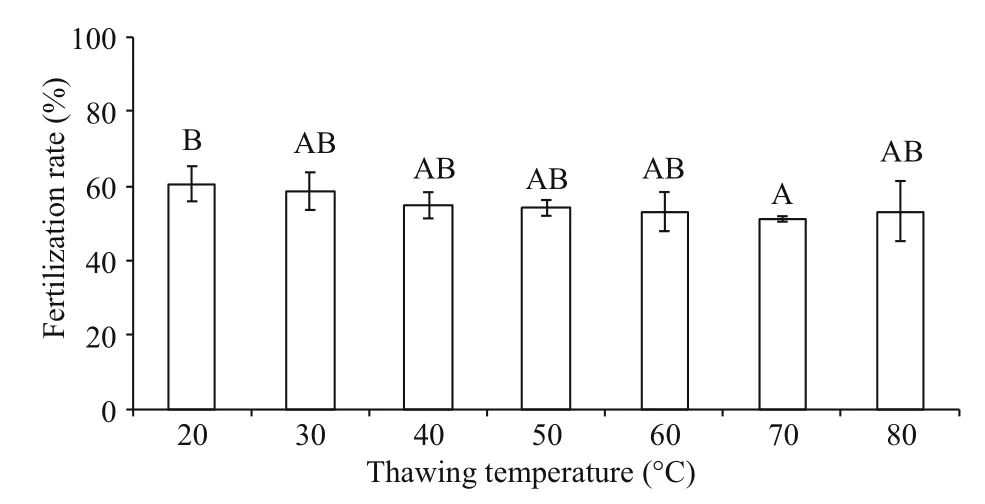
Fig.2 Post-thaw sperm fertilization rates (%) after cryopreservation in 8% DMSO and thawed at different temperatures ( n=3) of M. galloprovincialis

Fig.3 Post-thaw sperm fertilization rates (%) after being frozen to different endpoint temperatures in a programmable controller before being transferred into LN ( n=3) of M. galloprovincialis
3.2 Effects of thawing temperatures on post-thaw sperm fertilization rate
Post-thaw sperm fertilization rates fluctuated from 51.2%±0.7% to 60.6%±4.7% at different thawing temperatures (Fig.2). Significant difference (P<0.05)was found between 20°C (60.6%±4.7% fertilization rate) and 70°C (51.2%±0.7% fertilization rate) thawing temperatures, whereas each of these two temperatures had no significant difference with others (P>0.05).
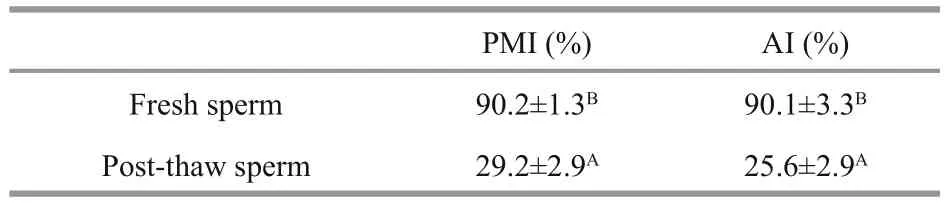
Table 1 Sperm plasma membrane integrity (PMI) and acrosome integrity (AI) in fresh and post-thaw sperm ( n=3) of M. galloprovincialis
3.3 Effects of endpoint temperatures on post-thaw sperm fertilization rate
The highest post-thaw sperm fertilization rate of 60.2%±4.0% was achieved at the endpoint temperature of -30°C which was significantly higher than other endpoint temperatures (Fig.3).
3.4 Assessment of sugar type and concentration on post-thaw sperm fertilization rate
No significant improvement in post-thaw sperm fertilization rate was found in all sugar types and concentrations evaluated (P>0.05; Fig.4), ranging from 22.6%±5.1% to 45.1%±4.9%. Instead the postthaw sperm fertilization rate decreased with the increase in the sugar concentrations.
3.5 Effects of different sperm to oocyte ratios on post-thaw sperm fertilization rate, PMI and AI
Figure 5 showed that the post-thaw sperm fertilization rate increased with the increase of sperm to oocyte ratio, reaching the highest of 91.4%±6.0%at ratio of 50 000:1. However, no significant difference was found between sperm to oocyte ratio from 30 000:1 to 50 000:1 (P>0.05). The PMI and AI values in post-thaw sperm were significantly lower than those in fresh sperm (P<0.01; Table 1).
4 DISCUSSION
In this study a programmable freezing technique has been developed for the blue mussel sperm collected with the strip spawned method. The addition of glucose, sucrose or trehalose in the cryoprotectant could not further improve the post-thaw sperm fertilization rate. On the other hand, the fertilization rate of post-thaw sperm increased with the increase in sperm to oocyte ratios.
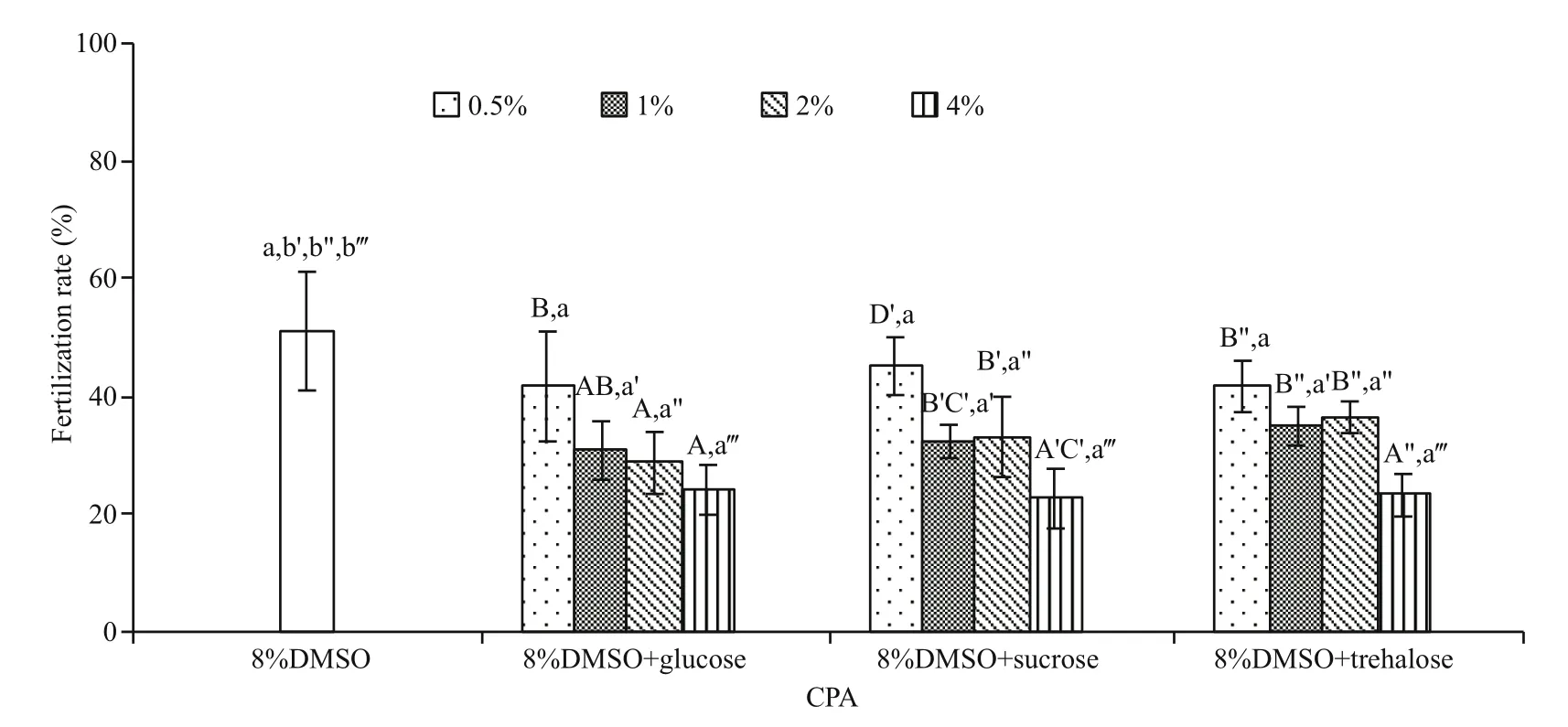
Fig.4 Comparison of post-thaw sperm fertilization rates (%) after being frozen in 8% DMSO and its combination with different sugar types and concentrations ( n=3) of M. galloprovincialis
In cryopreservation, an optimal cooling rate could balance the effects from the formation of intracellular ice and the increase in solute concentrations, leading to maximal maintenance on sperm quality (Liu et al.,2015a). In the present study, a programmable freeze controller was selected as it could strictly regulate the cooling rate. The best post-thaw sperm fertilization rate was produced at -4°C/min. This cooling rate is faster than that optimised in European flat oyster,Ostreaedulis(-3°C/min; Vitiello et al., 2011), but slower than most other species investigated so far,includingH.laevigata(-5°C/min; Liu et al., 2016b),disc abalone,H.discushannai(-50°C/min; Kang et al., 2004), small abalone,H.diversicolorsupertexa(-12 or -15°C/min; Gwo et al., 2002), red abalone,H.rufescens(-16°C/min; Salinas-Flores et al., 2005),Pacific oysters,Crassostreagigas(-6°C/min; Ieropoli et al., 2004), scallopsChlamysfarreri(-20°C/min; Li et al., 2000) and macha surf clams,Mesodesma donacium(-18°C/min; Dupré and Guerrero, 2011).
Thawing temperature evaluation is one of the important steps for the development of sperm cryopreservation as optimal thawing temperature could minimise recrystallization and reduce osmotic stress to sperm (Gao and Critser, 2000; Herráez et al.,2012). In the current study, the thawing temperature of 20°C produced the highest post-thaw sperm fertilization rate, which agrees with the study in Pacific oysters (Adams et al., 2004). However, this temperature is lower than most of the studies in marine mollusk (Liu et al., 2015a) as well as the study in the same species (Liu et al., 2016a). The difference in optimal thawing temperature revealed by Liu et al.(2016a) may be due to the fact that a nonprogrammable freezing technique was used in their investigation.

Fig.5 Post-thaw sperm fertilization rates (%) at different sperm to oocyte ratios after cryopreservation in 8%DMSO ( n=3) of M. galloprovincialis
Theoretically, the optimal endpoint temperature should be species specific while the temperatures lower or higher than the optimal temperature or temperature range will affect the post-thaw sperm survivals (Gwo, 2008). In this study, the highest postthaw sperm fertilization rate was achieved at -30°C endpoint temperature, which is much higher than the-90°C reported in the small abalone (Gwo et al.,2002). In the current study, reasons causing significant reduction in post-thaw sperm quality at -20°C endpoint temperature were not clear. It might be due to inadequate sperm dehydration, therefore causing severe damage by intracellular ice formation in the subsequent freezing steps. This phenomenon has also been observed in farmedH.laevigata(Liu et al.,2016b), marine shrimp,Sicyoniaingentis(Anchordoguy et al., 1988) and African catfish,Clariasgariepinus(Viveiros et al., 2001) where sperm viabilities were also lower at higher endpoint temperatures.
In livestock and fish sperm cryopreservation, sugar is often consisted in cryoprotective medium. The addition of sugar would reduce the formation of intracellular ice, stabilize the membrane during freezing and serve as an energy source (Suquet et al.,2000; Purdy, 2006; Cabrita et al., 2010). In this study,no positive effect on post-thaw sperm fertilization was obtained by the addition of trehalose, sucrose or glucose. These results were in agreement with the findings on Japanese pearl oysters,P.fucatamartensii(Kawamoto et al., 2007) and the same species with the non-programmable freezing method (Liu et al.,2016a), where the post-thaw sperm quality could not be improved by the addition of sugar. In contrast, Liu et al. (2014b, 2015b) have found that the addition of glucose significantly improved the post-thaw sperm fertilization rate in farmed greenlip and blacklip abalone.
A higher sperm to oocyte ratio is normally needed in fertilization to compensate cryodamages on sperm after cryopreservation (Liu et al., 2016a). In this study, higher sperm to oocyte ratios (30 000:1 to 50 000:1) were required to produce 85% post-thaw sperm fertilization rates, which are 1 500 to 2 500 times higher than the ratios used in fresh sperm. These ratios were higher than those reported in most published studies in marine mollusk (Liu et al., 2015a,2016a). The reason causing this higher sperm to oocyte ratio may be partly due to the fact that the sperm PMI and AI were severely damaged during the cryopreservation process (Table 1) and potential high variations in sperm maturity when they were stripspawned.
5 CONCLUSION
The current study has developed a programmable freezing technique for sperm collected with strip spawned method in the blue mussel,M.galloprovincialis. A post-thaw sperm fertilization rate of >85% has been achieved when the sperm were cryopreserved in 8% DMSO at the cooling rate of-4°C/min from 2°C to -30°C endpoint temperature,thawed in a 20°C seawater bath and fertilized at the sperm to oocyte ratio of 30 000:1 to 50 000:1. The addition of sugars in 8% DMSO did not improve the post-thaw sperm fertilization rate.
6 DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGEMENT
We thank Mr Andy Dyle for the provision of blue mussel broodstock and Mr Mark Gluis of SARDI for technical support.
 Journal of Oceanology and Limnology2018年6期
Journal of Oceanology and Limnology2018年6期
- Journal of Oceanology and Limnology的其它文章
- Neuroanatomy and morphological diversity of brain cells from adult crayfish Cherax quadricarinatus*
- Effects of feeding time on complement component C7 expression in Pelteobagrus vachellii subject to bacterial challenge*
- Pf- D mrt4, a potential factor in sexual development in the pearl oyster Pinctada f ucata*
- Specific genetic variation in two non-motile substrains of the model cyanobacterium Synechocystis sp. PCC 6803*
- Functional characterization of a Δ6 fatty acid desaturase gene and its 5′-upstream region cloned from the arachidonic acidrich microalga Myrmecia incisa Reisigl (Chlorophyta)*
- The expression characteristics of vitellogenin (VTG)in response to B(a)p exposure in polychaete Perinereis aibuhitensis*
