Antioxidative and antimicrobial activities of intertidal seaweeds and possible effects of abiotic factors on these bioactivities*
XU Peihang (徐佩杭) , TAN Huaqiang (譚華強) , JIN Weiguang (金偉光) ,LI Yanfei (李燕飛) , C. SANTHOSHKUMAR , LI Ping (李平) , ,LIU Wenhua (劉文華) ,
1 Marine Biology Institute, Shantou University, Shantou 515063, China
2 Guangdong Provincial Key Laboratory of Marine Biology, Shantou University, Shantou 515063, China
3 STU-UNIVPM Joint Algal Research Center, College of Sciences, Shantou University, Shantou 515063, China
Abstract To overcome acute physiological stress from frequent exposure to high irradiance levels and emersion stress, seaweeds colonizing intertidal zones generate valuable secondary metabolites. Their chemical composition is influenced by spatial changes in environmental parameters, and bioactivities closely linked to specific compounds change accordingly. We measured antioxidative and antimicrobial activities of 26 species in ten intertidal zones of eastern Guangdong, China, and examined the possible effects of abiotic factors. Most brown algae exhibited higher antioxidative activity and total phenol content than red algae and green algae, while most brown algae and green algae revealed more efficient antimicrobial activity than red algae. Their activities were also affected by the environment of their intertidal habitats. Similar antioxidative ability or total phenol content were found in seaweeds settled in high-, middle- and low-tide zones, while more positively antimicrobial ability was discovered in seaweeds colonizing the low-tide zone than in those in middle- and high-tide zones. These differences were mainly caused by the different stress levels of sun exposure, nutrition and desiccation, as well as temperature and salinity fluctuations in various tidal regions.Seaweeds colonizing coastal waters and experiencing stresses such as low salinity, limited dissolved oxygen(DO) or rich nutrition exhibited superior antioxidative ability and total phenol content. This result was further strengthened by the finding that the antioxidative ability and total phenol content of Ulva fasciata were positively affected by nutrition and negatively influenced by DO or salinity. The antioxidative activity and total phenol content of Sargassum vachellianum were also positively affected by ammonium-nitrogen. No similar trend was found in the antimicrobial activity of seaweeds. Our results suggested an effect of abiotic factors on the antioxidative and antimicrobial activities of intertidal seaweeds in the wild, and may allow for selective gathering high-activity category in terms of algal species and local environmental conditions.
Keyword: abiotic stress; antioxidant activity; antibacterial activity; phenol content; intertidal zone; macroalgae
1 INTRODUCTION
Seaweeds are very diverse and have scores of secondary metabolites with biological uses for commercial application in cosmetics, pharmacy, food and other natural products. Certain seaweeds,especially those settled in intertidal zones, are exposed to extreme conditions such as broad fluctuations in salinity and desiccation, and high solar radiation(Cruces et al., 2012; Balboa et al., 2013). Such seaweeds have multiple characteristics that equip them well in coping with such stressful conditions.One example is the synthesis of specific secondary metabolites including pigments, polysaccharides and phenolics (Stengel et al., 2011; Balboa et al., 2013).This synthesis has been the subject of intense scrutiny,mostly regarding composition, bioactivity and extraction methods (Oumaskour et al., 2013; Alassali et al., 2016); potential use for industry; and food and pharmacological applications (Wijesekara and Kim,2015; Venkatesan et al., 2016). Most seaweeds in these studies were collected from the wild. It is known that natural temporal and spatial changes, including temperature, salinity, nutrients, light, pH and pollution, can have a profound influence on the chemical composition of populations of seaweeds in the wild (Ritter et al., 2008; Stengel et al., 2011;Fernández et al., 2014). Biological activities are also generated by variations in these changes.
A review has found that chemical components of seaweeds, including polysaccharides, phenolics,pigments and lipids, display significant diversity within taxa and are always influenced by environmental parameters (Stengel et al., 2011). For example, the cell-wall accumulation of pigments and polysaccharides in the red algaGelidiumsesquipedalewas different under various light qualities (Torres et al., 1995). An increase in UV-B radiation resulted in a significant increase in phlorotannin concentration in the brown algaAscophyllumnodosum(Pavia et al.,1997). A reduction in salinity decreased total phenol contents and changed the phenolic composition ofA.nodosumandFucusvesiculosus(Connan and Stengel, 2011). Previous studies also revealed the effect of translation of seasonal variation on the antioxidative and antimicrobial activities of seaweeds(Stirk et al., 2007; Manilal et al., 2009; Fariman et al.,2016). In comparison to other months of the year, the highest antimicrobial activity ofUlvarigidaextracts was observed in samples collected during spring and summer; the highest free radical scavenging activity was detected inU.rigidaextracts collected in late winter and early spring (Trigui et al., 2013). These findings can be explained: seasonal effects of factors like the maturity of seaweeds and levels of exposure to the sun impact chemical composition, resulting in differences in bioactivity (Sampath-Wiley et al.,2008; Fariman et al., 2016). A few studies also have reported the spatial effects of abiotic factors on the bioactivity of seaweeds (Zubia et al., 2009). Seaweeds from shallow waters (0–3 m) show higher antioxidative activity than those from depths of more than 70 m(Kelman et al., 2012). The rapid responses of soluble phlorotannins that correlate with the antioxidative activity is triggered by UV radiation (Cruces et al.,2012). However, in contrast to temporal variation, the effects of spatial variation on the antioxidative activity and antimicrobial activity of seaweeds still need more attention.
The chemical composition, bioactivities and commercial applications, etc., of a number of seaweeds in China have been explored to date (Zheng et al., 2001; Zhang et al., 2007; Luo et al., 2010; Li and Kim, 2011). There are certain limitations on the locations and range of species among these investigations. In comparison to seaweeds studied on the coasts of Shandong and Fujian provinces (Zheng et al., 2001; Luo et al., 2010), seaweeds on the eastern Guangdong coast have received less attention. The eastern Guangdong coast is rich in algal biodiversity and is home to species of considerable ecological,economic and social potential. However, only few species likeGracilarialemaneiformisandPorphyra haitanensisin eastern Guangdong are exploited to extract agar-agar and for use in the food industry,respectively. The pressure on these traditional species could be decreased with the development of other algae species. Despite these advances, little is yet known about the total phenol content, and the antioxidative and antimicrobial activities, of seaweeds from the eastern Guangdong coast.
The aim of this study was to explore the total phenol content and the antioxidative and antimicrobial activities of intertidal seaweeds in eastern Guangdong coastal waters, and to learn some effects of their habitats. This may, ultimately, allow for selective gathering of species falling into the category of showing high levels of activity, and arranging them by species and local habitat. Two major aspects were taken into account concerning the differences in seaweed habitats: (1) Although tidal exposure exerts considerable ambient stress on all intertidal seaweeds,those at emersion are exposed to higher irradiance levels, desiccation, air-temperature and salinity fluctuations than those at immersion (Connan et al.,2007). (2) Indexes reflecting the water quality(temperature, salinity, pH, dissolved oxygen (DO)and nutrition) were also considered when examining the spatial changes from living in various intertidal zones. In the current research, the total phenol content,and the antioxidative and antimicrobial activities of three taxa of seaweeds (green, red, brown) collected from ten different intertidal zones, were investigated.
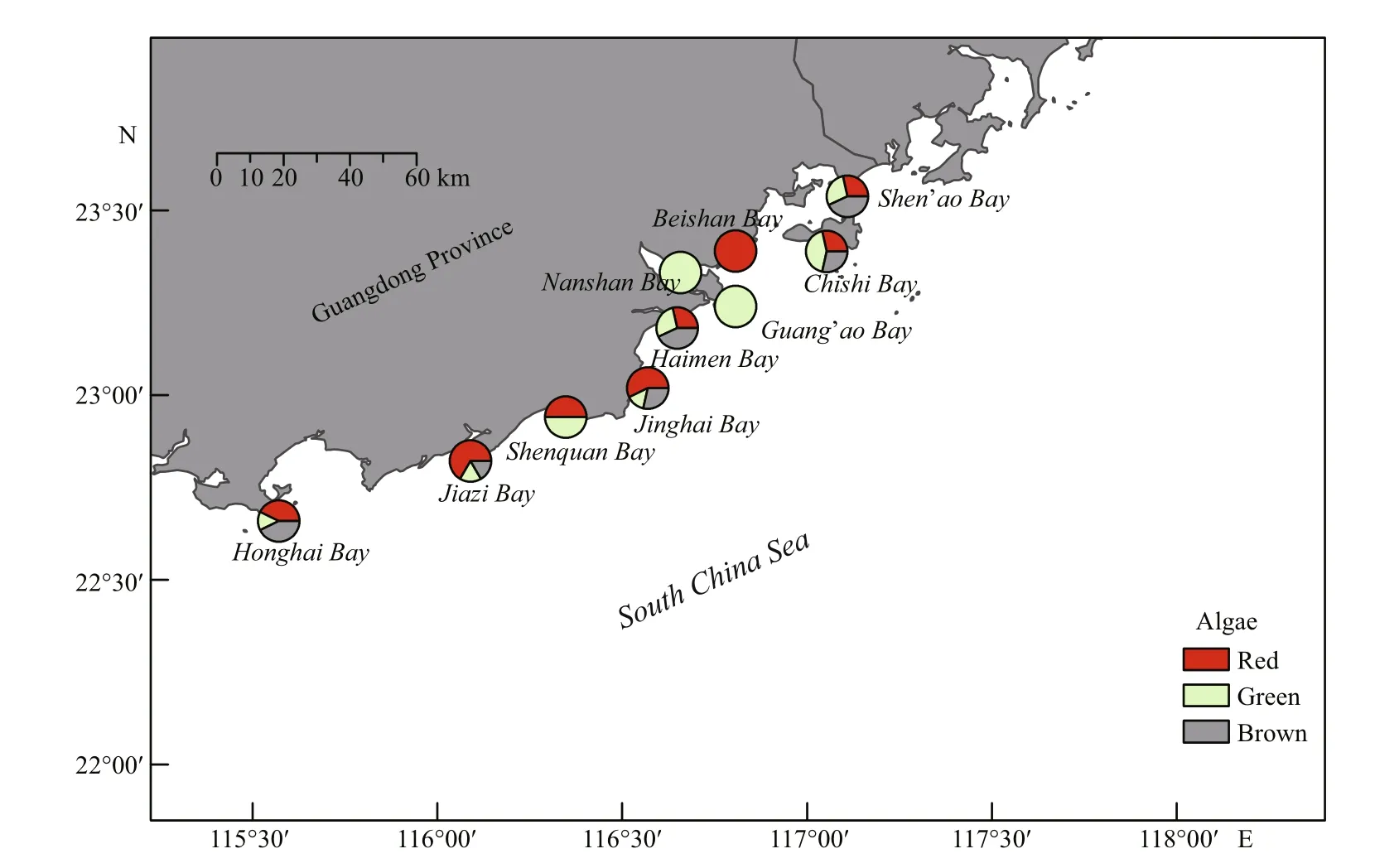
Fig.1 The survey section and distribution of algae collected in the intertidal zone of eastern Guangdong
2 MATERIAL AND METH OD
2.1 Chemicals
Ethanol, acetone, disodium hydrogen phosphate(Na2HPO4·12H2O) and sodium monobasic phosphate(NaH2PO4·2H2O) were purchased from Xilong Chemical Co., Ltd. (Guangzhou, China). Potassium hexacyanoferrate (K3Fe(CN)6), trichloroacetic acid,ferric chloride (FeCl3) and sodium chloride (NaCl)were purchased from the Fuchen Chemical reagents factory (Tianjing, China). Sodium carbonate anhydrous (Na2CO3) was purchased from Guangcheng Chemical reagents factory (Tianjing, China). Folin-Ciocalteau’s phenol reagent was purchased from Sigma-Aldrich Shanghai Trading Co., Ltd. (Shanghai,China). Gallic acid was purchased from Damao Chemical reagents factory (Tianjing, China).Gentamicin susceptibility test discs (OXOID,10 μg/disc) were purchased from Laboratory Biology Technology Co., Ltd. (Beijing, China). Mueller Hinton agar, tryptone, yeast extract and agar were purchased from Huankai Microbial Science &Technology Co., Ltd. (Guangzhou, China).
2.2 Field site and sample preparation
Seaweed samples were collected from ten different intertidal zones along the coastline of eastern Guangdong, China, in May and June 2015 (Fig.1).Species were identified taxonomically with the help of seaweed taxonomist Dr. TAN Huaqiang, Shantou University. The seaweed samples were washed in tap water to remove sand and epiphytes, dried in a vacuum-freeze dryer (Beijing Huaxing Technology Development Co., Ltd., Songyuan, Beijing, China),crushed into a fine powder (60–100 mesh) and stored in a refrigerator (-20℃).
The samples were extracted with ethanol (100%)and acetone (100%) in 1:20 (w:v) in a conical flask at room temperature for 24 h and 48 h, respectively.Extraction with different solvents was carried out separately. The extracts were then filtered through Whatman No. 1 filter papers. Sample preparation was replicated three times. Finally, algae extractions were concentrated by rotary evaporation (RE-52C, Yarong Instruments Co., Ltd., Zhengzhou, China) at 40℃and stored in a refrigerator (-20℃) for further study.The ethanol extracts were dissolved in 1 mg/mL ethanol for determination of total phenol contents and antioxidative assays. The acetone extracts were solubilized in 100 mg/mL acetone for use in antimicrobial assays.
2.3 Environmental factors
At each location the abiotic factors: water temperature, salinity, pH and DO were measured by YSI556MPS (YSI Inc., Ohio, USA). The nutrient content of the water samples: ammonia-nitrogen,nitrate-nitrogen, nitrite-nitrogen and phosphate were tested in the laboratory by referencing GB 17378.4-2007 (National Standards of the People’s Republic of China, 2008) and Schnetger and Lehners (2014).
2.4 Antioxidative activities
Antioxidative activity of algae extracts was measured by ferric reducing antioxidant power(FRAP) (Dorman et al., 2003). Each 1.0 mL of the sample solution was mixed with 1.0 mL 0.2 mol/L phosphate buffer (pH 6.6) and 2.5 mL 1% potassium hexacyanoferrate. The mixture was then incubated at 50℃ for 20 min and, once cooled, 1.5 mL of 10%trichloroacetic acid was added. The mixture was centrifuged for 10 min and then 2.0 mL of the upper layer was mixed with 2.0 mL distilled water and 0.5 mL 0.1% aqueous FeCl3. Absorbance of the final mixture was measured at 700 nm by Uv-vis spectrophotometry (Shanghai Spectrum Instruments Co., Ltd. Shanghai, China). The reducing power of algae extracts was presented as gallic acid equivalents(GAE) in milligrams of gallic acid per gram of dry extract. The calibration curve for gallic acid wasy=0.021 5x+0.010 7 (R2=0.996). Every sample was triplicated.
2.5 Determination of total phenolic content
Total phenolic content (TPC) was determined as described previously (Velioglu et al., 1998; Duan et al., 2006). One milliliter of each sample solution was mixed with 1.5 mL of deionized water and 0.5 mL of 0.1 mol/L Folin-Ciocalteu reagent. After 1 min,1.0 mL of 20% sodium carbonate was added. The controls contained all the reaction reagents except the sample. The mixture was mixed thoroughly and incubated at 37℃ for 30 min, and the absorbance was then measured at 750 nm. TPC was expressed in terms of mg GAE/dry extract. The gallic acid calibration curve wasy=0.004 0x+0.009 2 (R2=0.998).Every sample was triplicated.
2.6 Antimicrobial activities
Antimicrobial activity of algae extracts was measured by the diffusion method (Burkholder et al.,1960). Sterile discs, 5.5 mm in diameter, were prepared by pipetting 10 μL extract onto each disc,placing them on Mueller Hinton agar (pH 7.4±0.2)and incubated at 37℃ for 24 h. The width of the clear halo encircling each disc on the cultivated agar plates was measured as the inhibition results. Acetone(100%) without algal extract was used as a negative control and no antimicrobial activity was observed.The gentamicin susceptibility test discs were used as the positive control. The antimicrobial activity was classified from low active (+: diameter of inhibition <10 mm), moderately active (++: 10 mm ≤ diameter of inhibition < 15 mm) to highly active (+++: 15 mm ≤diameter of inhibition) and inactive (-: no/very hazy inhibition zone) (Val et al., 2001; Oumaskour et al.,2013). Ten bacteria strains were used for measuring the antimicrobial activity of each sample. All tests were at least triplicated. The strains used wereStaphylococcusaureusCMCC(B)26003 (Sa),BacilluscereusCMCC(B)63303 (Bc),B.subtilisCMCC(B)63501 (Bs),Aeromonashydrophila(Ah),Proteusmirabilis(Pm),VibriofluvialisATCC33810(Vf),V.mimicusATCC33653 (Vm),V.parahaemolyticusATCC17802 (Vp),V.vulnificusATCC27562 (Vv) andV.alginolyticusATCC33787(Val). The microorganisms were obtained from the Department of Biology, College of Science, Shantou University.
2.7 Statistical analysis
Seaweeds harvested from more than three intertidal zones were used for identifying the relevance between antioxidative/antimicrobial activities or total phenol content of algae extracts and each environmental parameter. This was analyzed by Pearson’s correlation test (Rvalues,P<0.05) with Origin 9.0 software(Originlab Corporation, Hampton, USA). The correlation between antioxidative activity and total phenol content of algae extracts was also calculated by Pearson’s correlation test (Rvalues,P<0.01). Oneway ANOVA was performed on the antioxidative/antimicrobial activities with GraphPad Prism 5(GraphPad Software, Inc., La Jolla, USA).
3 RESULT
The 26 seaweed species, representing 47 samples,were collected from ten intertidal zones (Table 1), and their distribution is shown in Fig.1. Abiotic factors in these zones were found at different levels: water temperatures, DO, salinity and pH were in the range 24.23–29.97℃, 4.59–8.03 mg/mL, 22.00–34.91 and 7.95–8.49, respectively. Ammonia-nitrogen, nitratenitrogen, nitrite-nitrogen and phosphate varied from 0.01 to 0.20 mg/L, from 0.04 to 0.47 mg/L, from 0.00 to 0.06 mg/L and from 0.02 to 0.08 mg/L, respectively.In comparison with other bays, Beishan Bay,Guang’ao Bay and Nanshan Bay were considered to be in poor condition in having low DO, low salinity or rich nutrition.

Table 1 Total phenol content, antioxidative and antimicrobial activity of seaweed species

Table 1 Continued

Fig.2 Differences in antioxidative activity of seaweed extract among high-, middle- and low-tide belts
3.1 Antioxidative activity
The FRAP of ethanol extracts of all collected seaweeds are summarized in Table 1. The data indicate that, except fromGrateloupiaturuturu, the majority of brown algae showed a higher capacity than most of the red or green algae. The brown algaPadina arborescensexhibited the greatest antioxidative activity (77.0±3.6 mg GAE/g of extract). The red algaPolysiphoniahainanensisalso showed high activity.
Ten intertidal zones were classified into three areas on the basis of region. Shen’ao Bay and Chishi Bay,parts of Nan’ao Island, are near marine fisheries and aquaculture. In contrast, Beishan Bay, Guang’ao Bay,Nanshan Bay and Haimen Bay, parts of Shantou city,lie at the mouth of the Rongjiang River where there is a higher concentration of inorganic nitrogen and chemical oxygen demand. There was little anthropogenic disturbance in bays near the city of Shanwei, including Shenquan Bay, Jinghai Bay, Jiazi Bay and Honghai Bay. All antioxidative data were divided according to the tidal belt position and area of seaweed (Fig.2). As the graphical analysis indicates,there was no significant difference among the high-,middle- or low-tide belts for all taxa in every area,and some species settled in the high- or middle-tide belts had even greater activity than those in the lowtide belt.
When considering seaweeds settled in different intertidal zones, it was found that most seaweeds could not stand the very poor condition of low DO,low salinity or rich nutrition. However, seaweeds normally had higher antioxidant activity when they survived in such poor conditions, just as 1 g of the ethanol extract ofCaloglossananaoensis(Beishan Bay) inhibited reducing activity equivalent to 18.6±0.9 mg gallic acid. InUlvaconglobata(Guang’ao Bay) it was equivalent to 13.9±1.0 mg gallic acid; and inU.fasciata(Nanshan Bay) it was equivalent to 8.7±0.1 mg gallic acid (Table 1). These were all higher than the majority of other similar species settled in mild conditions (Table 1). Salinity,DO and nutrition were correlated significantly with the reducing power ofU.fasciatain eight bays(Pearson’s correlation test;P<0.05;R=-0.914,R=-0.948,R=0.955, 0.938, 0.941 and 0.923 respectively;Table 2). Ammonium salts correlated significantly with the reducing power ofSargassumvachellianumin four bays (Pearson’s correlation test;P<0.05;R=0.959; Table 2); however, the correlation was not found inCaulaanthusokamurai,U.conglobataorPachydictyoncoriaceum(Table 2).
3.2 Total phenolic content
The TPCs of all collected seaweeds are presented in Table 1. Most of the brown algae had a higher total phenol content than most of the red or green algae.The ethanol extract of the brown algaPadina arborescens, which showed highest antioxidative activity, also contained the highest amount of phenolic compounds (102±3.6 mg GAE/g of extract). A high correlation between the antioxidative activity and the total phenol content was found among the 47 seaweed samples (R=0.949,P<0.01). This suggests that the phenolic compounds of seaweeds make a significant contribution to their antioxidant capacity. While comparing the total phenol content of seaweeds in different tidal belt positions and areas, no significant difference was found among the high-, middle- or low-tide belts of green and brown algae in every area(Fig.3). In Nan’ao and Shantou, red algae settled in the high- or middle-tide belts had a higher total phenol content than those in the low-tide belt. In comparison,collections made near the city of Shanwei showed that red algae settled in the low-tide belt had a higher total phenol content than those in the middle-tide belt.
Seaweeds that survived in poor conditions seemed to attain a higher total phenol content more easily than other similar varieties settled in mild conditions; for exampleCaloglossananaoensis(Beishan Bay,33.4±1.4 mg GAE/g of extract),U.conglobata(Guang’ao Bay, 35.6±1.5 mg GAE/g of extract) andU.fasciata(Nanshan Bay, 20.6±0.5 mg GAE/g of extract) (Table 1). The correlation between environmental parameters and total phenol content of ethanol extract of seaweeds is presented in Table 3.Salinity and phosphate were correlated negatively with total phenol content ofCaulaanthusokamuraiin three bays (Pearson’s correlation test;P<0.05;R=-0.999,R=-0.998 respectively; Table 3). DO and nutrition were correlated significantly with total phenol content ofU.fasciatain eight bays (Pearson’s correlation test;P<0.05;R=-0.766,R=0.862, 0.893,0.839 and 0.874 respectively; Table 3). Temperature,ammonia-nitrogen and phosphate were correlated significantly with total phenol content ofS.vachellianumin four bays (Pearson’s correlation test;P<0.05;R=-0.973,R=0.958,R=0.968,respectively; Table 3). No association was found inU.conglobataandPachydictyoncoriaceum(Table 3).
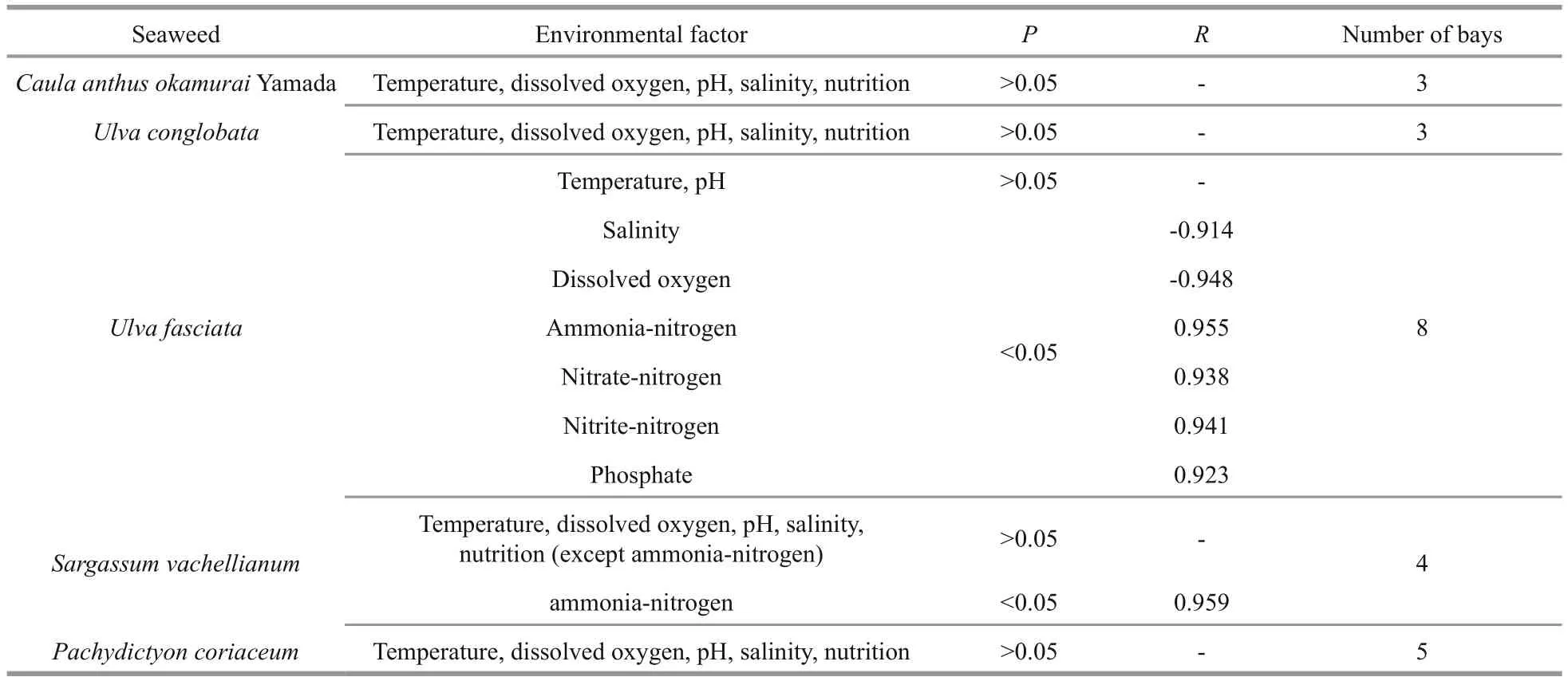
Table 2 Correlation analysis between environmental parameters and antioxidative capacity of ethanol seaweed extract

Fig.3 Differences in total phenol content of seaweed extract among high-, middle- and low-tide belts

Fig.4 Differences in antibacterial activity of seaweed extract among high- (H), middle- (M) and low- (L) tide belts
3.3 Antimicrobial activity
Of all species, the highest percentage of active taxa was discovered in Chlorophyta (100%), followed by Phaeophyta (86%) and Rhodophyta (47%) (Table 1),which is similar to previous reports (Salvador et al., 2007; Ibtissam et al., 2009). OnlyPolysiphonia hainanensisshowed activity against nine microorganisms, whileS.vachellianumandPachydictyoncoriaceum,ChaetomorphaantenninaandCodiumcylindricumshowed high activity against Gram-positive bacteria (Sa, Bc or Bs). The great majority of algae settled in the low-tide belt exhibited higher activity than those settled in the middle- or high-tide belts (Fig.3).

Table 3 Correlation analysis between environmental parameters and total phenol content of seaweed ethanol extract

Table 4 Correlation between environmental factors and antimicrobial activity of seaweed acetone extract
We found that antibacterial activity in seaweeds surviving in very poor conditions of low DO, low salinity or rich nutrition was not significantly different from those settled in moderate conditions. When comparing congener seaweeds inhabiting various tidal zones, perfect sensitive bacteria to their extracts were considered, shown on Table 4.Caulaanthusokamurai,S.vachellianum,P.coriaceum,U.conglobataandU.fasciataappeared in at least three intertidal zones, so they were used in the analysis. No correlation was discovered between the environmental parameters and the antimicrobial activity of extracts ofC.anthusokamurai,U.conglobataorU.fasciata(Pearson’s correlation test;P>0.05; Table 4). DO was significantly correlated with the antimicrobial activity of the acetone extract ofS.vachellianuminStaphylococcusaureusandVibrioparahaemolyticus(Pearson’s correlation test;P<0.05;R=0.997 and 0.963, respectively; Table 4).Ammonia-nitrogen was negatively correlated with the antibacterial activity of acetone extract ofP.coriaceuminBacilluscereus(Pearson’s correlation test;P<0.05;R=-0.921; Table 4). Ammonia-nitrogen,nitrite-nitrogen and phosphate were also negatively correlated with the antibacterial activity of the acetone extract ofP.coriaceuminStaphylococcusaureus(Pearson’s correlation test;P<0.05;R=-0.948, -0.921 and -0.936, respectively; Table 4).
4 DISCUSSION
Ethanol extract from Phaeophyta revealed higher total phenol content and antioxidant activity than extracts from Chlorophyta and Rhodophyta. A positive association was found between the total phenol content and antioxidative activity in the FRAP assay system, in agreement with previous studies(Duan et al., 2006; Zhang et al., 2007). This means the higher phenol content of an algae extract may contribute to a higher antioxidative activity. Acetone extract from Phaeophyta and Chlorophyta exhibited better antibacterial activity than extract from Rhodophyta. This can be ascribed to the various levels and composition of secondary metabolites in the three kinds of seaweed (Vijayabaskar et al., 2012;Pérez et al., 2016). In all species collected, the brown algaPadinaarborescensexhibited the greatest antioxidative activity and highest total phenol content.The red algaPolysiphoniahainanensisshowed the broadest antibacterial spectrum; and the brown algaeSargassumvachellianumandPachydictyon coriaceum, as well as the green algaeChaetomorpha antenninaandCodiumcylindricum, showed high activity against Gram-positive bacteria (Sa, Bc or Bs).
Previous studies have reported on the temporal variation in secondary metabolites and antioxidative activity of seaweeds (Fariman et al., 2016), and their changeable components when adapting to ambient stress (Stengel et al., 2011). However, there is still very little information on the association between antioxidative or antimicrobial activities of seaweeds and the differences in their habitats.
This study revealed two phenomena of particular interest with regard to the effects of abiotic factors caused by emersion of intertidal seaweeds. The first is that the antioxidant capacity and phenol content of seaweeds settled in high- or middle-tide belts were similar to, or even higher than, those settled in lowtide belts. This was not related to anthropogenic disturbance, although human activities affected the species and quantities of seaweeds in intertidal regions. Previous study has found that the phenol content of seaweeds settled in a high tidal zone(Pelvetiacanaliculata,Ascophyllumnodosum) had significant correlations with measured air temperature,which was not found in seaweed settled in a low tidal zone (Bifurcariabifurcata) (Connan et al., 2007).High UV-B radiation always leads to a fast accumulation of phenols and pigments in seaweeds,and this is related to their strong antioxidant protection(Hupel et al., 2011; Cruces et al., 2012; Balboa et al.,2013; Zubia et al., 2014; Fariman et al., 2016).Nutrient availability may also result in variations in phenol concentrations of some seaweeds (Yates and Peckol, 1993). As has been noted, seaweeds living in high-tide belts were exposed to more extreme stress,including high irradiance levels, desiccation, less nutrition, air temperature and salinity fluctuations,compared to those living in low-tide belts (Connan et al., 2007). These stresses must result in the first phenomena mentioned above. The second is that seaweeds in low intertidal zones were more likely to have higher antimicrobial capacity than seaweeds in other zones. Although phenol compounds of seaweeds possess antimicrobial activity, a higher total phenol content in seaweeds cannot lead to higher antimicrobial activity. In addition to phenol compounds, other secondary metabolites in extracts, which also present antimicrobial activities, adapted to abiotic stress in different ways. Various hydrophilic compounds can be found in acetone extracts of seaweeds, such as polysaccharose (Balboa et al., 2013), lipids, volatiles(Plaza et al., 2010; M?ki-Arvela et al., 2014) and pigments (Si et al., 2011). Polysaccharide contents reduced when faced with salt stress (Aghaleh et al.,2009). The content and composition of lipids in algae changed as water temperature increased (Sanina et al., 2008), and species of seaweeds in deeper water accumulated greater quantities of lipids (Gerasimenko et al., 2012). The lack of nitrogen nutrition made polyunsaturated fatty acid decrease and also promoted saturated fatty acid and monounsaturated fatty acid(Pinchetti et al., 1998). The varying composition and content of these components in seaweeds help to explain the antimicrobial activity.
This study also found that seaweeds had higher antioxidative activity and total phenol content when they survived in the very poor conditions of coastal waters with low DO, low salinity or rich nutrition.This might not apply to antibacterial activity. These results partially overlapped those of Ksouri (Ksouri et al., 2008), who suggested that extreme climate conditions, in terms of high radiation, salinity and low rainfall, were likely related to the better antioxidant potentialities ofCakilecaritima(Jerca Seashore,Tunisian), than toC.caritima(Tanarka, Tunisian).Among the seaweeds collected,U.fasciata(the most widely distributed) showed strong relevance between its antioxidative capacity and nutrition (positively)and DO or salinity (negatively). The total phenol content ofU.fasciatahas a positive correlation with nutrition and a negative correlation with DO. Seawater salinity may primarily be influenced by tides,evaporation and rainfall in general, but disturbance by fresh water from the estuary should also be considered responsible for the salinity differences among the ten bays. In particular, Nanshan Bay, near the Rongjiang River estuary, had low salinity seawater. Living under moderate hyposaline conditions leadsU.fasciatato polyamine and malondialdehyde accumulation (Lee and Chen, 1998; Chen and Zou, 2015), to increasing availability of antioxidants and the activities of antioxidant enzymes (Lu et al., 2006). These results partially explain the negative relevance between antioxidative activity ofU.fasciataand seawater salinity levels from 27 to 35. Nutrient levels in coastal bays were always affected by anthropogenic activity,such as pollution from the estuary and marine aquaculture. It had been proven that the nitrogen and phosphorus levels were closely related to the nearby aquafarm in Shen’ao Bay, Nan’ao Island; further, the nutrition level was lower from March to October than in other months due to the mass-production of phytoplankton and seaweeds (Zheng, 2009). Another resource of nitrogen and phosphorus on the coastline of eastern Guangdong is the rich nutrients of deep ocean water stemming from off shore upwelling activities (Zeng, 1986). This should be considered the key factor influencing the seawater nutrient content in bays with little anthropogenic interference, such as Honghai Bay and Jinghai Bay. Both nitrogenenrichment and nitrogen-depletion stress can promote differences in biochemical composition ofU.fasciata,including fatty acids (Pinchetti et al., 1998) and amino acids (Taylor et al., 2006). The interaction of temperature and nitrogen also affected phenolic compounds inU.fasciata(Figueroa et al., 2014).These components, especially the phenolic component, might all help in increasing antioxidant capacity whenU.fasciatalives in nutrient-enriched seawater. The present study also suggested that the antioxidative activity and total phenol content ofU.fasciatawas negatively related to concentrations of DO. Surprisingly, however, no reports revealed a relationship between the biochemical composition ofU.fasciataand DO in seawater. The fluctuation of DO in seawater can be ascribed to other abiotic factors. For instance, waves infiltrating through groundwater leads to an increase in DO (Bozkurt and Kabdasli, 2013), and nitrogen content has a negative effect on the concentration of DO in surface water during the summer (Luo et al., 2005). Rainfall,temperature and salinity also contributed to the content of DO in seawater. These abiotic factors have been proven to be the key stressors affecting the antioxidant activity and phenolic compounds ofU.fasciata, which helps explain the aforementioned result. The present study also revealed that the antioxidative capacity ofS.vachellianumis positively affected by nutrition. Additionally, the total phenol content ofS.vachellianumhas a significant relationship with temperature (negative) and nutrition(positive). Seasonal variation in biomass, and in nutritional and chemical composition such as in lipids and carbohydrates, have been evaluated inSargassum(Marinho-Soriano et al., 2006; Kumar et al., 2015;Hoang et al., 2016). Few reports, however, have uncovered the relationship between abiotic factors and antioxidant activity or phenol content ofSargassum. The total phenol content ofCaulaanthus okamuraiis negatively related to salinity and phosphate. The antibacterial activity ofS.vachellianumorPachydictyoncoriaceumis highly relevant to DO (positive) or nutrition (negative) in several bacteria. However, this relevance could not be applied to all seaweeds. Similar situations appeared in some terrestrial plants, but no significant regularity can be found in how environmental factors affect the antibacterial activity (Kachoie et al., 2013; Riahi et al., 2015).
5 CONCLUSION
Among all the 26 species,Padinaarborescensexhibited the greatest antioxidative activity(77.0±3.6 mg GAE/g of extract) and highest phenol content (102±3.6 mg GAE/g of extract).Chaetomorphaantennina,Codiumcylindricum,S.vachellianumandPachydictyoncoriaceumshowed high activity against Gram-positive bacteria (Sa, Bc or Bs). These species should be further exploited because of their excellent performance in accumulating a high total phenol content and for their bioactivity. In comparison with terrestrial plants, seaweeds used in previous relevant studies have almost entirely been collected in the wild, where the environmental factors are complex and interactive. The current research has demonstrated, for the first time, the effect of abiotic factors caused by emersion on antioxidative and antimicrobial activities of intertidal seaweeds.Seaweeds collected from low-tide belts have higher antibacterial activity than—though similar antioxidative activity to—those collected from highor middle-tide belts. Coastal waters with specific features such as low DO, low salinity or rich nutrition have a positive impact on the antioxidative activity of seaweeds, especiallyU.fasciataandS.vachellianum.Antibacterial activity ofU.fasciata,S.vachellianumorP.coriaceumis highly relevant to dissolved oxygen(positive) or nutrition (negative) in several bacteria.These discoveries may permit the selective harvesting of seaweeds with high specific activity.
6 DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.
7 ACKNOWLEDGMENT
We are grateful to ZHANG Quanliang, WU Yinglin, YU Fei, and HE Gaoyang and several members of Marine Biology Institute in Shantou University for their help in collecting samples. We thank Elaine Monaghan, BSc(Econ), from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac),for editing the English text of a draft of this manuscript.
 Journal of Oceanology and Limnology2018年6期
Journal of Oceanology and Limnology2018年6期
- Journal of Oceanology and Limnology的其它文章
- Neuroanatomy and morphological diversity of brain cells from adult crayfish Cherax quadricarinatus*
- Effects of feeding time on complement component C7 expression in Pelteobagrus vachellii subject to bacterial challenge*
- Cryopreservation of strip spawned sperm using programmable freezing technique in the blue mussel Mytilus galloprovincialis*
- Pf- D mrt4, a potential factor in sexual development in the pearl oyster Pinctada f ucata*
- Specific genetic variation in two non-motile substrains of the model cyanobacterium Synechocystis sp. PCC 6803*
- Functional characterization of a Δ6 fatty acid desaturase gene and its 5′-upstream region cloned from the arachidonic acidrich microalga Myrmecia incisa Reisigl (Chlorophyta)*
