Carbon distribution strategy of Aurelia coerulea polyps in the strobilation process in relation to temperature and food supply*
WANG Nan (王楠) , LI Chaolun (李超倫) , , WANG Yantao (王彥濤) ,FENG Song (馮頌)
1 Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences,Qingdao 266071, China
2 Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology,Qingdao 266237, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Abstract Mass occurrences of moon jellyfish have been observed in coastal waters. Strobilation directly determines the initial population size of adult jellyfish, but energy distribution during the strobilation process is not well understood. In this study, strobilation was induced in polyp of Aurelia coerulea by elevating temperature. The different stages in the strobilation process, including polyp budding, strobilation and body growth, were investigated at six temperature levels (8, 10, 13, 15, 17 and 19°C) and five food supply levels(0, 30, 60, 100 and 150 μg C/L). The results showed that the duration of strobilation preparation stage (SP)remarkably decreased with increasing temperature. Food level positively affected the production of buds and ephyrae and the body growth of parent polyps. Of the six temperatures tested, 13°C was optimal for strobilation. At 13°C, strobilation activity was enhanced, and this treatment resulted in the greatest energy distribution, highest ephyrae production and longest duration of strobilation stage (SS). Polyps tended to allocate 6.58%–20.49% carbon to buds with sufficient food supply regardless of temperature. The body growth of parent polyps was highest at lower temperatures and higher food levels. This study is the first to provide information on carbon-based energy distribution strategy in the polyp strobilation process. We concluded that budding reproduction is a lower-risk strategy for A. coerulea polyps to increase populations.Even during strobilation season, polyps prioritize budding, but at the optimal strobilation temperature,polyps utilize a portion of the energy stored for budding to release ephyrae. The body carbon content of parent polyps may be considered as strategic energy reserves, which could help to support budding activities and strobilation during harsh conditions.
Keyword: Aurelia coerulea; temperature; food supply; carbon distribution strategy; strobilation
1 INTRODUCTION
Increasing jellyfish population and bloom frequency are worldwide concern in the recent decades (Condon et al., 2013). In the East Asia,outbreaks of large jellyfishes are considered serious ecological disasters that negatively impact the marine ecosystem, fisheries, coastal facilities and tourism,resulting in severe environmental and economic problems (Lucas et al., 2014; Sun et al., 2015b).Studies suggest that jellyfish blooms are attributed to the anthropogenic coastal environmental changes such as climate change, overfishing, eutrophication,development of aquaculture, and habitat modification(Bakun and Weeks, 2006; Purcell et al., 2007; Lo et al., 2008; Richardson et al., 2009).
Moon jellyfish in the genusAureliaare the most common species of large jellyfishes and have a global distribution ranging between 70°N to 40°S (M?ller,1980; Lucas, 1996). Because of their remarkable adaptability to a wide range of environment factors,Aureliaspp. have caused problematic blooms in many coastal embayments, fjords and estuaries. Outbreaks of these species have been frequently reported in the East Asia seas, including Jiaozhou Bay, Liaodong Bay, Tokyo Bay and Shiwa Lake (Toyokawa et al.,2000; Wang et al., 2012; Hong et al., 2013; Wang and Sun, 2015). Massive coastal occurrences ofAureliaspp. can clog the cooling water intakes of coastal power and nuclear plants, damaging coastal enterprises and the maritime economy (Purcell et al.,2007; Richardson et al., 2009).
Aureliaspp. have a metagenic life cycle comprised of a benthic asexual polyp stage and a planktonic,sexual medusa stage. The perennial polyps can asexually bud new individual. Podocysts are another means of asexual reproduction and produced in response to food shortage in the harsh environments.When the environment improves, the excystment of podocysts contributes to the increase of polyp population. The polyps form transverse constrictions and then liberate numerous ephyrae. This process is called strobilation, and it plays a vital role in determining the population size of the later medusa stage. Previous studies have suggested that the strobilation process can be affected by abiotic and biotic factors, including temperature (Pascual et al., 2015; Soko?owski et al.,2016), salinity (Purcell et al., 2009), pH (Winans and Purcell, 2010), light (Liu et al., 2009), DO (Ishii et al.,2008) and food level (Wang et al., 2015a, b). To better predict the scale and tendency of jellyfish blooms, it is essential to understand the details of energy distribution through budding reproduction, ephyra production and population growth at the polyp population level. In these studies, the measurement units for recorded indicators were not unified, thus reducing data comparability. For instance, the common unit for buds,ephyrae and podocysts is the individual, while the unit for somatic growth is length (μm, mm, cm, etc.);comparison among these variables is thus difficult.Moreover, even though the unit of measurement for buds, ephyrae and podocysts is uniform to be wet or dry weight, they differ in water content and biochemical components, making the comparison less precise. The previous studies on bioenergetics mainly focused on respiration and ingestion rates of planulae larvae,polyps, ephyra and medusae (Schneider and Weisse,1985; B?mstedt et al., 1999; Hansson et al., 2005; Uye and Shimauchi, 2005; Ikeda et al., 2017). Little is known about energy distribution between asexual reproduction (budding reproduction and strobilation)and body growth of adult polyps. This knowledge is essential for illuminating the processes and mechanisms associated with jellyfish outbreaks.
The present study evaluated the effects of temperature and food supply onAureliacoeruleapolyp population proliferation, strobilation (both the different stages of the process and ephyra production)and body growth of parent polyps at the polyp population level. Carbon distribution among budding reproduction, ephyra production and body growth under each condition was also quantified. The aim of this study are to better understand the effects of environmental factors on carbon distribution in polyps during strobilation season, and to provide practical data for modeling research.
2 MATERIAL AND METHOD
2.1 Sources of A. coerulea polyps
The sessile polyps ofA.coeruleaused in this experiment were from the stock culture population reared in the laboratory at the Institute of Oceanology,Chinese Academy of Sciences, Qingdao. The polyps were derived from planulae kept by mature medusae captured in Jiaozhou Bay in June 2014. The stock polyps were cultivated in the 30 L aquarium tank at 20°C in sand-filtered seawater (30 cm× 50 cm corrugated plates, natural light, salinity 30–31, feed with adequateArtemianauplii every day).
Water quality parameters, zooplankton abundance andA.coeruleaabundance in Jiaozhou Bay were shown in Fig.1 (Wan and Zhang, 2012; Wang and Sun, 2015). The mean surface water temperature varied between 4 and 27.4°C (Fig.1). Total zooplankton(<500 μm) was high from May to July, and the highest of 486.9 ind./ m3was observed in May (Fig.1). In 2009,Aureliaepyhra was observed from April to June with its abundance increasing from 0.1 to 2.9 ind./m3,while medusa was captured only in July (Fig.1).
2.2 Controlled laboratory experimental design
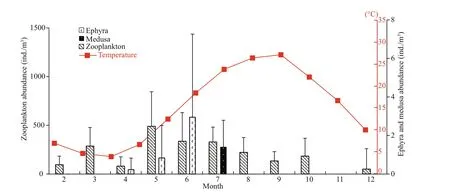
Fig.1 Temperature, zooplankton abundance and A. coerulea ephyra and medusa abundance in Jiaozhou Bay
In this experiment, the strobilation process was induced by directly elevating the culture temperature to target temperature regimes. For warming induction,the initial test polyps were thus acclimated in a 25-L container with sand-filtered seawater outside in winter before the experiment began. During the acclimation,no temperature control was taken, and the culture seawater temperature changed with the external seawater temperature increasing from 4 to 8°C.
After 50-day acclimation at the low temperature,the corrugated plate with polyps (mean calyx diameter: 0.88±0.08 mm) was cut into 2 cm × 3 cm pieces. Budded polyps, podocysts, and impurities on each section were removed carefully with a dissecting needle, leaving 5 healthy polyps. Each section was placed in the bottom of an individual 200 mL glass beaker containing 200 mL of filtered seawater(0.45 μm mixed membrane).
Two orthogonal treatment sets were established.The temperature was maintained at six different levels(8, 10, 13, 15, 17 and 19°C) by incubator (Jiangnan SPX), and the food supply was maintained at five levels (0, 6, 12, 20 and 30 newly hatchedArtemianauplii). The mean carbon weight ofArtemianauplius individuals was 1.00 μg C (measured with ELEMENTAR-EL), thus the prey concentrations represented 0, 30, 60, 100 and 150 μg C/L. Every temperature and food supply orthogonal treatment consisted of three replicates, or 15 polyps in total. The entire experiment lasted 86 d.
The water in each beaker was replaced daily with seawater with the same temperature, and the numbers of buds, released ephyrae, and unconsumedArtemianauplii were recorded. To estimate body growth, the calyx diameters of polyps were measured weekly using an ocular micrometer under a dissecting microscope. The average long and short calyx diameters of the polyp were used as its calyx diameter.The calyx diameters of the 5 polyps were averaged as the representative values of polyp size in each group.Newly produced buds and ephyrae were removed using dissecting needle and pipette. Observations and measurements of each beaker took less than 5 min.
The strobilation preparation stage (SP) was defined as the period from the start of the experiment to the liberation of the first ephyra. The strobilation stage(SS) was defined as the period from the first to last liberation of ephyra.
2.3 Carbon content conversion
Conversion factors and formulae in this section were applied to convert buds, ephyrae and parent polyps growth to carbon weight equivalents.
2.3.1 The conversion factor of buds
In the experiment, each of the collected buds was stored separately at -20°C. The buds were assigned into five groups randomly, with 50 buds in each group.The samples were freeze-dried, weighed, and kept in a desiccator until further analysis. For the analysis of C contents, samples were coated by tin boats and measured using an ELEMENTAR- EL elemental analyzer (Elementar Company). The factor used to convert the number of buds to carbon weight equivalents was 3.35±0.18 μg C/polyp.
2.3.2 The conversion factor of ephyrae
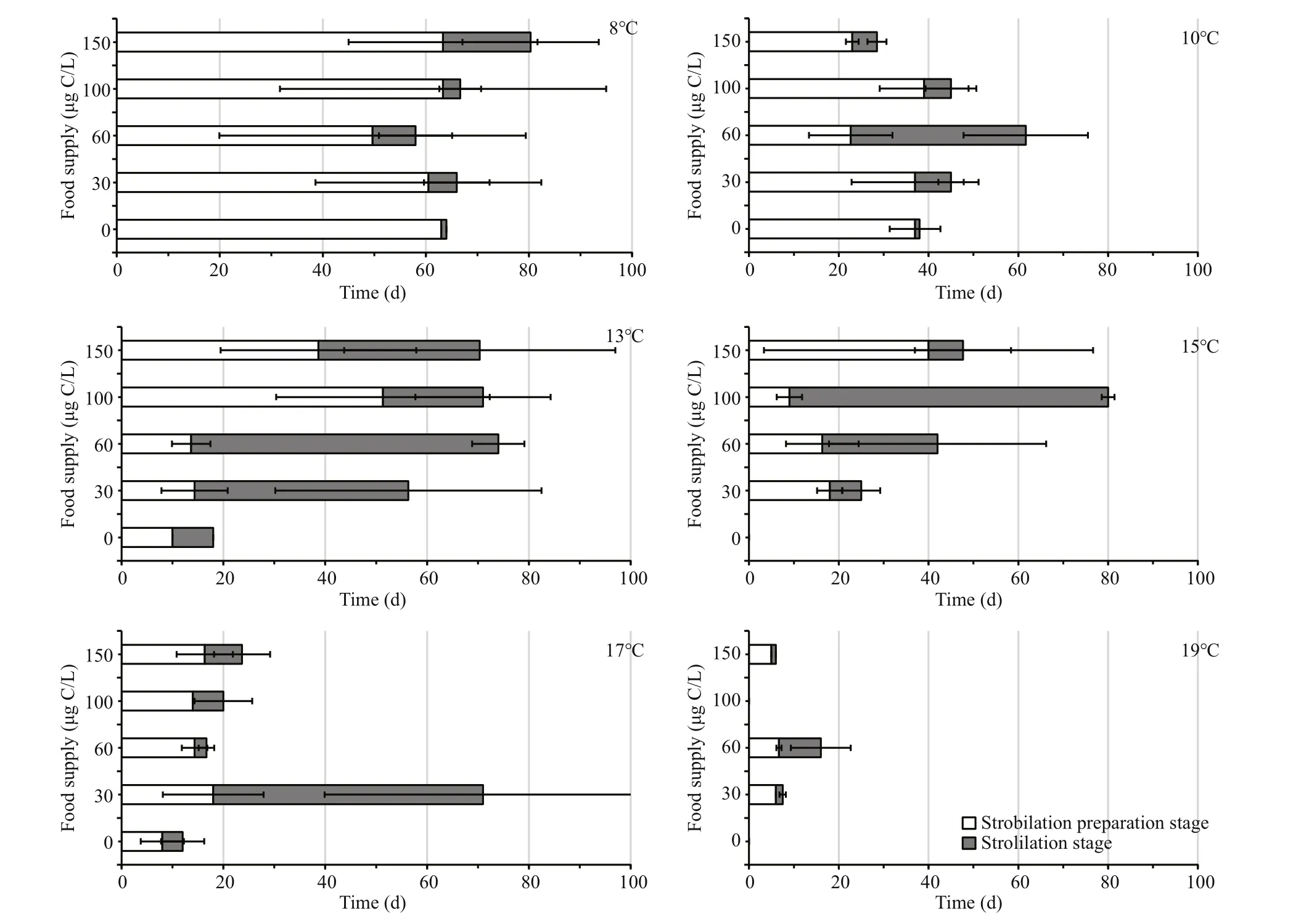
Fig.2 Mean duration of the strobilation preparation stage (SP) and strobilation stage (SS) of A. coerulea polyps under each combination of six temperatures and five food supply levels
In the experiment, ephyra production did not reach the detection threshold of the elemental analyzer.Hence, more than 1 000 polyps were induced by reducing the cultivated temperature to 13°C to liberate adequate numbers of ephyrae. The newly liberated ephyrae were collected and assigned into five groups randomly, with 100 ephyrae in each group. The carbon weight of each group was measured using the ELEMENTAR-EL elemental analyzer (sample preparation process with 2.3.1), and the factor used to convert the number of ephyrae to carbon weight equivalents was 4.58±0.23 μg C/ephyra.
2.3.3 The conversion formula for growth
Fifty similar sized polyps were collected in the same group. The calyx diameter of the group (D, mm)was calculated as the average calyx diameter of one polyp. The total carbon weight of the fifty polyps in this group was measured with the elemental analyzer,and carbon weight of the group (C, μg) was calculated as the average carbon weight of one polyp. To obtain the relationship between polyp calyx diameter and carbon content, we established ten groups of different sizes and measured their carbon weights. Power function fitting was performed to analyze the relationship betweenDandCusing the following conversion formula:C=15.300D1.7581(R2=0.753,P<0.05).
2.4 Statistical analysis
The combined effects of temperature and food supply on bud productions, ephyra liberation and body growth of parent polyps were tested by two-way ANOVA after testing data for normality and equal variance. If the overall ANOVA results were significant, Tukey's HSD pair-wise comparisons were performed to evaluate differences between experimental combinations. All statistical tests were performed using SPSS 16.0.
3 RESULT
3.1 Strobilation duration
During the 86-day experiment, polyps strobilated under most combinations of temperature and food level, except for those in the 15°C-0 μg C/L, 19°C-0 μg C/L and 19°C-100 μg C/L (Fig.2).

Table 1 Statistical analysis results
The statistics results showed that there was a significant effect of temperature on SP and SS duration; however, the duration of SP and SS did not differ significantly among the different food levels(Table 1). The SP stage lasted longest (59.9±5.9 d) in the 8°C groups. SP shortened with increasing temperature (Fig.3a). In the 19°C groups, it took less than 6.7 d for polyps to release the first ephyra. The maximum average duration of the SS stage was 32.3±20.2 d at 13°C, and values then decreased at higher temperatures (Fig.3b). The minimum average duration of the SS stage was 4.7±3.9 d at 19°C.
3.2 Change in carbon weight
3.2.1 Budding reproduction
The carbon weights of total buds in each temperature and food level combination are illustrated in Fig.4. At the level of population consisted of 5 polyps, the highest production was 383.00 μg C recorded at 15°C with a food supply level of 150 μg C/L, while the lowest production was 1.1 μg C/population at 8°C with 0 μg C/L.
Two-way ANOVA results suggested that temperature significantly affected budding production(P<0.001). Bud production did not increase with increasing temperature (Fig.4). The Tukey’s HSD pairwise comparisons showed that bud productions showed no significant difference (P<0.001) among 8,13 and 19°C, lower than those at 10, 15 and 17°C.

Fig.3 Duration of SP (a) and SS (b) at six temperatures
Food condition significantly affected budding production (P<0.001). The pairwise comparisons showed that budding reproduction increased significantly with increasing food supply. Over the course of the experiment, the production of buds was highest at a food level of 150 μg C/L and lowest at 0 μg C/L for all temperatures.

Fig.4 The average carbon weight of total buds produced under each combination of 6 temperatures and 5 food levels during the 86-day experiment

Fig.5 The average carbon weight of total ephyrae liberated under each combination of 6 temperatures and 5 food levels during the 86-day experiment
3.2.2 Ephyra production
No strobilation occurred in the 15°C-0 μg C/L,19°C-0 μg C/L and 19°C-100 μg C/L groups (Fig.5).Ephyrae release was highest under the combination of 13°C and 150 μg C/L (total: 102.30 μg C/population),followed by the combination of 13°C and 100 μg C/L(68.70 μg C/population). Excluding the three 0-ephyra combinations, the lowest production was 1.53 μg C/population at 8°C in 0 μg C/L.
Two-way ANOVA results suggested that temperature significantly affected ephyrae production (P<0.001).The Tukey’s HSD pairwise comparisons showed that at 13°C, ephyrae production was significantly higher than all other temperatures, while the other five temperature groups did not significantly differ.
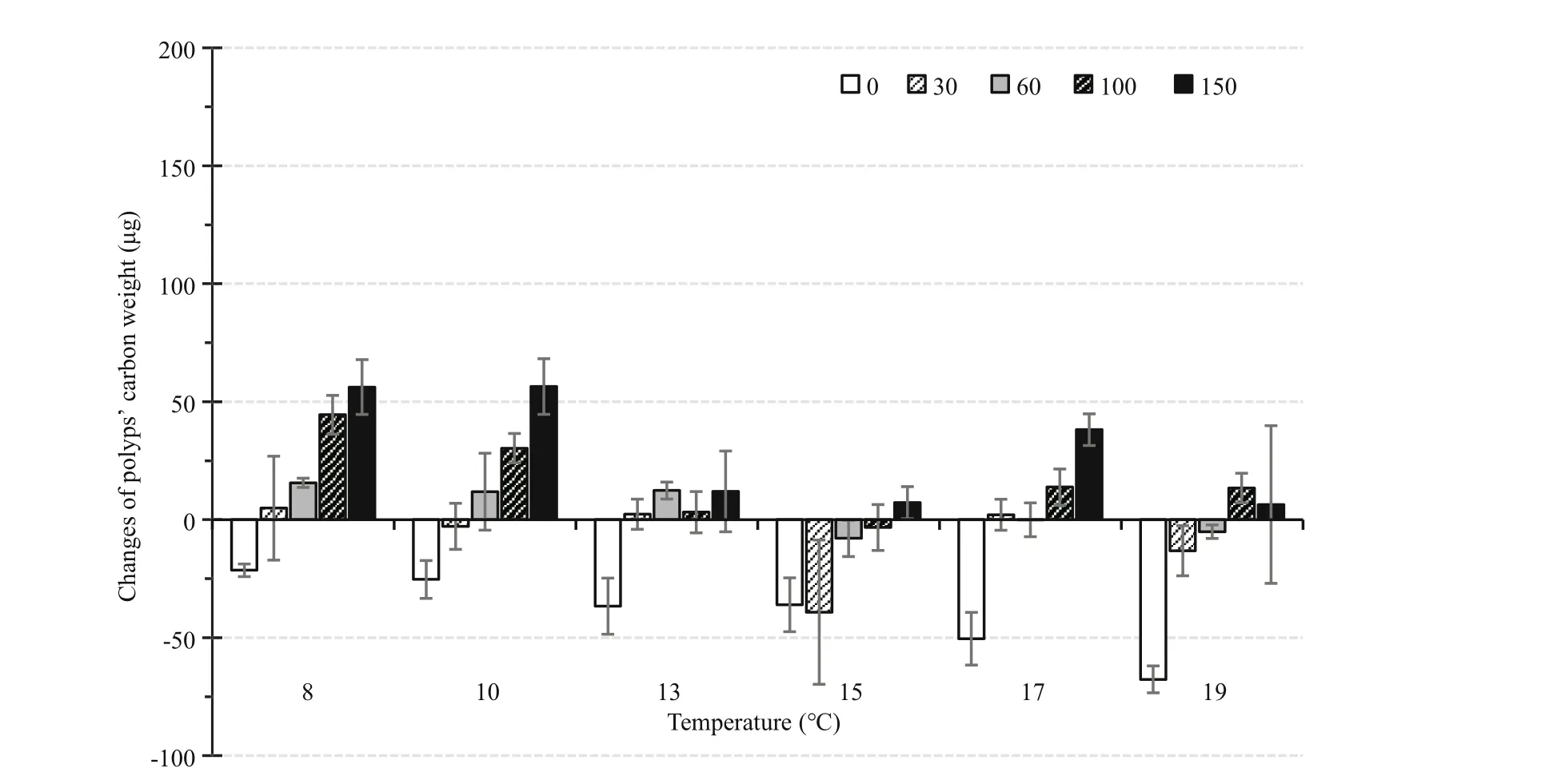
Fig.6 Changes in body carbon weight of parent polyps under each combination of 6 temperatures and 5 food levels during the 86-day experiment
Food conditions also had significant effects on the carbon weight of total ephyrae (P<0.001). The Tukey's HSD pairwise comparisons showed that ephyrae production in the 0 μg C/L groups was significantly lower than the other groups, and production in the 150 μg C/L group was significantly higher than the other groups. The 30, 60 and 100 μg C/L groups did not significantly differ.
3.2.3 Polyp somatic growth
When food was not sufficient, the change of body carbon weight was negative (Fig.6). After 86 d,somatic growth of the parent polyps was highest(56.44±11.80 μg C) in the combination of 10°C and 150 μg C/L, and lowest (-67.67±5.71 μg C) in the 19°C and 0 μg C/L group.
The two-way ANOVA showed significant effects of temperature on the change in body carbon weight(Table 1); however, the change of body carbon weight did not differ significantly at 8 and 10°C (Tukey’s HSD pair-wise comparisons). The growth of parent polyps generally decreased with increasing temperature.
Food supply also significantly affected the growth of parent polyps (P<0.001). At each temperature, the change of body carbon weight was generally correlated with increasing food supply. However,13°C-100 μg C/L, 17°C-60 μg C/L and 19°C-150 μg C/L did not follow this trend.
3.2.4 Carbon allocation
The qualitative data of the ratios of buds, ephyrae,and growth carbon weight in the total carbon source were showed in Table 2. The total carbon source comprised by two parts: the carbon intake and the initial body carbon weight. When food supply was higher than 0 μg C/L, the ratio of buds carbon weight was the highest in all temperature and food supply combinations, with the percentage of 6.58%–20.49%in the total carbon source. The ratios of ephyrae carbon weight were highest at 13 °C except for those in 0 μg C/L groups, with percentage of 4.20%–6.69%in the total carbon source. Lower temperature and higher food supply increased the ratio of polyps growth carbon weigh in the total carbon source. In the 19°C-0 μg C/L group, polyps consumed the highest initial body carbon storage (95.78%) with the investment rate to budding production was 11.06%.When food supply was 0 μg C/L, the total carbon source mainly came from the polyps initial body carbon storage, leading to the negative growth of polyps. And the initial carbon storage was distributed to reproduction.
3.3 Cumulative carbon weight of budding reproduction, ephyrae production and parent polyps growth
The cumulative carbon weights of buds and ephyrae and somatic growth of parent polyps were compared to describe the changing processes and to quantify the carbon distribution among them (Fig.7).

Fig.7 Changes in the cumulative carbon weight of buds and ephyrae and growth of parent polyps in each combination of 6 temperatures and 5 food levels during the 86-day experiment
At 8°C, when food supply was low (0 and 30 μg C/L), the carbon weight of buds, ephyra and parent polyps growth were lower, while in better food conditions (≥60 μg C/L), 137.35–198.77 μg carbon was allocated to new buds, and less to the growth of parent polyps (15.64–56.23 μg C) and strobilation(24.43–50.38 μg C). During the SS stage, the cumulative carbon weight in somatic growth was higher than that of strobilation in the 100 and 150 μg C/L groups.
Carbon distribution patterns between 8° and 10°C were similar; however cumulative carbon weight in growth increased over ephyrae only in the 150 μg C/L groups.
At 13°C under scarce food supply (0 and 30 μg C/L), the cumulative carbon weight of ephyrae was equal to that of buds. When food supply was higher,the cumulative carbon weight was ranked in decreasing order as buds, ephyrae and then body growth. When food supply was 100 and 150 μg C/L,ephyrae were continually released during the SS stage.
Carbon distribution patterns were similar at 15, 17 and 13°C, except for the 15°C-0 μg C/L group (i.e.,no strobilation occurred). However, the cumulative carbon weight of ephyrae at 15 and 17°C was lower than at 13°C.
At 19°C with food levels of 0, 30 and 60 μg C/L,the carbon weight was low for buds, ephyrae, and parent polyps growth. At 100 and 150 μg C/L carbon weight in growth varied little over the 86 d, and both the carbon weight of ephyrae and adult polyps growth stayed at low levels. Under adequate food conditions,more carbon was allocated to new buds.
4 DISCUSSION
4.1 Budding reproduction
Bud production did not monotonically increase with increasing temperature. The highest production appeared in the 15°C-150 μg C/L group, consistent with our report in another experiment examining decreasing temperature (Wang et al., 2015a). At 8,13 and 19°C, bud production was significantly lower than the other three temperatures under the same food regimes. The daily feeding efficiency at 8°C was lowest (range from 89.54% to 92.40%) at each food levels except for 30 μg C/L (F5,66=10.644,P<0.001). At the colder temperature of 8°C, the efficiency of predation was reduced due to weaker mobility of polyp’s tentacles, which would lead to relatively less energy intake. Furthermore, except for no food condition, the carbon allocated to growth was largest at 8°C among all temperature levels, which would lead to lower budding production as well. While the daily feeding efficiency at 19°C was the highest (range from 93.31% to 94.83%) in all food levels. At a higher temperature of 19°C, respiration rate in polyps increased and they consumed more energy(Mangum et al., 1972). At 13°C, polyps invested more energy to strobilation than budding. Higher bud production at increased temperatures has been reported in the previous studies (Ma and Purcell,2005; Hoover and Purcell, 2009; Han and Uye,
2010; Wang et al., 2015a). Conversely, Liu et al.(2009) reported that theA.auritabud production decreased with increasing temperature. In our experiment, no linear relationship between buds and temperature was found. It might be attributed to two different mechanisms. First, higher metabolism rate at high temperature (19°C), and second, differences in strobilation status. For example, Han and Uye chose a temperature range in which strobilation does not occur, and the effect of temperature and food was investigated at the level of individual polyp (Han and Uye, 2010),while our experiment used temperature rang that are favorable for strobilation at the population level. There was also a temperature-dependent energy tradeoff among buds, ephyrae and polyp growth during the strobilation process.
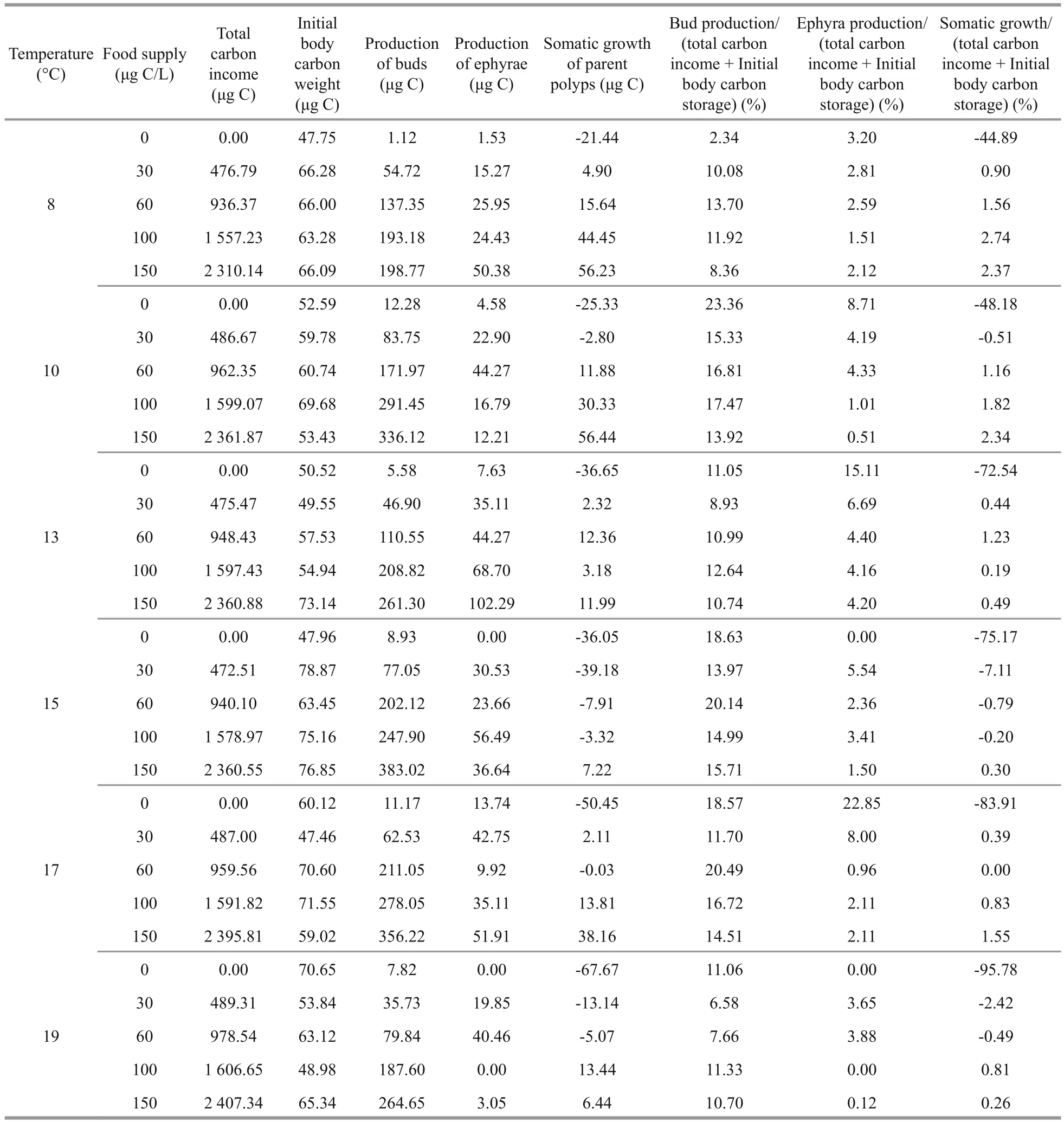
Table 2 The percentage of carbon distribution to budding, strobilation and growth under each combination of 6 temperatures and 5 food levels
Food supply had a remarkable effect on bud production in this experiment. Providing more food led to greater bud production as the previous reports(Keen and Gong, 1989; Han and Uye, 2010; Wang and Li, 2015; Wang et al., 2015a, b). In sustained low food levels, budding activity did not stop, but production was lower. Hence, sufficient food was advantageous for polyps to increase populations through budding activity.
4.2 Strobilation
It is widely acknowledged that temperature triggers the strobilation process (Prieto et al., 2010). The strobilation temperature ofAureliaspp. varies among geographic populations (Miyake et al., 1997). In our experiment, the duration of the SP and SS was markedly influenced by temperature inA.coeruleapolyps; however, neither stage was affected by food supply. The temperature-depending mechanism obtained in the present study seems to mostly agree with values from the literature (Liu et al., 2009;Wang et al., 2015a). Warmer temperature accelerated the release of the first ephyra and shortened the duration of the SP (Fig.3a). In field monitoring, Purcell et al.(2009) showed thatAureliapolyps strobilated earlier when pre-strobilation temperature was higher. The time until first strobilation ofAurelialabiatadecreased by 50% between 7°C and 15°C (Purcell, 2007). The 13°C treatment produced the longest SS as has been found in our previous studies (Wang et al., 2015a),and the highest total carbon weight of the released ephyra (Fig.5). Hence, 13°C was the optimal temperature for strobilation among the tested temperatures.
Specifically, we observed that single polyp had repeatedly conducted strobilation at the constant temperature of 13 and 15°C under abundant food conditions. As the culture temperature remains constant in the SS, maintaining temperature in the strobilation favored temperature range contributed to continuous strobilation. Hence, strobilation can also occur with culture temperature held constant within temperature ranges that favor strobilation.
There were no strobilation groups at 15 and 19°C(15°C-0 μg C/L, 19°C-0 μg C/L and 19°C-100 μg C/L), but these groups differed in other ways. At 15°C when food supply was higher than 0 μg C/L,strobilation was active, and the SS lasted longer(Fig.2). The absence of strobilation at 15°C-0 μg C/L was due to its second lowest initial body carbon weight. Although the initial body weight at 8°C-0 μg C/L was the lowest, strobilation occurred as well. It might be due to the less consumption of respiration at 8°C. Therefore, the absence of strobilation occurred at 15°C, when the food was rather scanty, and the initial body weight could not support both budding and strobilation. For 19°C, the duration of SP was less than 6.7 d. If polyps could strobilate at 19°C,considering the short duration of SP, they would strobilate again over the course of the 86-d experiment.However, even in the most adequate food groups, the polyps liberated ephyra in approximately 1 d, and after that they did not strobilate again for the duration of the experiment. This suggests that strobilation would not be induced with temperature held constant at 19°C.
At 19°C, although strobilation occurred in the 30,60 and 150 μg C/L groups (Fig.2), the duration of SP and SS was shorter than in other treatments. During the winter acclimation prior to the experiment, the culture temperature was elevated from 4 to 8°C, and polyps were ready to strobilate. Before morphological changes were observed, the switch of strobilationrelated genes had already turned on, leading to the accumulation of strobilation-related products(author’s unpublished data). We chose the normal appearing polyps for this experiment, but we could barely control the expression of strobilation-related genes. In the experiment, elevating the temperature to 19°C induced polyps that had already accumulated strobilation-related products to release the ephyrae rapidly. As the result, the average length of SS stage was less than 6 d, and after quick release, strobilation did not continue and did not occur for the duration of the experiment.
Consequently, the strobilation favored temperature range forA.coeruleapolyps ranged from 8 to 17°C,which agrees with results found for ephyrae-favorable temperature in the field survey. In Jiaozhou Bay,A.coeruleaephyrae were trawled in April, May and June when the sea temperature was approximately 7 to 17°C (Wan and Zhang, 2012;Wang and Sun, 2015).
Temperature also significantly affected ephyra production. In this experiment, as ephyra production was highest at 13°C, this was suggested to be the optimal strobilation temperature. In our previous experiment with decreasing temperatures, most ephyra released at 13°C as well (temperature levels:10, 13, 15°C) (Wang et al., 2015a). Shi et al. (2017)also reported that the rate of strobilation was highest at the moderate temperature of 12°C (temperature levels: 9, 12, 15, 18°C).
Our result showed that food conditions significantly affected ephyra production. ForA.coeruleapolyps,more ephyrae were liberated with adequate food supply. This same trend has also been shown forAureliaspp.,N.nomurai,C.nozakiiandR.pulmo(Thein et al., 2013; Schiariti et al., 2014; Feng et al.,2015; Sun et al., 2015a). The strobilae ofA.auritaare usually polydisc (Purcell et al., 2009). In this experiment, well-fed polyps formed more constrictions, further developing into numerous ephyrae. However, under scarce food conditions, only a small number of polyps liberated ephyrae, and the ephyra output was much smaller. Average ephyra production in the 0 μg C/L groups was 4.58 μg C(equal to 1 ephyra). In the field when zooplankton biomass was low, strobilation either did not occur, or monodisc or moderately polydisc strobilation occurred (Lucas, 2001). This is probably because strobilation should be fueled by the stored energy in polyps. Russel (1970) also suggested that initial polyp calyx diameter affects ephyra production; thus, we suggest that continuous starvation have not been the factor which could inhibit the strobilation. During the strobilation season, abundant food will result in higher ephyra production, providing the foundation for a jellyfish bloom.
4.3 The growth of parent polyps
The body growth of parent polyps typically decreases with increasing temperature. With increasing temperature, a greater food supply is needed to balance the negative growth caused by higher respiratory consumption. This trend was also reported by Han and Uye (2010), who found that at low temperatures polyps tended to be larger than at high temperatures. Compared to those at warmer temperatures, polyps had a greater percentage of larger individuals and slower population growth rate at lower temperatures (Willcox et al., 2007).
Our results also showed that body growth did not differ significantly between 8 and 10°C. This may be due to insufficient food intake at 8°C and the lower ephyra production at 10°C. We recorded more uncapturedArtemianauplii at 8°C in the daily observations, and the absolute carbon income was lower than those at the other 5 temperatures (Table 2).This result is consistent with another study onN.nomuraipolyps, in which tentacle movements and feeding frequency became lower at lower temperatures(Sun et al., 2015a).
Food supply had a strong positive effect on the body growth of parent polyps. This positive relationship has been shown in other studies onAureliaspp. (Willcox et al., 2007; Han and Uye,2010; Webster and Lucas, 2012; Chi and Javidpour,2016). The same increasing trends had been reported inCyaneanozakii(Sun et al., 2012). In our 0 μg C/L groups, parent polyps showed negative growth, and there was even some mortality at 19°C. In the 19°C-0 μg C/L group, polyps began to die from the 44th day. When the experiment was over, the mortality of the three repetitions in 19°C-0 μg C/L group was 100%, 40% and 60%, respectively. This might be due to energy consumption to maintain polyp basic life activities. At 19°C-0 μg C/L, the respiration consumed highest carbon (48.49 μg C, 68.63% of the initial body carbon weight). Nevertheless, budding reproduction and strobilation did not cease even under insufficient food levels. We suggest that the energy used to produce buds and ephyrae was fueled by stored energy of polyps. Therefore, in addition to respiratory consumption, budding and strobilation led to negative polyp growth under continuous starvation as well.
4.4 The carbon distribution strategy related to temperature and food supply
This study presented the first data on the carbon distribution among budding reproduction, strobilation,and parent polyps growth under different combinations of temperature and food supply. In the present study,the units of buds, ephyrae and parent polyps body growth were converted to carbon weight. The purpose of the unit conversion is twofold: to improve data comparability and to facilitate analysis of energy allocation. Therefore, the dynamics of energy allocation could be accurately quantified by analyzing cumulative carbon weight during the three life stage processes under different treatments throughout the experiment.
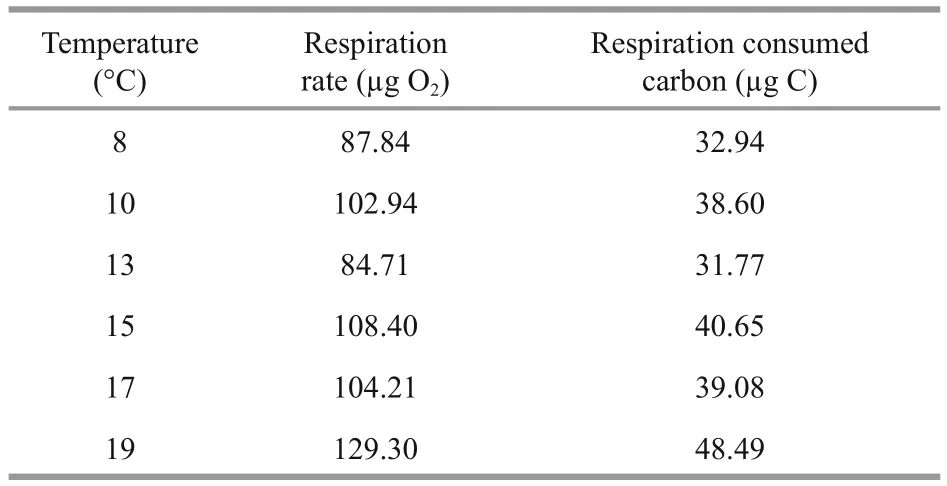
Table 3 The respiration rate and consumed carbon at 6 temperatures in the 86-day experiment (food supply=0 μg C/L)
In general, the polyps continually allocated 6.58%–20.49% carbon to budding reproduction when food supply was no less than 30 μg C/L. The carbon of buds was 1.3–86.7 (average: 10.3) times higher than to that of ephyrae. The ratios of buds accounted highest in total carbon source (Table 2). When food supply was higher, budding reproduction showed a clear advantage. Budding is the asexual reproductive manner which can directly produce child polyps(Vagelli, 2007).A.coeruleapolyps tended to assign priority to budding in all temperature levels in the experiment. It seemed that budding reproduction was a lower-risk strategy for the maintenance and propagation of the population, because the off spring produced by budding would face a known habitat.
Polyps tended to distribute energy to strobilation within a specific temperature range. At population level, the ephyrae were not released daily in SS, the time interval between ephyrae releasing varies. At the optimal strobilation temperature, providing adequate food condition polyps distributed more energy to ephyrae, and the ephyrae were continually released with shorter time interval. The optimal strobilation temperature in our experiment was 13°C, at which strobilation occurred actively with clearly higher ephyrae production, longer duration of the SS stage and highest ratio (4.20%) of strobilation carbon weight. Moreover, with sufficient food the polyps continually liberated ephyrae during the latter half of the SS stage. Strobilation is a riskier strategy, because releasing ephyrae would float with the current and face an unpredictable environment. The higher mortality caused by predation, space and food competition, as well as the possible unsuitability of new habitat, could potentially affect the future of the population. The other risk is the shrinkage of parental polyps after strobilation, suggesting that they face on lower predation ability and growth potential. Based on our data, the carbon each polyp lose after strobilation was 19.45±8.28 μg C accounting for 57.08%±9.88% of the initial carbon before strobilation. Hence, polyps trended to strobilate in the favored temperature range in order to match the zooplankton peak. It was proved in the field investigation. In Jiaozhou Bay, the strobilation temperature range of 8–17 was from late April to early June, and the annual peak of zooplankton abundance appeared in the same period. Furthermore,the ephyrae were also sampled in the high productive season (Fig.1). It is noteworthy that under scarce food conditions, polyps tended to sacrifice body organic storage to release ephyra, and the energy contributed to buds and ephyrae became comparable. This suggests that starved polyps prefer to extend their habitat by releasing planktonic ephyrae as a strategy to ensure survival of the population.
Parent polyps tended to utilize more energy for body growth at lower temperatures and higher food levels. At lower temperatures (8°C) when food supply was sufficient, polyps tended to grow larger and accumulate highest ratio (2.37%) of carbon to gain greater fecundity. It was reported that when parent polyps got abundant food, new ephyrae had a better nutritional foundation and higher starvation resistance(Wang and Li, 2015). The body carbon content of parent polyps could be considered as strategic energy reserves, which may help to support budding activities and strobilation under harsh conditions. In consideration of seasonality, the energy reservation at lower temperature season may be energy support for the budding at the following warmer season.
To quantify the respiratory consumption, we used the exponential equation between carbon weightspecific respiration rate ofA.auritapolyps and temperature reported by Ikeda et al. (2017) to calculate the respiration rate of polyps in our experiment. As polyps were starved in Ikeda’s respiration rate experiment, the equation was applied to our no food groups (Table 3). Without food, polyps had to consume their initial body carbon storage to maintain the respiration, budding and strobilation. The result showed that the initial carbon storage could cover the carbon consumption of respiration, budding and strobilation,and the parent polyps showed negative growth.
In Jiaozhou Bay, the water temperature ranges from 4–26°C during the spring-summer temperature elevation process, and the peak of zooplankton abundance was in May (Fig.1A of Wan and Zhang,2012). Seasonal polyp population dynamics in Jiaozhou Bay could be surmised as follows: The experimental strobilation favored temperature range(8–17°C) usually occurs from April to June in Jiaozhou Bay. In March, with relative higher zooplankton abundance, well-fed parent polyps store more organic content and grow larger in preparation for the coming strobilation season. In April, the temperature is about 8°C, although the zooplankton abundance is second lowest around the year, the carbon stored in March could be utilized, the ingested and stored carbon is firstly incorporated into producing buds to expand the polyp population, then strobilation.In May, when sea temperature increases to 13–14°C,the zooplankton abundance reaches its annual peak,polyps metamorphose into polydisc strobilae to liberate more ephyrae, which results in energy expenditure by parent polyps. The carbon invested to strobilation was higher than that in other months,leading to relative lower growth carbon. If the optimal strobilation temperature lasted longer, the duration of the strobilation stage would be prolonged correspondingly. In June, when temperature is elevated above the upper limits of the favorable temperature range, polyps release ephyrae quickly over about one week and halt strobilation in future days. In June, zooplankton abundance When temperature is generally over 19°C, polyp population is also expanded through budding, but the body growth of parent polyps stays at lower levels. As better food conditions undoubtedly contribute to greater budding, strobilation and body growth, this is likely to be the driving force forA.coeruleajellyfish blooms.
5 CONCLUSION
Temperature and food conditions affected the budding reproduction, strobilation and body growth ofA.coeruleapolyps in several aspects: (1) Polyps produced more buds at 15°C, and sufficient food supply contributed to greater bud production. (2)Strobilation can occur at 8–17°C. Within this temperature range, polyps release ephyrae earlier at higher temperatures. Among the six temperatures tested, 13°C was the optimal temperature at which more ephyrae were liberated and the duration of SS stage was longest. Sufficient food supply ensured greater ephyrae production, but scarce food conditions could not limit the occurrence of strobilation. (3) The body growth of parent polyps generally increased with decreasing temperature and increasing food supply.
The analysis of carbon distribution in the strobilation processes revealed thatA.coeruleapolyps utilize different strategies for allocation among budding reproduction, strobilation and body growth.Our results suggested that polyps prioritize budding.Budding reproduction might be a lower-risk strategy forA.coeruleapolyps to expand their populations. At the optimal strobilation temperature, polyps utilize part of their budding energy to release ephyrae. The body carbon content of parent polyps may be considered strategic energy reserves, which could help to support budding activities and strobilation under harsh conditions.
6 DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGEMENT
We thank Ms. ZHANG Zhenghua forcaring for the polyps. We are grateful to WANG Shiwei and WANG Pengpeng for help with samplingA.coeruleamedusae. Editing services were provided by Wiley editing services.
 Journal of Oceanology and Limnology2018年6期
Journal of Oceanology and Limnology2018年6期
- Journal of Oceanology and Limnology的其它文章
- Neuroanatomy and morphological diversity of brain cells from adult crayfish Cherax quadricarinatus*
- Effects of feeding time on complement component C7 expression in Pelteobagrus vachellii subject to bacterial challenge*
- Cryopreservation of strip spawned sperm using programmable freezing technique in the blue mussel Mytilus galloprovincialis*
- Pf- D mrt4, a potential factor in sexual development in the pearl oyster Pinctada f ucata*
- Specific genetic variation in two non-motile substrains of the model cyanobacterium Synechocystis sp. PCC 6803*
- Functional characterization of a Δ6 fatty acid desaturase gene and its 5′-upstream region cloned from the arachidonic acidrich microalga Myrmecia incisa Reisigl (Chlorophyta)*
