Effects of the heavy metal cadmium on photosynthetic activity and the xanthophyll cycle in Phaeodactylum tricornutum*
JI Yan (季琰) , XIE Xiujun (解修俊) , WANG Guangce (王廣策) ,
1 School of Biological and Chemical Engineering, Qingdao Technical College, Qingdao 266555, China
2 Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
3 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
A bstract Cadmium (Cd) is one of the most common and widespread heavy metals in the environment.Cd has adverse effects on photosynthesis that are countered by photosystem I (PSI) and photosystem II(PSII); however, the protective responses of these photosystems to heavy metal stress remain unclear.Using the model diatom Phaeodactylum tricornutum, a biological indicator that is widely used to assess the impact of environmental toxins, we simultaneously measured the effects of Cd on PSI and PSII and examined the levels of pigments in response to high light treatments before and after Cd exposure. Cd significantly reduced the quantum yield and electron transport rates of PSI and PSII. The quantum yield of non-photochemical energy dissipation in PSI due to donor side limitation increased faster than the quantum yield due to acceptor side limitation. The Cd treatment activated the P. tricornutum xanthophyll cycle under artificial light conditions, as indicated by an increased diatoxanthin content. Xanthophyll is important for photoprotection; therefore, the accumulation of diatoxanthin may down-regulate PSII activities to reduce oxidative damage. Together, our results suggest that the rapid reduction in PSII activities following Cd exposure is an adaptive response to heavy metal stress that reflects the variable exposure to external stressors in the native P. tricornutum environment.
Keyword: heavy metal; cadmium; photosystem I; photosystem II; Phaeodactylum tricornutum
1 INTRODUCTION
Heavy metal pollution is one of the most serious types of pollution confronting mankind, and continues to increase with industrialization, particularly in fastgrowing developing countries. Therefore, heavy metal pollution poses an increasing threat to the environment and human health. Heavy metals adversely affect phytoplankton ( Farga?ová et al.,1999; Payne and Price, 1999; Kola and Wilkinson,2005; Miao and Wang, 2006; Kapkov et al., 2011) and these can be used as indicators to evaluate heavy metals contamination in aquatic ecosystems (Guanzon et al., 1994; Wang and Pan, 2012; Wang et al, 2013).Moreover, phytochelatin photosynthesis in plants and algae may be one of the most sensitive biochemical processes to heavy metal stress (Clemens and Ma,2016).
Many studies have shown that photosystem II(PSII) activities are significantly affected by heavy metals (Dewez et al., 2005; Perales-Vela et al., 2007;Pan et al., 2009), with inhibition of electron transport,quantum yield and photochemical efficiency; as well as reduced chlorophyll synthesis (Dewez et al., 2005;Rocca et al., 2009; Zhang et al., 2010; Ouyang et al.,2013). PSII produces large amounts of reactive oxygen species when there is an excess amount of absorbed light energy; for example, under high light conditions (Pospí?il, 2016). The xanthophyll cycle,one of the most important photoprotection mechanisms, participates in the dissipation of the excess light energy into harmless heat (Lepetit et al.,2017). This process is crucial for plants and algae to endure high light stress. The extent to which heavy metal exposure affects the xanthophyll cycle is thought to determine how tolerant plants and algae are to heavy metals. However, data supporting this hypothesis is rare.
In contrast to PSII, photosystem I (PSI) is more tolerant to various stressors (Yamori and Shikanai,2016). Nevertheless, because PSI and PSII coordinately operate on the thylakoid membrane and the activities of PSI may be directly influenced by both the donor side (PSII) and the acceptor side (for example, ferredoxin and the Calvin Cycle). By simultaneously tracing changes in PSI and PSII activities, it is possible to investigate the effects of heavy metals on the photosynthetic apparatus of PSII and PSI and the interactions between the two photosystems. Klughammer and Schreiber (1994)developed an updated method of determining PSI quantum yield using a so-called Dual-PAM-100 system, which can simultaneously measure the chlorophyll fluorescence and changes in P700+absorbance. This system has been used to investigate the responses of PSI and PSII to various stressors(Huang et al., 2010; Deng et al., 2013).
Cadmium (Cd) is one of the major heavy metal pollutants due to its wide use in industry and its high solubility in water, which together have led to a wide distribution in aquatic ecosystems. Biologically, Cd has structural and enzymatic roles in cells; however,elevated concentrations are highly toxic to humans,animals and plants (Pinto et al., 2003; Nawrot et al.,2006; Zhou et al., 2006; Monteiro et al., 2011). Cd may alter the PSII activity by directly acting on the oxidizing side or on the reducing side of PSII, may inhibit the activity of the photosynthetic apparatus by acting on the water-splitting system and oxygen evolution rate (Siedlecka and Krupa, 1996; Zhou et al., 2006; Perreault et al., 2011), and may alter gene expression (Masmoudi et al., 2013).
In this study, we investigated the activities of PSI and PSII inPhaeodactylumtricornutum. The genome of this diatom has been sequenced and it is a valuable model that can be used as an indicator for biological monitoring. The quantum yields, electron transport rates and excess energy dissipation rates of PSI and PSII were simultaneously measured to find how Cd treatment differentially affects their activities. We also investigated how the abundances of xanthophyll cycle pigments change before and after treatment with high intensity light under different Cd concentrations.
2 MATERIAL AND METHOD
2.1 P. tricornutum culture
Phaeodactylumtricornutumwas cultured in f/2 medium at 20°C under white fluorescent illumination at approximately 100 μmol photons/(m2·s), and a 12-h light to 12-h dark cycle. The cells were harvested for Cd treatment after one week of culture(approximately 7.9×106cells/mL).
2.2 Cd treatments
A 200-mmol/L CdCl2(reagent grade, 99.9% purity;JingShanTing Chemical, China) stock solution was prepared in distilled water and added to harvestedP.tricornutumcells at (25, 50, and 100 μmol/L CdCl2final concentrations). Cells without the addition of CdCl2were used as controls. Measurements were taken at 0, 1, 2, 3 and 4 d after the start of the treatments.
2.3 Slow inductive curve and rapid light curve measurements
The activities of PSI and PSII were measured simultaneously using a Dual-PAM-100 system (Walz,Germany). The liquid containing the algal cells was injected into a quartz glass cuvette that was reserved for this system, and the cuvette was inserted between the emitter and the detector heads. The samples were adapted to the dark for 5 min before the start of each measurement. Slow inductive curves were determined using the automated induction program (Dual PAM v1.19; Walz). The samples were illuminated with a very low intensity light (less than 1 μmol photons/(m2·s)) to induce the minimal fluorescence(Fo). Maximum fluorescence (Fm) was determined by applying a saturation pulse light (SP, 10 000 μmol photons/(m2·s)) with a 300-ms duration. Next, the samples were illuminated with far-red light to oxidize the P700, and the maximal change in P700 signal between the maximal oxidized state (Pm) and maximal reduced state (Po) was determined as described by Klughammer and Schreiber (1994, 2008).
After the determination ofFo,FmandPm, the actinic light (AL) was applied at an intensity of 103 μmol photons/(m2·s) and the slow induction curve was employed to analyze photosynthesis under light. A series of SPs, each with a duration of 300 ms, was applied every 20 s after the start of the AL to determine the maximum fluorescence signal (Fm′) and maximum P700+ signal (Pm′) under the AL. Just before each SP,the chlorophyll fluorescence was denoted byF, and the P700 signal was denoted byP. The slow induction curve was recorded over a 300-s duration until photosynthesis steady state was reached, and then the AL was turned off . The data derived from the final SP was used to analyze the activities of PSI and PSII.The quantum yields of PSI and PSII were detected during the slow induction curve and then determined automatically using the Dual-PAM software. For PSII, the effective photochemical quantum yield was calculated according to the equation: Y(II)=(Fm′-F)/Fm′. The effective photochemical quantum yield of PSI was calculated according to the equation:Y(I)=1-Y(ND)–Y(NA); where the quantum yield of non-photochemical energy dissipation in the reaction center due to donor side limitation was calculated by the equation: Y(ND)=(P-Po)/Pm, and the quantum yield of non-photochemical energy dissipation in the reaction center due to acceptor side limitation was calculated by the equation: Y(NA)=(Pm–Pm′)/Pm. The data were recorded during the measurement of the slow induction curve.
After the induction curve was determined, the samples were illuminated with 13 different levels of photosynthetically active radiations (PARs) to determine the rapid light curve. The duration of each PAR was 30 s, and at the end of each PAR, an SP was triggered to simultaneously assess the effective quantum yield of PSII and PSI. Using the Y(I) and Y(II) values, the relative electron transport rates(ETRs) through PSI and PSII (ETR(I) and ETR(II),respectively) were calculated as follows:

2.4 High light treatment and measurement of pigment contents
To determine whether Cd treatment has any effect on the activation of the xanthophyll cycle, the algal solution was treated with a high light intensity(2 000 μmol photons/(m2·s)) for 30 min. Before the high light treatment, the algal samples were illuminated under the culture light conditions(100 μmol photons/(m2·s)). All of the algal samples(before and after the high light treatment) were cryopreserved by freezing in liquid N2prior to pigment analysis using high-performance liquid chromatography (HPLC; Rigol L-3000; Rigol,China). The pigment extraction and HPLC analysis were carried out as described by Zhao et al. (2014).The activity of the diadinoxanthin cycle, which includes reversible conversion of diadinoxanthin(Ddx) and diatoxanthin (Dtx), was determined by measuring the de-epoxidation state (DEPS). This is calculated by the formula: DEPS=Dtx/(Ddx+Dtx).
2.5 Statistics
Each treatment was replicated four times, and the mean and standard deviation (S.D.) calculated.Statistical differences in the DEPS values between the groups were analyzed by one-way ANOVA using the SPSS Statistics 19 package (IBM, USA).
3 RESULT
3.1 Effects of Cd on the Y(I), Y(II), Y(ND) and Y(NA)
At day 1 of treatment and at the lowest Cd concentration tested (25 μmol/L), the Y(I) was significantly reduced in comparison with the control(Fig.1). Even greater reductions were detected at days 2 and 3 with higher Cd concentrations. In contrast, the Y(ND) increased significantly after the Cd treatment,and at days 2 and 3 it had increased to approximately five times that of the control; suggesting that Cd significantly stimulates the Y(ND). However, the Y(NA) showed no obvious changes over the first three days of Cd treatment, but at day 4, there was an obvious increase for the higher Cd-concentration treatments. The Y(II) also clearly decreased upon Cd treatment, with an inverse correlation between the two parameters. However, an effect of the treatment time could not be detected because the Y(II) dropped similarly irrespective of the number of days of treatment.
3.2 Effects of Cd on the ETR(I)
Cd had immediate effects on the ETR(I), which was reduced from day 1 irrespective of the Cd concentration being tested (Fig.2). Over time, Cd significantly reduced the ETR(I) under all light intensities. At day 2, the ETR(I) values were decreased to half that of the control under 25 μmol/L Cd; and at higher concentrations, the ETR(I) was decreased to even lower values (one third of that of the control). At days 3 and 4, the ETR(I) values were decreased to even lower proportions of the control; in particular,under higher light intensities.
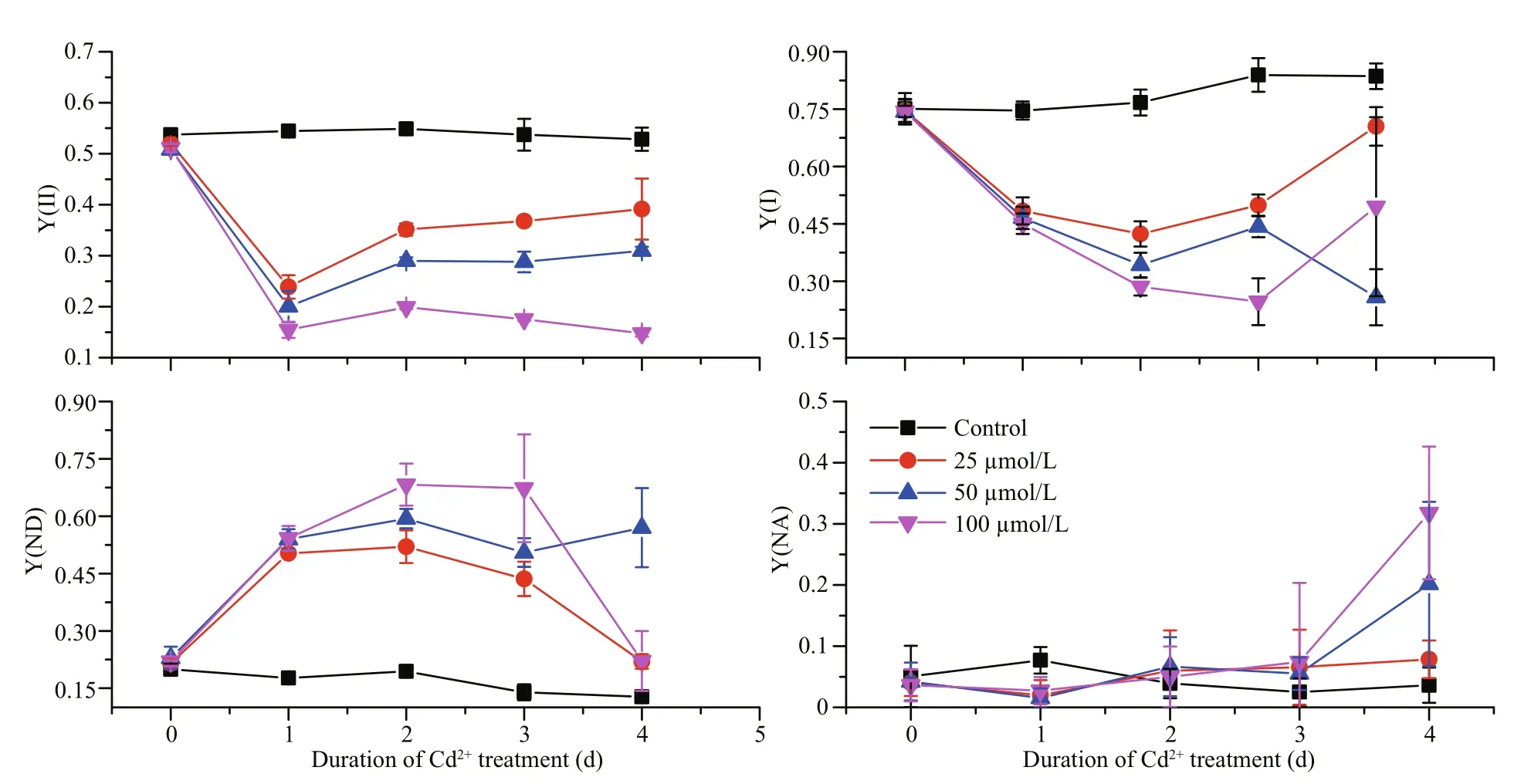
Fig.1 The effects of different Cd concentrations on the effective quantum yields of PSI (Y(I)) and PSII (Y(II)), the quantum yield of non-photochemical dissipation due to donor side limitation (Y(ND)), and the quantum yield of nonphotochemical dissipation due to acceptor side limitation (Y(NA)) for P. tricornutum over days 0–4 of treatment

Fig.2 The effects of different light intensities on the electron transport rate of PSI (ETR(I)) for P. tricornutum under different Cd concentrations
A high Cd concentration had immediate effects on the electron transport rate of PSII (ETR(II)),decreasing the ETR(II) values immediately after the 100-μmol/L Cd treatment of (Fig.3). The Cd treatments also had long-term effects on the ETR(II).At day 1, the ETR(II) dropped dramatically to one third or lower for all three concentrations being tested,in comparison with the control. However, the ETR(II)recovered over the following three days for all Cd concentrations being tested; with the exception for the highest concentration at day 4, which decreased further. In contrast, the ETR(II) for the lowest Cd concentration being tested (25 μmol/L) recovered to 75% of the control. Therefore, it appears that the ETR(II) is more tolerant to Cd than the ETR(I).

Fig.3 The effects of different light intensities on the electron transport rate of PSII (ETR(II)) of P. tricornutum under different Cd concentrations
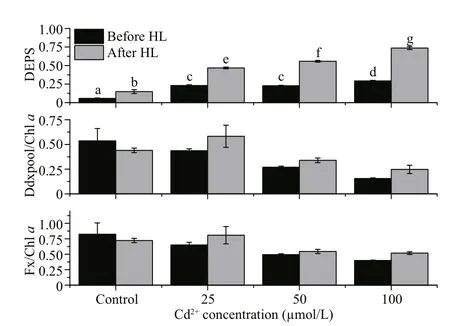
Fig.4 The effects of different Cd concentrations on various pigment contents of P. tricornutum before and after high light treatment
3.3 Effects of Cd on pigments
Treatment with high light (2 000 μmol photons/(m2·s)) and Cd had significant effects on the protection against high light in terms of the DEPS(Fig.4,P<0.05). For all Cd concentrations, the DEPS more than doubled after the high light treatment, with the rise in DEPS correlating with the Cd concentration.
4 DISCUSSION
In this study, we examined the effects of Cd on the Y(I) and Y(II), the ETR(I) and ETR(II), the Y(ND)and the Y(NA), and activation of the xanthophyll cycle. Y(I) maintained relatively higher values than those of Y(II) under all the Cd concentrations tested during the 4-day time interval. This indicates the relatively higher capacity of PSI to maintain its physiological activities under stress. It has been reported in plants and algae that PSI is more tolerant than PSII to stress conditions such as dehydration(Gao et al., 2011; Huang et al., 2011) and low temperature (Huang et al., 2010). Maintenance of PSI activity and PSI-associated cyclic electron flow(CEF1) may be crucial for the survival ofP.tricornutumfollowing Cd exposure. CEF1 is important for establishing the proton gradient across the thylakoid membrane that is a prerequisite for the activation of non-photochemical quenching (NPQ)on PSII (Yamori and Shikanai, 2016). CEF1 is also required for ATP synthesis, which is important for providing the energy required to extrude or sequester the harmful Cd (Masmoudi et al. 2013).
Examining the PSI reaction center (P700; Fig.1),we found that during the first 3 d of treatment, the Y(ND) values of the Cd-treated groups were significantly higher than those of the control group.This increase in Y(ND) may arise from a reduction in the PSII activities. The Y(NA) values increased significantly after 4 d of Cd treatment because there were insufficient electron acceptors, perhaps as the Calvin Cycle slowed down. Together, these data suggest that the enzymatic reactions in theP.tricornutumchloroplast stroma are affected relatively late after Cd exposure, while PSII on the thylakoid membrane responds to Cd earlier.
The Y(II) dropped significantly after the Cd treatment, even at day 1 and under the lowest concentration of tested (25 μmol/L; Fig.1). It is well known that a decrease in PSII activities may occur following damage to the PSII reactive core (for example, the D1 protein) or after down-regulation via non-photochemical quenching (For example, NPQ).In diatoms, NPQ is closely correlated with the diadinoxanthin cycle, which is one of the special xanthophyll cycles that has reversible conversions between Ddx and Dtx. When the absorbed light energy is in excess, we expect that Ddx would be converted into Dtx, and this conversion might be reflected by the measured increase in DEPS. Dtx binds to fucoxanthin chlorophyll protein (FCP) and triggers conformational changes, and then excess light energy is dissipated as heat (Ruban et al., 2012;Goss and Lepetit, 2015). We found that the DEPS value for the control group ofP.tricornutumbefore high light was lower than those of the Cd-treated groups (Fig.4). This finding suggests that the presence of Cd exerts stress effects onP.tricornutumcells and under this stress the normal illumination level(100 μmol/(m2·s)) becomes excessive, leading to Ddx cycle activation. It has been reported that Cd inhibits the epoxidation of Dtx to Ddx inP.tricornutum(Bertrand et al., 2001). Therefore, it is reasonable to assume that the accumulated Dtx during the light period would not be converted back to Ddx during the dark period because of the inhibitory effects of Cd,and the constant Dtx levels would reduce the Y(II)(Fig.1). Since we did not examine the effects of Cd treatment on the D1 protein content we cannot exclude the possibility that the Cd treatment affected D1 directly; and thereby, reduced the PSII activities.However, after the high light treatment, the DEPS value significantly increased (Fig.4), suggesting that the Ddx cycle remains active in the cells treated with various concentration of Cd. This observation is in line with the results obtained previously by Bertrand et al. (2001).
According to Biller and Bruland (2012), the Cd concentration in the Pacific Ocean is less than 1 nmol/L. However, for some heavily polluted coastlines, the Cd concentration reaches 19.14 mg/kg dry weight (Naser, 2013). The 100 μmol/L Cd used in our study is higher than most Cd concentrations found in the environment; however, it may provide valuable information about how microalgae respond to acute heavy metal treatments. We found that even at the lowest Cd concentration we tested (25 μmol/L), the quantum yield and ETR declined from day 1,suggesting thatP.tricornutumis very sensitive to Cd.The DEPS value rose significantly with the increased Cd concentrations as well as with the high light treatments, suggesting that Cd and high light independently stimulate the photoprotection mechanism. These two factors should be considered as important external stressors to which microalgae may be exposed.
5 CONCLUSION
Cd treatment reduced the quantum yield and ETR inP.tricornutum. Cd reduced both PSI and PSII’s activities, but PSII was more sensitive to Cd. Cd stimulated the xanthophyll protection mechanism against high light, as indicated by elevated Dtx contents. Dtx accumulation may down-regulate PSII activities to reduce oxidative damage. In conclusion,our results suggest that the reduced PSII activities ofP.tricornutumexposed to Cd may be an adaptive response to external stressors in its environment.
6 DATA AVAILABILITY STATEMENT
The data used in the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGEMENT
We thank Shelley Robison, PhD, from Liwen
Bianji, Edanz Group China (www.liwenbianji.cn/ac),
for editing the English text of a draft of this manuscript.
 Journal of Oceanology and Limnology2018年6期
Journal of Oceanology and Limnology2018年6期
- Journal of Oceanology and Limnology的其它文章
- Neuroanatomy and morphological diversity of brain cells from adult crayfish Cherax quadricarinatus*
- Effects of feeding time on complement component C7 expression in Pelteobagrus vachellii subject to bacterial challenge*
- Cryopreservation of strip spawned sperm using programmable freezing technique in the blue mussel Mytilus galloprovincialis*
- Pf- D mrt4, a potential factor in sexual development in the pearl oyster Pinctada f ucata*
- Specific genetic variation in two non-motile substrains of the model cyanobacterium Synechocystis sp. PCC 6803*
- Functional characterization of a Δ6 fatty acid desaturase gene and its 5′-upstream region cloned from the arachidonic acidrich microalga Myrmecia incisa Reisigl (Chlorophyta)*
