Quantum chemical study on micro extractionsand electrocatalytic oxidation of bisphenolA at ferric hydroxide colloidal particles modified carbon paste electrode
, , , , ,
(1. Institute of Catalysis for Energy and Environment, Shenyang Normal University, Shenyang 110034, China; 2. College of Chemistry and Chmical Engineering, Shenyang Normal University, Shenyang 110034, China; 3. College of Material Science and Engineering, Hebei University of Engineering, Handan 056038, China; 4. Chemistry Section, Beipiao First Senior Middle School Liaoning, Beipiao 122100, China)
Abstract: The graphite powder surface is chemically modified by ferric hydroxide colloidal particles, and used to make a carbon paste electrode. At the modified electrode, biphenol A shows an electrochemical catalytic oxidation with oxidation peak potentials negatively shifted about 246 and 262 mV, and peak current increase of 42.7% and 10 times compared with unmodified CPE and carbon electrode with same geometric area. Respectively. In order to understand this catalytic behavoir, a PM7 Semi-empirical quantum chemical method in MOPAC2012 software was used in the study of electrochemical catalytic activity of the system based on the molecular cluster models of a piece of graphene with 38 carbon atoms as the surface of graphite powder, BPA under vacuum conditions. The quantum chemical results are further analyzed with molecular descriptors, and the results are accord well with the experimental results.
Key words: PM7 semi-empirical molecular orbital theoretical method; biphenol A; chemical modified carbon paste electrode; electrochemical catalytic oxidation; ferric hydroxide colloidal particle
0 Introduction
Carbon paste electrode (CPE) being composed of graphite powder as solid phase and organic binder as liquid phase[1-2]has been used for many years in the electro analysis and electrochemical studies. In order to increase its functionality, CPEs have been modified by different methods such as adsorptions, chemically modifying or intercalating modifiers on the surface of graphite powder[3-7], as well as the imbedded modifiers in the organic binders[8-11]. But the theoretical or quantum chemical studies on the CPEs have very few reports in literature due to the difficulty in modeling the graphite powder surface[12-13]and its chemical modifications. There is also a report shown that the pure grapheme surface has no redox catalytic behavior without modifications[14]. The modified CPEs have been widely used in electroanalytical chemistry[15-18]and chemical researches[19-20]in aqueous system. Biphenol A(BPA, 2,2-bi-4-hydroxyl benzyl) protane, chemical formula is C15H16O2, is an important raw material of chemical engineering for the productions of polycarboxylate, epoxyl resin, polysulfone, tetrebromine bisphenol A as a flame retardant agent. Recently BPA was also found as a kind of endocrine disrupter chemicals(EDCs)[21-22]. EDCs can act as the natural hormone or inhibit the activity of natural hormones, and interference the human normal neuro system, immune system and internal secretion systems, and show a great influence on human health. Electrochemical methods have also been used for the detection of BPA[23-25].
In the present paper, a CPE was composed of methyl silicone oil andgraphite powder chemically modified with ferric hydoxide colloidal particles, and shows an electrochemical catalytic behavior to pyrogallol in aqueous solution[26]as well as the micro extraction behavior. In order to understand deeply the electron transfer mechanics from quantum chemical view, the graphite powder surface was simply modeled as a piece of graphene with 38 carbons, and whole working electrode was modeled as molecular cluster composited of graphene, ferric hydroxides and BPA molecules as well as the silicon oil as microliquid extraction reagendand electrochemical catalyst. The molecules and molecular clusters were calculated with semi-empirical quantum chemical method MP6 in MOPAC2012 software[22-23], and the calculated results were analyzed in thermodynamic and frontier orbital theoretical concepts. Some interesting results are reported here.
1 Experimental
1.1 Instrument and reagents
Allelectrochemical experiments were carried out on a CHI620b electrochemical instrument(CHI Co., Shanghai, China) with three-electrode system, including a home-made platinum wire electrode as the counter electrode, a ferric hydroxide colloidal partical modified carbon paste electrode as the working electrode and a KCl saturated calomel electrode(Model 232, Shanghai, China) as the reference electrode, and all potential reported here was respect to this reference electrode.
Polyamide resin(Tianjin, China), epoxy resin (Wuxi, China) methyl silicone oil(Shenyang, China) and Graphite powder(Spectrographic pure) were all used for the preparation of basic carbon electrode. Potassium ferrocyanide(Analytical pure) was prepared into 5.00 mM as the stock solution. Aluminum chloride(Analytical pure) was prepared into 1.00 mM as a stock solution. Potassium chloride(analytical pure) was prepared into 1.0 M as an electrolyte solution. Ferric hydroxide colloidal particles was prepared with a ferric chloride of 10% aqueous solurtion as described below. All chemicals were purchased from Shengyang Chemical Co, and all solutions were prepared with ultrapure water (18.2 MΩ, USA) for the experiment. All experiments were carried out at room temperature with 1.0 atmosphere.
The basic carbon electrode was prepared as previous work[24]. Graphite powder, epoxy resin and polyamide resin was mixed into paste, filled into a clean glass tube about 6~7 cm in length and 5.0 mm in diameter. A piece of copper wire was inserted into the paste from other end of the tube as electronic lead. The electrode was solidified in air for about 72 hours. After the solidification, the top of the electrode was polished on sand paperas the cargen electrode, and tip out some paste from the top of the electrode, and left a cavity about 1mm depth to hold carbon paste as the CPE electrdodes.
A red-bright color ferric hydroxide colloidal particle suspension solution was prepared with adding ferric chloride solution(10% in weight) of 5.0 mL was dropped into 95.0 mL boiling water in 200 mL beaker in a stirred bath25, which was stable for several weeks. 10 mL prepared ferric hydoxide colloidal solution and 6.6 g spectropured graphite powder were mixed in 50 mL beaker, heating and stirring until the mixture become a dry powder again. In this case, the ferric hydroxide nanoparticles were modified on the surface of graphite powder.
The modified powder was mixed with methyl silicone oil at mass ratio of 2∶1 on a clean glass plate into a paste with glass bar. The carbon paste was filled into the cavity of the basic carbon electrode, polished on glassy paper into mirror-like surface, and served as the modified carbon paste electrode(Modified CPE) in all of this work. If the paste was prepared with unmodified graphite powder and methyl silicone oil, the prepared CPE was called the unmodified carbon paste electrode.
1.2 Molecular cluster models and computational details
All quantum chemical calculation in this work was performed with semi-empirical quantum chemical methods of MP6 in MOPAC2012 software[22-24]on personal computers. All molecules and molecular clusters were designed by PCMOD 7.0[26]and further optimized with MOPAC2012 software before the energy and molecular orbital calculations. The heat of formations and frontier orbital were obtained from the semi-empirical calculations in vacuum, and don not consider the influence of solvent effects, partly due to the calculation complexity and the methyl silicone oil of organic phase mixed with the graphite powder and greatly reduced the influences of water molecules on the molecule and molecular clusters. All of the obtained data were used in analysis and evaluation of the stabilities and the electron transfer properties of each molecular cluster.
In this study, BPA is first in aqueous solution, it process a liquid microextract into organic phase of silicone oil in carbon past electrode. The related molecule clusters models as described in Fig.1A. BPA in aqueous solution is an hydrolated molecule arounded by 15 water molecules of a box model and indicated as BPA-W15, after extract into silicone oil phase we used box model of BPA interacts with 2 silicxone oil moleucles as the first process.
Another process is solid phase micro extraction, the related cluster models are discribed in Fig.1B. In this process Graphite powder is commonly considered as a stratification of multiple graphenes. The exposied surface of graphite powder can be simply modelled as a piece of graphene sheet[27-28]. In this study, Graphite powder is commonly considered as a stratification of multiple graphenes. The exposied surface of graphite powder can be simply modelled as a piece of graphene sheet[27-28]. In this study, a piece of graphene sheet was composed of 38 carbon atoms in match-join form of twelve six-rings, which was described as C38 in Fig.1B. The molecule of biphenol A can be shorted as BPA in the Fig.1B. The ferric hydroxide colloidal particle surface can be simply modelled as three ferric oxides bridged with oxygens together with two chlorides as the opposite part of the ferric oxide colloidal particle positive charges, and described as FeO in Fig.1B. The modified C38 with ferric oxide is described as FeO in Fig.1B. the interaction of C38 with BPA as C38+BPA. The interaction of C38-FeO with BPA as C38-FeO+BPA in Fig.1B.

Fig.1A The molecule or molecule cluster models related to the liquid microextraction process

Fig.1B Molecule or molecular clusters related to biphenol A solid phasemicroextraction and catalytic electrochemical oxidation process
2 Results and discussions
2.1 Experimental results
2.1.1 Cyclic voltammetric behavior of BPA at the modified CPE
The cyclic voltammogram of biphenol A at the modified CPE shows one irreversible oxidation peak located at 0.546 V(51.1 μA) as curve 1 shown in Fig.2A, while at the unmodified carbon paste electrode, an oxidation peak at 0.792 V(35.8 μA) and at carbon electrode with same geometric area shows a oxidation peak located at 0.808 V(4.68 μA) as curve 2 shown in Fig.2A.
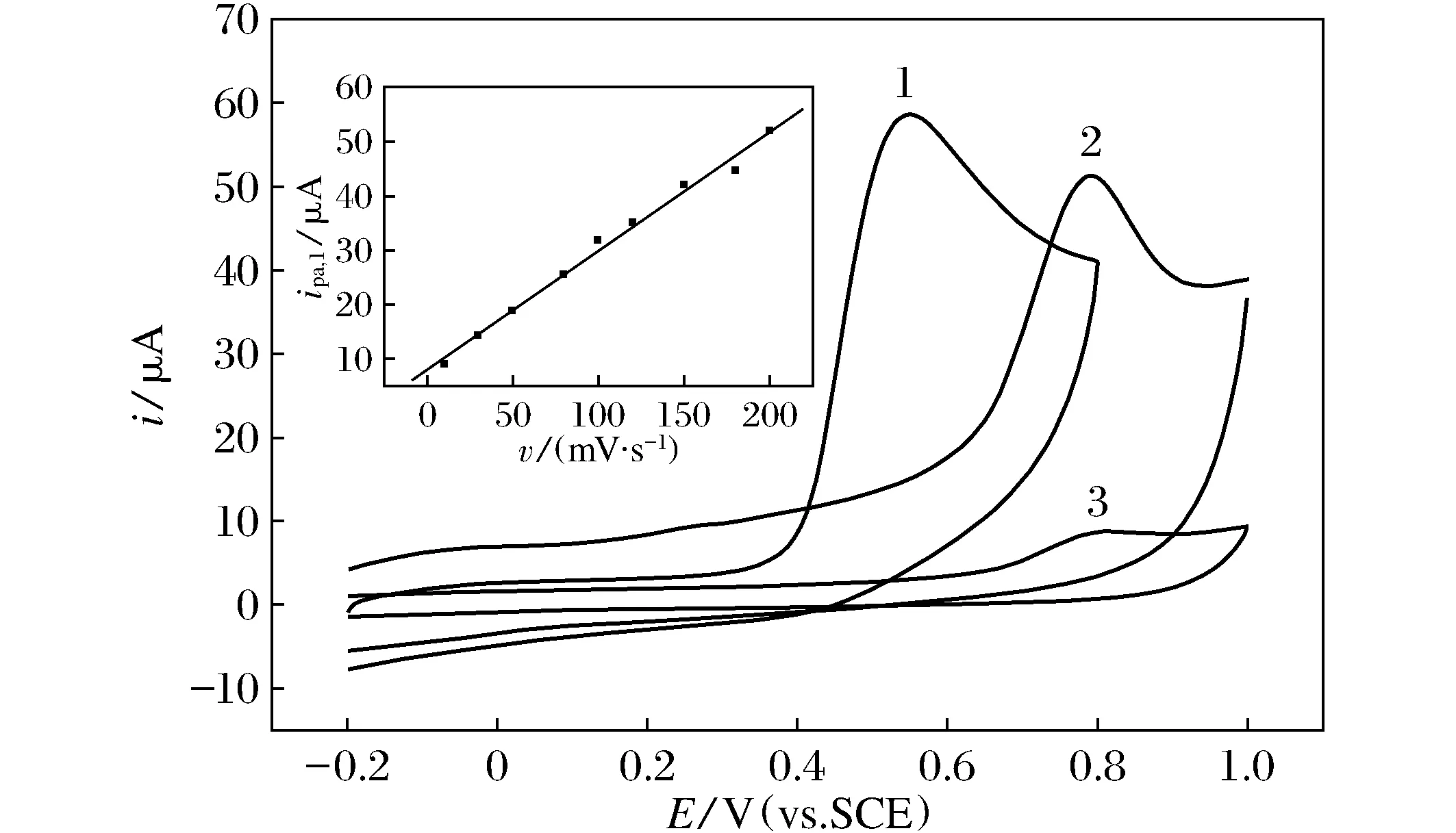
Fig.2 The CVs of BPA on the modified CPE(1) and CPE without modification (2) and carbon electrode with same geometric area (3). pH 8.0 of 0.2 M KCl electrolyte solution including 0.5 mmol/L BPA, Quiet time=2 s, scan rate=100 mV/s.
Compared the oxidattion peak current and peak potenial on modified and unmodified CPE there peak current increase about 42.7% electrodes shown in Fig.2 curve 1, and 2, and peak potential negative shift about 246 mV. The larger oxidation peak potentials negative shifts means the electrochemical catlytic oxidartion ocurs. The oxidation peak current increase about 10 times but the peak potential only negative shift about 16 mV.
Potential at theunmodified CPE and carbon electrode with similar electrode geometry area shown in FIG1, curve2, and curve 3, indicates this current increase mainly atribute from the liquid micro exctraction of the methyl silicone oil in the CPE suface. The peak current of BPA at modified CPE is linear changed with scan rate with a regression equation ofiaa=9.289 08v+0.209 02(R=0.990 6,SD=2.044 81). This relationship indicates that the irreversible oxidation process is surface controlled and the BPA is extracted on the graphite powder surface.
2.1.2 The effect of concentration of BPA
In 0.2 M KCl electrolyte solution (pH 8.0) including different concentration of BPA in the range of 2.0×10-81.0×10-3M,the differential pulse voltammetric experiments were performed at extraction potential of -0.50 V for 30 s. The oxidation peak current of BPA was plotted against the concentration of BPA; a weibull growth curve [16] was obtained with a regression equation ofipa=18.015-17.54e-0.000 677 3c0.919 4,R=0.999,SD=0.242 5 as shown in Fig.3. This result indicates the extraction follows a membrane growth process. According to Freundlich adsorption theory[17], the logarithm plot of Freundlich isotherm was two straight lines as shown in Fig.3 insertion.

Fig.3 The relation between oxidation peak current of BPA n DPV and its concentration and logarithm plot of Freundlich isotherm in the insertion. The experimental conditions were as followings: extraction potential -0.5 V, extraction time 120 s. the other conditions were the same as those in Fig.2
Since the detection related to the extraction process, which is an exponential function of the concentration of BPA in the monitoring solution, the commonly used linear working curve of peak current with concentration is not suitable in this case. As we known that in the potential measurement model, the potential and concentration are follows the Nerstian equation, we use the exponential of concentration related to measured potential changes and setup a relation for the working curve. With the exponential extraction process followed by the voltammetric measurement case, we adopt an exponential function of concentration of BPA as the working curve. In the detectable concentration range of BPA, 0.020 μm~5 mM, there are two linear working curves. In the lower concentration range of 0.020m~20M, the first working cure with the regression equation ofipa=0.457 6+0.013 87ln[c/nM](R=0.996 8,SD=0.002 91) as shown in Figure 3 insertion. In the higher concentration range of 0.20 mM ~ 5 mM, the second working curve with the regression equation ofipa=7.147+4.300ln[c/mM](R=0.986 4,SD=0.862 4) as shown.From the about working curves we can found the lower detection limit for BPA is 20 nM and the upper detection limit is 5 mM, the detection range for this method is very wider than commonly used electrochemical voltammetric methods due to the couping of solid phase nano-extraction of BPA onto the electrode surface, which takes the advance of the solid phaseand liquid phase microextractions for the preconcentration and separation prior to the electrochemical measurements.
2.2 Quantum chemical calculation results and analysis
2.2.1 The thermodynamic stabilities of molecule and molecular clusters
Heat of formation of molecule or molecular clusters can be easily obtained from the PM6 calculation in MOPAC2012, and used to evaluate the stability of molecule or molecular clusters. According to thermodynamic principle[29], the heat of a reaction can be calculated with heat of formation of products and reactants as,
(3)
Where,nandmare the numbers of products and reactants. The heats of formation of all the molecules and molecular clusters used in this work were calculated by PM6 semi-empirical quantum chemical method and listed in Table 1. The changes of reaction heat and free energys of each cluster formation were also calculated according to equation (3) and listed in Table 1. the BPA molecule in aqueous solution is not favorable than it in methyl silicone oil phase with negative free energy and reacion heat changes. So BPA molecule is favorable for micro extract from aqoues solution into the organic phase. Graphene. After BPA extracted into organic phase, it contact with ferric hydoxide and forms as a complexes with very larger negative formation heat and free energy changes as list in Table 2. So by the solid phase ectracttion of ferric hydroxide colloidal particles, the BPA molecule is even more favorable in the solid phase extraction process with the large oxidation peak current and the adsorption behavior in the Freundlich isotherm. Solid phase extraction not only increase the preconcentration of BPA, but also shows electrohemical catalytic oxidation activity. The futher calculations as disscussed in the next section.

Tab.1 Tthermodynamic parameters of molecule or molecular clusters for liquid phase microextraction
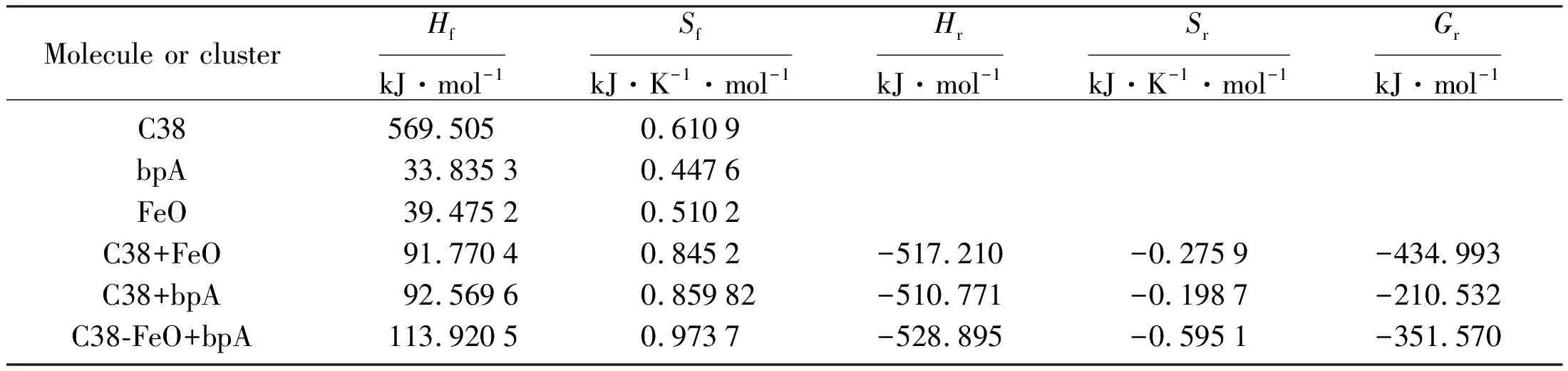
Tab.2 Thermodynamic parameters of molecule or molecular clusters for solid phase microextraction
All iterms are described in Fig.1
2.2.2 The frontier orbital analysis of molecule and molecular clusters
According tothe Kohn-Sham Theory, the chemical reactivity tendencies of a molecule with approximations may be useful for evaluation of electrochemical activity, and can be obtained from frontier molecular orbital (FMO) composited of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). Some descriptors have been reported such as the frontier orbital gap (ΔE), chemical potential (μ)[30], molecular hardness (η)[31], molecular electrophinicity (ω)[32], and charge exchange (ΔN) from A to B molecules[33-34]. All of these terms can be obtained from the frontier orbital energies of the HOMO (εHOMO) and LUMO (εLUMO), respectively. All these descriptors are defined as,
Here ΔNwas originally used to describe the electron transfer quantity from molecule A to molecule B, but in our case, it is used to describe the electron transfer from molecule A to another electrode or output electric circuit withμB,ηB=0, we called molecule B as environment, the new quantity may be called exchangeable electron. All energies of the frontier orbital and all the corresponding descriptors of the molecules and molecular clusters used in this work were calculated by PM6 in MOPAC2012 software, and listed in Table 2. In order to compare the electron transfer capacity of grapheme C38 and modified C38 and other molecular clusters, we used the difference of the exchangeable electrons between any molecular cluster (ΔNMC) and grapheme C38 (ΔNC38) to describes relative amount of exchangeable electrons included in the molecules or molecular clusters, we call it as difference of exchangeable electron (ΔΔN, as another descriptor) and may be defined as[24],
ΔΔN=ΔNMC-ΔNC38
(8)
The difference of the exchangeable electrons values (ΔΔN) can be positive or negative. The positive value means the exchangeable electron of a molecular cluster is more than that of grapheme, and may be likely to transfer the electron from the molecular cluster to grapheme, and the molecular cluster may likely to be oxidized. In the negative case, the molecular cluster has less exchangeable electron than that of grapheme, electron may be transferred from environment to the molecular cluster, and the molecular cluster through the grapheme is likely to be reduced. So the difference of exchangeable electron may be used to evaluate the electron transfer possibility of a molecular cluster in the CPE system combined with the experimental results. From the calculation results in the Table 2 we can seen that all ΔΔNs of molecular clusters have a positive values, which indicate potassium ferrocyanide is easy to lose it electron to be oxidized. Among them, the molecular cluster with modified graphene better than those with unmodified graphene. It is very interested to found the ΔΔNvalues of the molecular clusters with modified graphene are 14% larger than those with unmodified graphene, which is very similar with the oxidation peak current increase in the CV grams. That means the values of ΔΔNare responsible for the electrochemical current in the experimental results.
The change ofexchangeable electronof the modified CPE compared with the unmodified CPE is increased about 39.6%, which very closed to the oxidation peak current inrease of 42.7%.

Tab.3 The calculated descriptors of molecule and molecular clusters
All the iterms are described in Fig.1.
2.2.3 The component analysis of frontier orbital
From molecular orbital theory[35]we known, a molecule is composited ofnnumber of atoms with some atomic orbital (φi,j), and there are m number of molecular orbital (Φi) of the molecule composited of atomic orbital by the linear combination of the n number of atomic orbital of atoms,and can be described as
(9)

(10)
So in a normalized molecular orbital, the square of each coefficient of an atomic orbital is the fraction of this atomic orbital in this molecular orbital. The square of the coefficient multiply by number of electrons in the molecular orbital is the charge density of atomic orbital in this molecular orbital. The analysis of a molecular orbital composition can help us to understand deeply the molecular activities in a chemical reactions, as well as electron transfer property. In our case, we can localize which group of the molecule taking part in the electron transfer process and which molecular orbital in the molecular cluster is mainly responsible for electrochemical oxidation reaction. All the frontier orbital including the LOMO and HOMO as well as the next HOMO were analyzed until we found out the group of our interested one, and all results were plotted in Fig.4.

Fig.4 The plot of frontier orbital energy and their components of molecule and molec ular clusters. All the iterms here were the same as those described in Fig.1.
All of the first LUMO of the molecule and molecular clusters are composed of carbon pz atomic orbital from C38 graphene, and all the first two HOMOs of ferro related molecular clusters are composited of s, p and d orbitals from the atoms of ferric hydroxide and BPA molecule. The interacting of ferric hydoxide and BPA molecule offers electron to graphene molecule during the oxidation peocess. and increase the amount of exchangeable electron compared with the graphene own. The fomation of ferric-BPA complex improves electrochemical activity of BPA and increase the.
2.2.4 The relationship between redox potential and energies of frontier orbital
The frontier orbital of HOMO and LUMO are important in chemical reactions, especially in electrochemical reactions of receiving or losing electron from them.So as early as 1949, Maccoll showed the good relation between reduction potential of a conjugated system and the frontier orbital energy [35]. The oxidation potential,EOX, is proportional to the energy of HOMO,EHOMO, and the reduction potentialEREDis proportional to the energy of LUMO,LUMO, and all together the expression can be written as [36],
EOX/RED=a+bεHOMO/LUMO
(11)

Fig.5 The relationship between redox potential and energy of frontier orbital
WhereEOX/REDis the experimental potentials of oxidation and reductions, and a, and b are constants. In our system, there are two molecular clusters were chosen for the electrochemical redox reactions, one is the C38+ferro-e for the unmodified CPE and the other is the C38-AlQ+ferro-c for the modified CPE. The energies of frontier orbital of the C38-AlQ+ferro-c molecular cluster are -5.366 eV and -2.334 eV, and the energy of the second HOMO is -5.935 eV. The energy difference of the two HOMO orbital is about 0.569 eV, so during the electron transfer process, the second HOMO does not take part in the process. But in C38+ferro-e case, the energies of frontier orbital are -5.225 eV and -2.103 eV, with the energy difference of 0.275 eV. The second HOMO (-5.50 eV) may be take part in the electron transfer process, the exchangeable electron can be calculated as 0.363. From the probability of the Fe atom in the first HOMO is about 0.404, and the main iterm isdx2-y2orbital. but the propbility in the second HOMO the Fe atom orbital occupied for about 0.46, and the main iterm isdz2orbital. The plot of redox peak potentials against the energies of frontier orbital is a good linear equation (as shown in Fig.5) was obtained as,
Ep,redox/V(vs.SCE)=0.090 18±0.011+0.034 8±0.002 6EHOMO/LUMO/eV
R2=0.989,SD=0.008
(12)
This linear relationship of experimental peak potential and calculated frontier orbital energies further indicates that the quantum chemical calculations are accord well with the experimental results, and offers theoretical evidence for the electro-catalytic behavior of potassium ferrocyanide at the ferric hydroxide colloidal particles modified CPE.
3 Conclusions
In the present paper, graphite powder surface was simply modified withferric hydroxide, and shows electrochemical catalytic behavior for BPA. Semi-empirical quantum chemical method of PM6 in MOPAC2012 software was used in quantum chemical calculations based on the molecular cluster models including graphene with 38 carbons,modifier and ferric hydoxide. Some conclusions were summarized as the followings. The designed molecular cluster models are reasonable from the thermodynamic view. The catalytic behavior comes from the increase of exchangeable electron after the modification, which corresponding to the peak current increase in CV grams. The quantum chemical calculations combined with the molecular descriptors will help us to well understand the electrochemical catalytic behavior of the systems. The linear relation of the calculated energies of frontier orbital and redox potential offers another evident in understanding the electrochemical activity of a molecule from quantum chemical view.
Acknowledgements
The authors would like to acknowledge the financial supports of the Chinese National Science Foundation (2004DFA02700, 20875063), Liaoning Education Minister(2004-c022)and national key, and National Key Laboratory on Electroanalytical Chemistry (2006-06).