Detection and determination of undeclared synthetic caffeine in weight loss formulations using HPLC-DAD and UHPLC-MS/MS
Crine Vin,Gbriel M.Zemolin,Thís R.Dl Molin,Luin Gobo,Sndr Mri Ribeiro,Gbriel C.Lel,Gbriel Z.Mron,Lendro M.de Crvlho,
aGraduate Program in Pharmaceutical Sciences,Federal University of Santa Maria(UFSM),Santa Maria-RS,Brazil
bCenter of Health Sciences,Federal University of Santa Maria(UFSM),Santa Maria-RS,Brazil
cDepartment of Chemistry,Federal University of Santa Maria(UFSM),Santa Maria-RS,Brazil
Keywords:Undeclared synthetic caffeine Weight loss formulations HPLC-DAD UHPLC-MS/MS
A B S T R A C T Caffeine is present in products marketed for weight loss,with the purpose of increasing thermogenesis and lipid metabolism.The dosage declared by the product manufacturer,or even its presence,is not always correctly described on the label.This work aimed to investigate the undeclared synthetic caffeine in weight loss formulations by a high-performance liquid chromatography with diode array detection(HPLC-DAD)method.From one hundred products purchased through Brazilian e-commerce,seventeen contained caffeine,either naturally or synthetically added to formulation.The caffeine-containing samples were confirmed by an ultra-high performance liquid chromatography-tandem mass spectrometry(UHPLC-MS/MS)method,and adulteration was clearly proven in five products.The content highest caffeine contained 448.8 mg per dose.Other irregularities were also found;nevertheless,the most serious was the addition of synthetic drugs without asking the consumers.Additional drugs expose the consumer to more possible side-effects as well as deleterious drug interactions.Intentional adulteration with any unlabeled substance is typically motivated by a desire to increase or alter the claimed effect of the marketed product to gain a commercial advantage.
1.Introduction
Products marketed for weight loss often contain high amounts of caffeine from natural or synthetic sources in order to increase thermogenesis and lipid metabolism.Caffeine has been shown to heighten resting energy expenditure in adult humans(both normal and overweight)in a dose-dependent manner.Caffeine increases the metabolic rate through the inhibition of phosphodiesterase(PDE)and stimulation of adenosine receptors.This leads to an accumulation of intracellular 3,5-cyclic-adenosine monophosphate(cAMP),which is metabolically excitatory for cells[1,2].
The caffeine content of drinks varies according to the product.In eight ounces of coffee,for example,the content varies from 60 mg in espresso to 125 mg in brewed coffee.Soft drinks,mainly colas,range in caffeine 23–31 mg per 8-ounce bottle[3].Except for pregnant women,400 mg of caffeine is the recommended daily allowance for an average person[4].High doses of caffeine may produce insomnia,heart palpitations,anxiety,nausea,vomiting,increased blood pressure,muscle twitching,tremors,and increased cholesterol levels[5].Doses over 600 mg/day can cause significant side effects,including tachycardia,tremors,insomnia,nervousness,chest pain,and arrhythmias[6,7].The chronic use of high doses can lead to dependence and tolerance,thus requiring larger doses in order to produce the same effect.In these subjects,the abrupt discontinuation might cause a clinical situation called caffeine withdrawal syndrome.The American Psychiatry Association accepts “caffeine withdrawal”as a clinical diagnosis.Its symptoms include headache,marked fatigue or drowsiness,dysphoria,depressed mood or irritability,difficulty concentrating,and flu-like symptoms[8].The adverse effects might have distinct clinical relevance at different dosages according to a population group.Caffeine intakes from all sources of up to 200 mg per day by pregnant women do not raise safety concerns for the fetus.However,considering the reduced maternal clearance and prolonged half-life during pregnancy along with the exposure of the fetus to maternal caffeine plasma levels,the unborn child is the most vulnerable group for adverse effects due to caffeine among the general population.Subjects with hypertension and/or advanced atherosclerosis have an increased risk for cardiovascular diseases.The administration of a single dose of 200 mg of caffeine significantly increases blood pressure and decreases myocardial blood,and such changes could increase the risk of acute cardiovascular events in these population groups[4].
The US FDA has defined traditional pharmacy compounding as‘the combining or altering of ingredients by a licensed pharmacist in response to a licensed practitioner's prescription for an individual patient,which produces a medication tailored to that patient's special medical needs”[9].In Brazil,this sector is regulated by the National Health Surveillance Agency(ANVISA),and the de finition is the same as that of the American agency[10].There are approximately 8200 compounding pharmacies registered in the country,representing about 10%of the country's drug market[11].Nevertheless,as it is a highly competitive market,the pharmacies use digital technologies to increase sales.Phones,websites,drive-thru systems,message ordering,and cell phone applications have been applied to interact with customers.
Adulteration with synthetic drugs is a recurring problem with so-called natural medicines[12].Cases of adverse effects resulting from the intentional addition of synthetic drugs in herbal medicines have been reported[13].For example,fenproporex,chlordiazepoxide and fluoxetine were identified as adulterants in prescription diet pills,and users reported headaches,palpitations,chest pain,nausea,insomnia and fatigue[13,14].The clinical consequences can be serious and sometimes life-threatening.This is especially true when patients use medications with potential interactions or when patients have other predisposing medical conditions.These incidents emphasize the importance of detecting the presence of undeclared synthetic drugs in herbal medicines to ensure their safety.Food supplements,pharmaceuticals,and compounded drugs are under distinct regulations,and all are potential targets for adulterations[12,14–19].
This work aimed to investigate the addition of undeclared caffeine as an adulterant in compounded formulations for weight loss.Since caffeine may be present naturally in some vegetable raw materials of the formulations,all of the components were also analyzed separately.A high-performance liquid chromatography with diode array detection(HPLC-DAD)method was developed to analyze the undeclared synthetic caffeine present in different pharmaceutical products.The presence and content of caffeine were confirmed using a comparative ultra-high performance liquid chromatography-tandem mass spectrometry(UHPLC-MS/MS)method.The quality and safety requirements of health products have been a recurrent matter among regulatory agencies.Accordingly,this study also aims to highlight the critical points concerning the regulation of compounded and industrialized products containing caffeine.
2.Materials and method
2.1.Instrumentation and apparatus
The chromatographic separations were carried out on a Knauer(Berlin,Germany)HPLC system,which consisted of a Smartline Pump 1000 coupled to a Smartline Manager 5000,and a multichannel UV spectrophotometer detector based on diode array technology (Smartline UV Detector2600)equipped with ChromGate?(Knaeur)software(Version 3.3.1).The chromatographic runs were conducted at room temperature(21 ±2 °C)using a reverse-phase C18column(4.6 mm × 250 mm,5 μm;Thermo Scientific)with an Acclaim?120 C18guard cartridge(4.3 mm × 10 mm,5 μm;Dionex Corporation,Sunnyvale,USA).Samples were injected using a sample injector equipped with a 20 μL loop.The detection was performed at 220 nm.
Mass spectrometry experiments were performed on an Agilent 1260 In finity LC-MS chromatograph with automatic injection and an Agilent 6430 triple quadrupole mass detector(Santa Clara,CA,United States).The chromatographic column was a Poroshell 120 EC-C18(3.0 mm × 100 mm)with a 2.7 μm particle size.An electrospray ionization source(ESI)was used to ionize the chromatographic effluent generated until 7.0 min.The parameters for ESI were optimized to give the best response and signal stability of the analyte.The final optimized parameters were a nitrogen gas flow of 11 L/min,a nebulizer pressure of 30 psi,a capillary voltage of± 2.4 kV and a drying gas temperature of 250 °C(N2).The ionized compounds were subsequently analyzed in an Agilent 6430 triple quadrupole mass spectrometer operating in the multiple reaction monitoring(MRM)mode with a resolution of 0.7 m/z(FWHM).The quantification transitions were divided into three temporal segments of acquisition,and the dwell time for each transition was optimized to 20 ms.High purity nitrogen(99.999%)obtained from Linde(Munich,Germany)was used as the collision-inducing gas.The ions monitored in ESI-MS/MS for the qualitative confirmation of caffeine were m/z 195.10(precursor ion)and m/z 138.2(product ion).
2.2.Reagents and solutions
The certified reference material caffeine was purchased from Sigma-Aldrich(Germany)with a declared purity of 99.0%.A stock solution of caffeine(1.0 g/L)was prepared in ultrapure water,and working solutions were obtained by dilutions of the stock solution with water.
Phaseolus vulgaris,Caralluma fimbriata,Cassia nomame,Fucus vesiculosus,Equisentum sp.,Plantago psyllium,Cordia ecalyculata,green tea(Camellia sinensis),Spirulina maxima,Passiflora sp.,Rhamnus purshiana,Garcinia cambogia,Citrus aurantium,Glucomannan(Amorphophallus konjak),Ma Huang(Ephedra sp.),Paullinia cupana,chitosan,and Cassia augustifolia were obtained from local compounding pharmacies,and all were raw materials in their bulk forms.
HPLC-grade solvents methanol(MeOH)and acetonitrile(ACN)were obtained from the Tedia Company(USA).Orthophosphoric acid(H3PO4)(85%,m/v)was purchased from Merck(Darmstadt,Germany).The methanol for analysis by UHPLC-MS/MS was of Chromasolv LC-MS grade and supplied by Sigma-Aldrich(St.Louis,MO,United States).
2.3.Sample source
The samples were acquired from pharmacies that announced herbal weight loss products in nine different Brazilian states(i.e.,Ceará,Distrito Federal,Goiás,Minas Gerais,Parana,Rio de Janeiro,Rio Grande do Sul,Santa Catarina,and S?o Paulo).The samples were purchased by contacting the pharmacies by email,telephone or via their websites.A pool of 20 capsules was prepared for both the determination of caffeine by the HPLC-DAD method and confirmatory analysis by UHPLC-MS/MS.
2.4.HPLC condition
The HPLC determination of caffeine was carried out by using a constant eluent composition of 0.1%phosphoric acid-ACN(70:30,v/v)and a gradient flow rate from 0.8 to 1.5 mL/min.The flow rate conditions were as follows:0.8 mL/min(0–4.4 min),0.5 mL/min(4.5–5 min),0.5–1.5 mL/min(5–10 min),and 1.5 mL/min(10 min).The total run time was 10 min.A re-equilibration period of 5 min was used between individual runs.Before the daily chromatographic experiments,the C18column was conditioned with the mobile phase for 45 min.
The equivalent weight of one capsule was dissolved in 25 mL of hot water(90°C)in a volumetric flask in order to perform the HPLC-DAD analysis.After extracting the caffeine by infusion and sonication for 30 min,the quantitative determination was performed in triplicate.All extracts were diluted forty times and filtered through a 0.45 μm cellulose acetate membrane prior to injection into the HPLC system.For studying the caffeine content in bulk raw plant extracts,36 herbal materials were acquired from different compounding pharmacies in Southern Brazil(Rio Grande do Sul).The herbal extracts were analyzed by the same procedure as the herbal weight loss pharmacy-compounded products aforementioned.
2.5.UHPLC-MS/MS condition
For confirmatory analysis by UHPLC-MS/MS,an isocratic elution was performed using 0.1%acetic acid/methanol(50:50,v/v)under a flow rate of 0.4 mL/min.The injection volume was 5 μL,and the column was maintained at 30°C.The ESI ionization source was used in the positive mode.The drying gas was under a flow of 13 L/min at 350°C.The nebulizer pressure was 40 psi,and the capillary voltage was 4000 V.
The equivalent weight of one capsule was dissolved in 25 mL of methanol.Methanol was the best choice for sample extraction because it is more protic than acetonitrile,facilitating the ionization and increasing the caffeine signal.The sample was then sonicated for 15 min in an ultrasonic bath.All extracts were diluted 100 times, filtered through a 0.2 μm hydrophilic membrane and transferred to the vials for analysis.
2.6.Validation of HPLC-DAD method
The HPLC-DAD method was validated by determining the following operational characteristics:specificity,linearity,limits of quantification(LOQ)and detection(LOD),precision and accuracy[20,21].
The method specificity was evaluated by studying the interference of other amines(L-tyrosine,p-octopamine,p-synephrine,tyramine,and hordenine)in caffeine analysis.Phaseolus vulgaris,Caralluma fimbriata,Cassia nomame,Fucus vesiculosus,Equisentum sp.,Plantago psyllium,Cordia ecalyculata,green tea(Camellia sinensis),Spirulina maxima,Passiflora sp.,Rhamnus purshiana,Garcinia cambogia,Citrus aurantium,Glucomannan(Amorphophallus konjak),Ma Huang(Ephedra sp.),Paullinia cupana,chitosan and Cassia augustifolia were also used to determine the specificity of the method.These bulk raw materials were treated in the same way as the samples of compounded formulations.
The method linearity was evaluated by six-point calibration curves performed on three different days.The LOD and LOQ were calculated using the following equations:LOD=3.3×Sa/b and LOQ=10×Sa/b,where Sa is the intercept standard deviation and b is the slope.The precision was expressed by the variation coefficients expressed as the relative standard deviation(RSD)of results obtained in triplicate for three different analyte concentrations.The accuracy was evaluated as a percentage of recovery obtained from analyzing samples spiked with known amounts of a reference substance at three different levels.The percentage recovery was calculated using the formula proposed by the AOAC[22].
3.Results and discussion
3.1.Determination of caffeine by HPLC-DAD and UHPLC-MS/MS
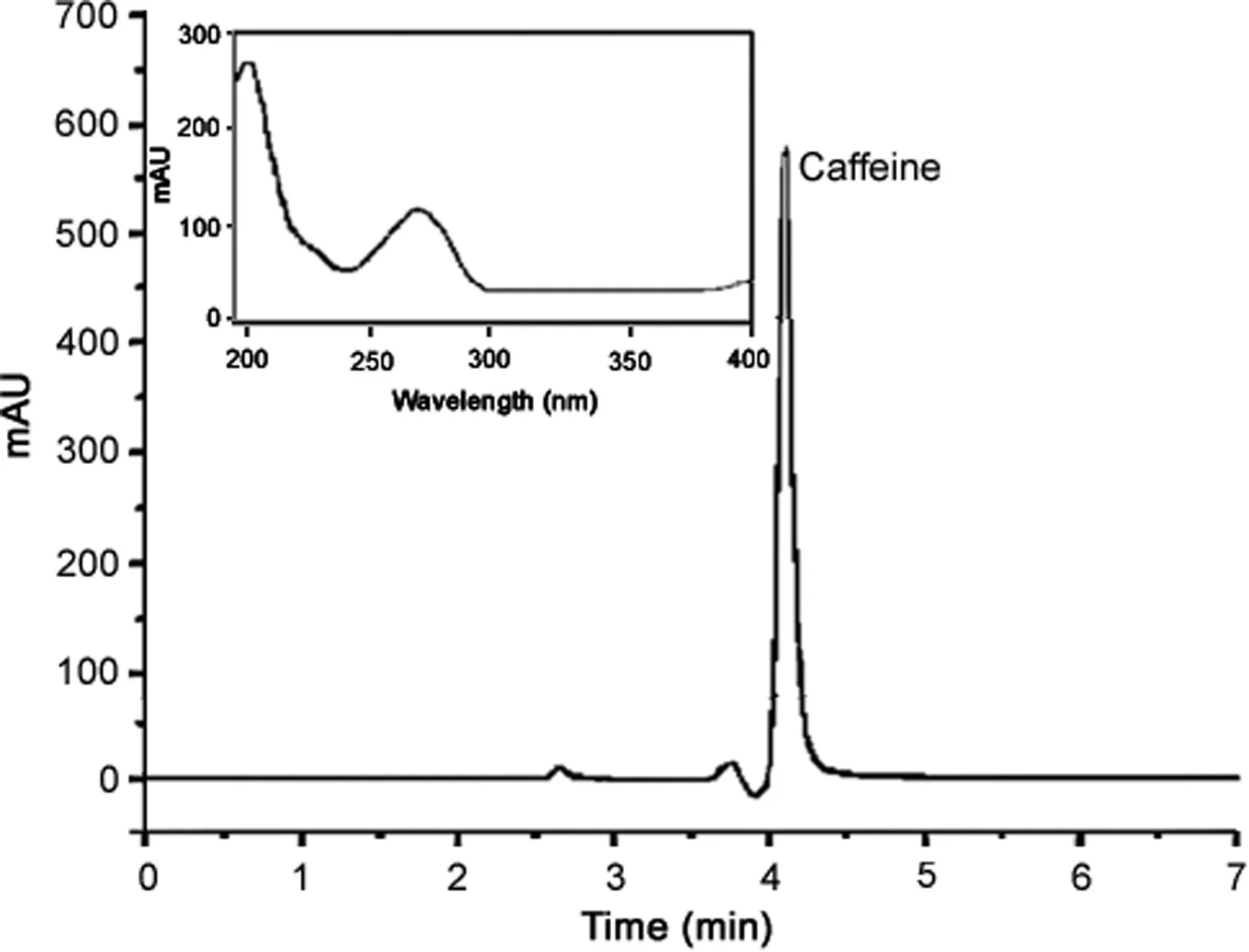
Fig.1.HPLC-DAD chromatogram obtained for caffeine.
In the present work,validation experiments were carried out for the determination of caffeine in different pharmacy-compounded products.The caffeine can be selectively determined in formulations for weight loss containing different plant materials,as observed in Fig.1.An HPLC method using acetonitrile/0.1%H3PO4(30:70,v/v)as the mobile phase and a gradient elution method with a programmed flow rate was optimized.The method showed to be selective for the determination of caffeine without interference from any other biogenic amines,such as synephrine,tyramine,L-tyrosine,p-octopamine,and hordenine.
The presence of caffeine was confirmed by a UHPLC-ESI-MS/MS comparative method.As can be observed in Fig.2,for sample “O”,caffeine can be determined in formulations containing plant materials and other components.The confirmation measurement was based on the MRM mode by evaluating the ion fragments at 195.1 m/z(molecular ion)138.1 m/z(quantifier ion)and 110.1 m/z(qualifier ion).The samples analyzed by the UHPLC-ESI-MS/MS confirmatory method were selected after the screening step by the validated HPLC-DAD method.
3.2.Validation of HPLC-DAD method for caffeine determination
The optimized method was validated based on the principal analytical validation parameters.Linearity data,analysis of variance(ANOVA),LOD and LOQ for determination of caffeine by the developed HPLC-DAD method are concise in Table 1.No interfering peaks were found in the chromatogram due to sample excipients.The linearity data were validated using ANOVA,which demonstrated significant linear regression(p<0.05)and no significant deviation from linearity(p<0.10).The sensitivity of the chromatographic system employed was assessed by determining the LOD and LOQ;the results were considered low and demonstrated good sensitivity for the method.Precision and accuracy data are summarized in Table 2.The low RSD obtained for all of the samples spiked with standards indicated good precision and repeatability for the method.The accuracy of the method was acceptable,as it was 97.2%with an SD value less than 2.2.
3.3.Sampling
In the present study,we searched websites of compounding pharmacies that advertised weight loss formulations.A total of 190 compounding pharmacies were contacted by email,telephone,via the pharmacy website or personally to request any available natural weight loss products.The appealing practice by pharmacies e-commerce could be observed,once the websites offer benefits that do not always correspond to the compounded product.Alluring claims such as"Belly's dry","Miracle weight loss formula",and"Lymphatic drainage in capsules"as well as the use of images that demonstrate the quick and effective weight loss are often used by pharmacy websites.Such claims have been employed with the unique purpose of improving sales,consequently promoting an indiscriminate use of these products.
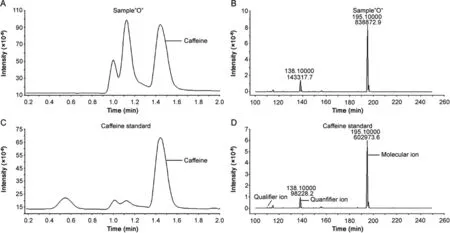
Fig.2.Total ion chromatograms(TIC)and ESI-MS/MS spectrum obtained for the caffeine analysis of(A and B)compounded formulation containing Citrus aurantium,L-carnitine,green tea,and chitosan(sample “O”)in comparison to(C and D)caffeine standard by UHPLC-ESI-MS/MS.

Table 1 Linearity data,analysis of variance,LOD and LOQ for determination of caffeine by the developed HPLC-DAD method.

Table 2 Precision and recovery for determination of caffeine by the developed HPLC-DAD method.
One hundred weight loss compounded formulations were received from seventy pharmacies.In disagreement with the regulations,all formulations were suggested without requiring any medical prescription[10].In addition,about 40%of the acquired formulations had brand names on their labels,such as“Compound for Reducing Body Size”, “Weight Loss Compound”, “Weight Loss Compound IV”,“Weight Loss Compound Magri II”, “Plus La Santa Slimming”, “Fine Fit M”, “Compound for Reducing Fat Mass”,“Slimming Compound”, “Compound for Reducing Appetite”,“Slimming Vegetable Compound”,“Oriental Cleaning”,and “Super Fat Burners M”.The names given to the products acquired in this work were appealing,with the clear purpose of stimulating consumption.A compounded product must contain only its formulation detailed on the label.
According to Brazilian regulations,industrialized capsules containing caffeine have been commercialized under"food for athletes"as a product category and can be sold with a brand name.The caffeine supplements for athletes should state the highlighted and bold warning,"This product should not be consumed by children,pregnant women,the elderly,and those who are sick".Their labels cannot induce the consumer to ingest a product,which does not have any action for weight loss,definition or the gain of muscle mass.The terms"anabolic","muscular hypertrophy","muscle mass","fat burning","fat burners","increased sexual ability","anticatabolic","anabolic",or similar terms cannot be present on the packaging[23].However,these requirements are not always followed by manufacturers,and some products end up being removed from the market[24–26].Warnings required for industrialized products do not exist for pharmacy-compounded capsules containing caffeine,once they are prescription-only healthcare products.
The pharmaceutical regulation is very diverse around the world,and the harmonization of such regulations is a subject of discussion among the main global regulatory agencies.The sampling is a useful tool to analyze merchant compliance with applicable regulations.In the USA,concerns have been also raised that some pharmacies were going beyond traditional drug compounding for individual patients and selling large quantities of drugs without meeting safety and other requirements applicable[9,27].

Table 3 Labeled vegetal compounds in all weight loss compounded formulations(n=100),their frequency and caffeine content in each component.
3.4.Determination of caffeine in compounded formulations for weight loss
Table 3 shows the vegetal compounds present in all of the weight loss products analyzed in this study(n=100),their frequency,and caffeine content by using the proposed HPLC-DAD method.Caffeine was present only in the following components:Cordia ecalyculata,Cassia angustifolia,green tea(Camellia sinensis),white tea(Camellia sinensis),Cassia nomame,and Paullinia cupana.Green tea(Cammelia sinensis)and Paullinia cupana had caffeine concentrations equivalent to those already described in the literature[28–30].In contrast,white tea(Cammelia sinensis)presented levelslowerthan those reported in the literature(43.080 mg/kg)[31,32],which represents about seven-fold more caffeine than the level found in this work.However,batch to batch variations are common in raw materials of plant origin.The presence of caffeine in Cassia angustifolia and Cassia nomame has not been previously reported.Herein,it is important to emphasize the possibility that both the herbal materials analyzed were not,in fact,the vegetal species that were declared in the product.In this case,our research tried to have access to the same bulk raw materials normally used in the weight loss formulations by the studied compounding pharmacies.Thus,we consider that the sellers are acquiring the herbal materials they really intend to use in terms of the natural active principle.Thus,C.angustifolia and C.nomame were considered as the species that the manufacturers really use for compounding the studied weight loss formulations.
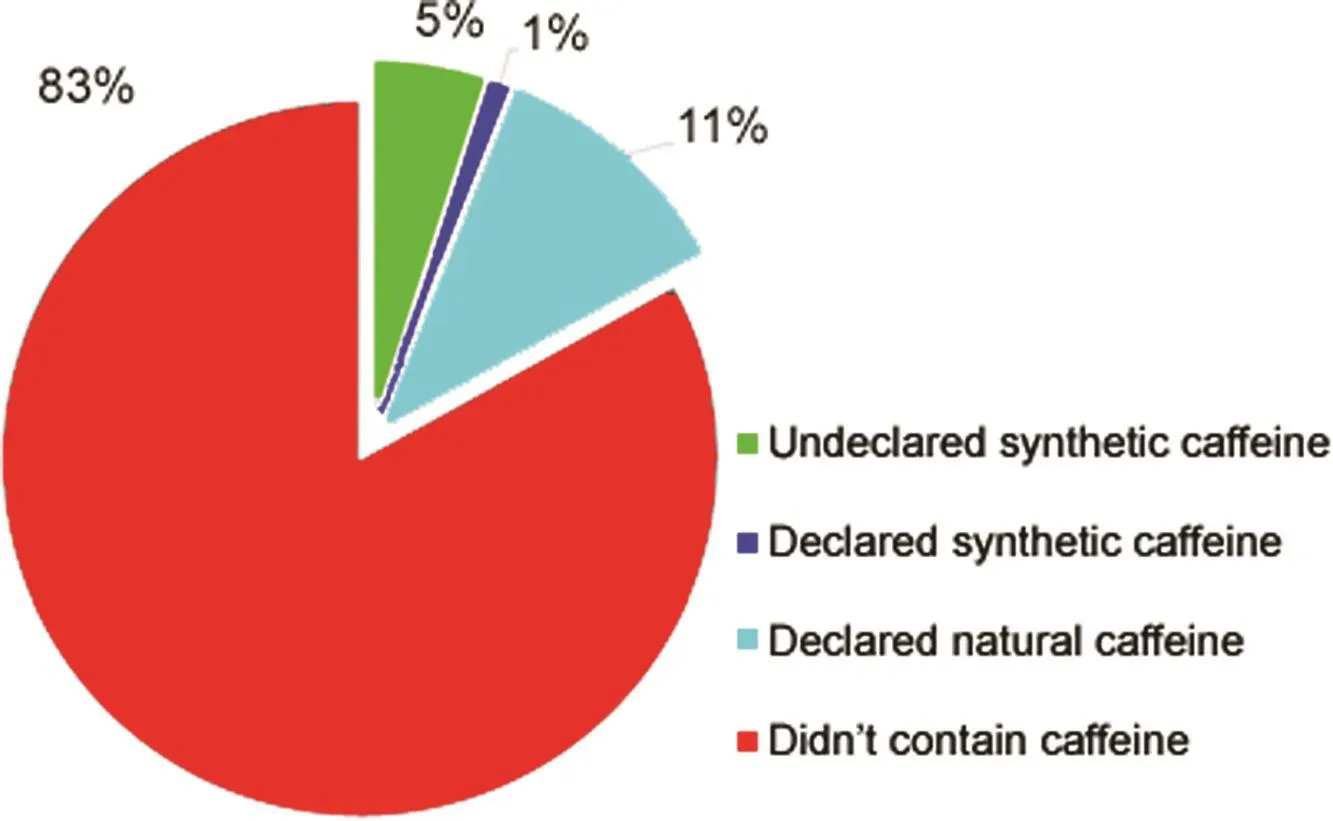
Fig.3.Incidence of caffeine in the analyzed samples.
Cordia ecalyculata presented a compatible caffeine content or one that was even higher than those presented by Camellia sinensis and Paullinia cupana,species that recognizably have high levels of this substance.This data may justify its frequent use in weight loss formulations.
All weight loss pharmacy-compounded products(n=100)were analyzed with regard to the caffeine content by using the validated HPLC-DAD method.Fig.3 shows the incidence of caffeine in the analyzed products;this substance was detected either from natural or synthetic sources in 17 samples.Although the differentiation between natural and added caffeine can be somewhat hampered by the possible batch-to-batch variability of caffeine contents in raw materials,levels much higher than those expected from natural sources(e.g.,Table 3)may be attributed to the intentional addition of a synthetic drug.Table 4 presents the formulation description,brand name,dose and caffeine content of those analyzed formulations that contained caffeine.The caffeine presence was qualitatively confirmed by monitoring the ions produced in ESI with m/z values of 195.10(precursor ion)and 138.2(product ion)in the comparative UHPLC-ESI-MS/MS method.In five analyzed samples(samples E,F,I,N,and O),the adulteration with synthetic caffeine was obvious,as the contents found were not declared on the labels and could not be attributed to any product of natural origin.Twelve percent of the samples had either the natural or synthetic caffeine content as stated on the label.
The samples with the highest caffeine content were sample I and sample N.After analyzing both samples,it is possible to note that the caffeine content does not come from the natural raw materials present in the formulations.Sample I contained 224.4 mg of caffeine per dose,and its recommended labeled dosage is two doses per day,which represents a daily intake of 448.8 mg caffeine.The sources of caffeine in this formulation are Cordia ecalyculata and Cassia nomame,which together could provide approximately 10.3 mg of caffeine per dose.In sample N,70.8 mg of caffeine per dose was found,and the pharmacy recommended three doses a day,which represents an intake of 212 mg of caffeine per day.White tea could provide a caffeine content of 1.2 mg per dose considering the value determined in this study.
The problem is that the consumers of these products are not aware of the risks associated with the caffeine added illegally.The user can present typical symptoms of this substance and even interactions of it with other drugs.The caffeine may increase sleep latency and reduce sleep duration in some adult individuals,particularly when consumed close to bedtime[4].Individual sensitivity to the effects of caffeine is well recognized[33].However,caffeine consumption can cause adverse effects such as increases in blood pressure,a rapid heartbeat,and tremors,and the caffeine-induced hypokalemia could contribute to ventricular arrhythmias[4,33].
Another irregularity found in the sampling process was the presence of hydrochlorothiazide(sample G),a potent diuretic,and benzocaine(sample M),an analgesic.Even if stated on the label,their inclusion can be considered an illegal practice since pharmacies inserted these drugs into the formulations deliberately.Theaddition of synthetic drugs,without first asking or warning the consumer,occurs in order to satisfy the consumer and ensure that the product works as intended as well as to provide quicker effects.In fact,for weight-loss products,consumers tend to quit using the products if they do not realize any initial effects.In contrast,if the product quickly succeeds in providing the desired results,more units are likely to be sold,thus increasing the pharmacy's profit.However,this practice exposes the user to potential drug side-effects and drug interactions.
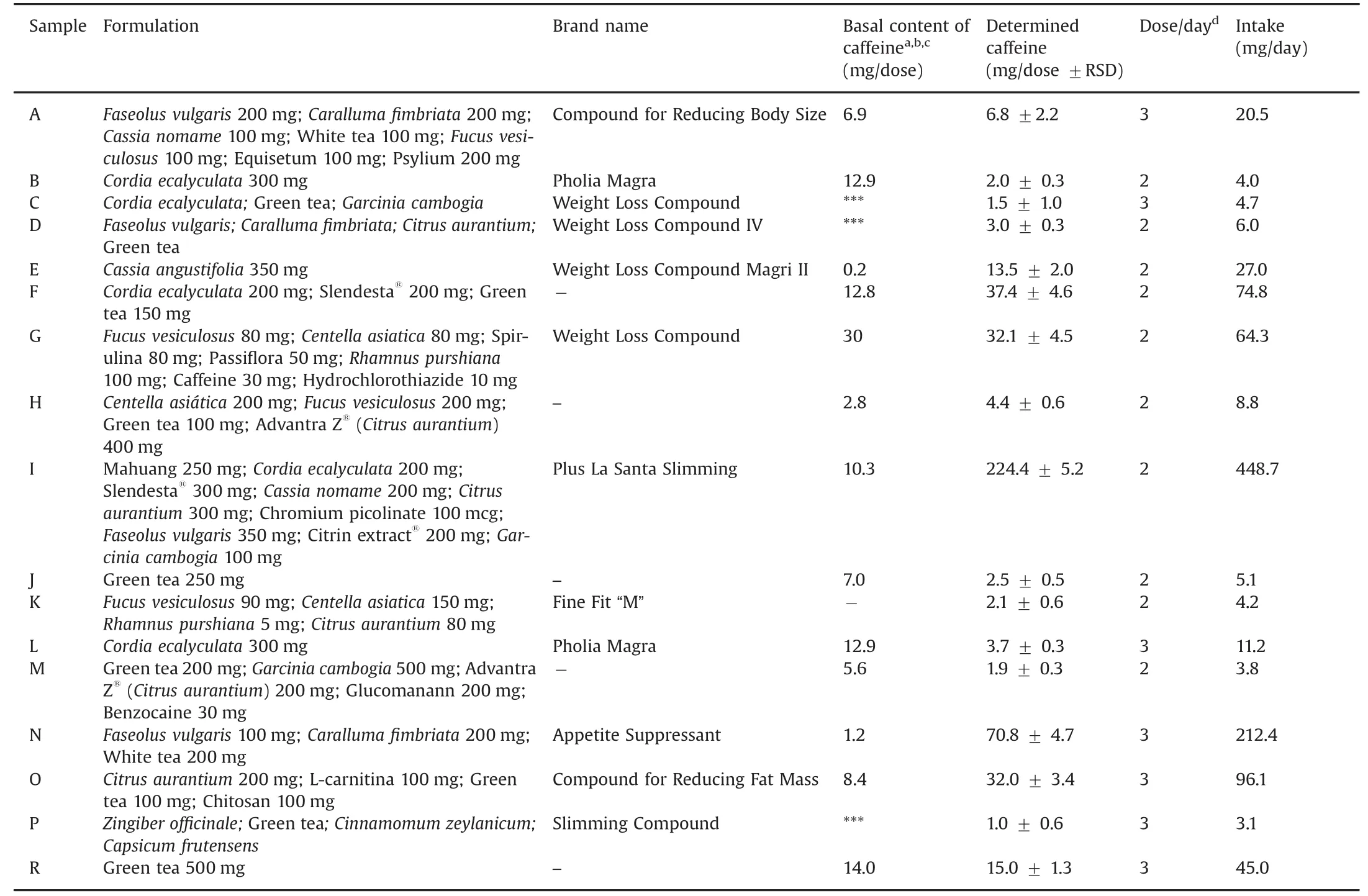
Table 4 Description of compounded formulation,brand name,doses and caffeine content of those analyzed pharmacy-compounded products that contained caffeine(n=17).
A final comparison with the industrialized dietary supplements would be relevant.It is noteworthy to point out that caffeine supplements for athletes cannot contain any other substances and must declare the dosage,which must be between 210 and 420 mg per serving[23].However,this does not guarantee that the dietary supplements available on the market adhere to these concentrations.Several product recalls have already been made for these reasons[17,18,33,34].
4.Conclusion
An HPLC-DAD method for the separation and identification of caffeine in compounded formulations was validated.From one hundred analyzed samples,this substance was present in seventeen.The adulteration was proven in five products by UHPLC-MS/MS method.The users of these products reported here could have increased sleep latency and a reduced sleep duration,particularly when consumed close to bedtime.Caffeine consumption acutely increases blood pressure and may also present adverse effects such as increases in a rapid heartbeat,tremors and arrhythmias.Moreover,the consumer may use other substances and/or medicines,and interactions deleterious to the health of consumers can occur.Other irregularities were also indicated such as formulations assigned a brand name,appealing labels,alluring claims by websites in order to improve the sales,and the addition of synthetic drugs without first asking the user.Lastly,it is noteworthy that due to the exponential increase in connectivity by the Internet,people involved in the manufacturing and marketing of intentionally adulterated health products have gained access to a wide marketplace.In countries that have very weak or non-existent health surveillance systems,adulteration can have an even bigger impact.This background plus the growing consumption of weight loss formulations indicates the need to take enforcement measures by competent health authorities in order to detect possible adulterations.
Conflicts of interest
The authors declare that there are no conflicts of interest.
 Journal of Pharmaceutical Analysis2018年6期
Journal of Pharmaceutical Analysis2018年6期
- Journal of Pharmaceutical Analysis的其它文章
- Novel ligand-based docking;molecular dynamic simulations;and absorption,distribution,metabolism,and excretion approach to analyzing potential acetylcholinesterase inhibitors for Alzheimer's disease
- Recovery rates of combination antibiotic therapy using in vitro microdialysis simulating in vivo conditions
- Evaluation of naproxen-induced oxidative stress,hepatotoxicity and in-vivo genotoxicity in male Wistar rats
- Cytotoxic effect of Rosa canina extract on human colon cancer cells through repression of telomerase expression
- Long-term stability of gentamicin sulfate-ethylenediaminetetraacetic acid disodium salt(EDTA-Na2)solution for catheter locks
- Highly sensitive LC–MS/MS method to estimate doxepin and its metabolite nordoxepin in human plasma for a bioequivalence study
