Evaluation of naproxen-induced oxidative stress,hepatotoxicity and in-vivo genotoxicity in male Wistar rats
Mir Hilal Ahmad,Mahino Fatima,Moarak Hossain,Amal Chandra Mondal
aSchool of Life Sciences,Jawaharlal Nehru University(JNU),New Delhi 110067,India
bInterdisciplinary Brain Research Centre,Faculty of Medicine,Aligarh Muslim University,Aligarh,India
Keywords:Genotoxicity Naproxen Wistar rat Antioxidants Oxidative stress DNA damage
A B S T R A C T Naproxen(NP),a nonsteroidal anti-inflammatory drug(NSAID),is used for the treatment of common pain,inflammation and tissue damage.Genotoxicity testing of NP is of prime importance as it represents the largest group of drugs to which humans are exposed.Not many genotoxic studies are reported on NP;therefore,the present study investigated the detailed genotoxic and oxidative stress properties of NP.Male Wistar rats were administered NP orally at the doses of 38.91 and 65.78 mg/kg body weight for 14 days.Reduced glutathione(GSH),superoxide dismutase(SOD),catalase(CAT)and lipid peroxidation(LPO)activities/levels were measured in the liver,kidney and brain tissues.The aspartate aminotransferase(AST),alanine aminotransferase(ALT),alkaline phosphatase(ALP)activities,and total bilirubin(TBIL)levels were measured in the liver tissues.Micronucleus frequency(micronucleus test MNT)and DNA damage(comet assay)were performed in the bone marrow cells and leukocytes,respectively.The results showed that NP treatment decreased the GSH levels and increased the SOD,CAT,LPO,ALT,AST,ALP and TBIL activities/levels compared to the control(p<0.05).Results of MNT showed an increased micronucleus induction and comet assay showed a significant increase in DNA damage in the NP treated animals(p<0.05).Treatment of NP resulted in the biochemical imbalance and induced oxidative stress that deteriorated the integrity of the cells,which caused significant damage to the genetic material and affected liver function in male Wistar rats.Therefore,NP is a potential genotoxic agent that induces genotoxicity and oxidative stress.
1.Introduction
NP(S)-6-(Methoxy-α-methyl-2-naphthalene acetic acid),the propionic acid derivative,is a non-steroidal anti-inflammatory drug(NSAID),widely used for the treatment of primary dysmenorrhoea,rheumatoid arthritis,osteoarthritis,ankylosing,tendinitis,bursitis,acute gout and juvenile arthritis[1,2].The main mechanism of action of NP is the inhibition of COX-dependent synthesis of proinflammatory algogenic prostaglandins by inhibiting cyclooxygenase(COX-1 and COX-2)activity[3].NSAIDs generate free radicals resulting in the generation of reactive oxygen species(ROS)[4].
ROS generation induces oxidative stress and is associated with cell death[4].Oxidative stress has been implicated as a general mechanism in the toxicity of many NSAIDs[5].Recent studies have reported that NSAIDs induce ROS production in cells[6]and elicit and/or contribute to oxidative stress[7,8].NSAIDs have been associated with liver injury;the mechanism is thought to be immunological idiosyncrasy[9,10].ROS produced in the cells results in error-prone DNA repair and increased susceptibility to apoptosis,which can all lead to cytotoxic,mutagenic,or carcinogenic events[11].
Genotoxic studies on NP are rather scarce,and there is very little information on the potential genotoxic effects of NP despite its wide range of applications,which is mentioned by another author as well[12].Nevertheless,it has been reported that NP can alter the biochemical biomarkers and genetic materials[13].Previous studies have reported a weak or no genotoxic effect of NP,employing either one or two parameters to assess the genotoxicity of NP.Further studies on this drug with additional factors are worth performing.In this milieu,a detailed study employing genotoxic and biochemical biomarkers in different organs was conducted in order to determine the possible organ-specific oxidative stress potential,hepatotoxicity and genotoxicity of NP.This will help to analyze its safety and efficacy,and furthermore can be interpreted and/or extended to the assessment of health risk to the humans.The set of tests used in this study can be considered as a reliable biomarker for the evaluation of NSAIDs toxicities in humans.
2.Materials and methods
2.1.Test animals
The study was conducted after obtaining Institutional Animal Ethical Committee's clearance.All protocols and experiments were conducted in strict compliance with ethical principles and guidelines provided by CPCSEA,New Delhi,India,after approval of Institutional Animal Ethics Committee(IAEC),Central Animal House Jawaharlal Nehru Medical College,Aligarh Muslim University,Aligarh(U.P),Registration No 401/RO/c/2001/CPCSEA.Male rats(Wistar strains),8–10 weeks old,weighing 180 ± 30 g,were brought to the laboratory one month before the start of the experiment for acclimatization to the laboratory conditions.Rats were housed in 3 different groups in separate cages,maintained under conditions of 12 h dark/light cycle under conditions of constant temperature(22±2°C)and humidity(60%–70%).Under standard laboratory conditions,the animals were allowed to access food and water ad libitum.
2.2.Test drug
The test drug used in the present study was NP,an NSAID.NP was procured from Sigma Chemicals Co.(USA).It was dissolved in DMSO and orally administered to the animals by oral gavage method.The administered DMSO concentrations did not cause any toxicity or affect the viability of the animals.
2.3.Experimental design
Rats were divided into 3 experimental groups:control,Treatment I,and Treatment II groups(6 rats/group).The control was administered with 10%dimethyl sulphoxide(75 μL DMSO/kg b.wt)for 14 days.Treatment I group was administered with 1/8th(38.91mg/kg b.wt)of LD50of NP for 14 days.Treatment II group was administered with 1/4th(65.78 mg/kg b.wt)of LD50of NP for the same duration.Rats were sacrificed by cervical dislocation,immediately dissected to obtain the bone marrow,liver,kidney and brain tissues.These tissues were utilized for biochemical estimations of GSH,SOD,CATand LPO activities/levels.Liver function parameters such as AST,ALT,ALP activities and TBIL level were determined in the liver tissues.For genotoxic studies,bone marrow was used for micronucleus test(MNT),and leukocytes for comet assay(single cell gel electrophoresis).
2.4.Statistical analysis
All the data were subjected to statistical analysis.The values were expressed as mean±SE.Statistical analysis was performed by one-way analysis of variance(ANOVA)followed by Tukey multiple comparison tests.p values<0.05 were considered as significant.
2.5.Biochemical assays
Biochemical assays were conducted for determination of nonenzymatic(GSH),enzymatic(SOD,CAT)and oxidative stress(LPO)biomarkers.For the estimation of biochemical parameters,tissues were homogenized and centrifuged to separate the post mitochondrial fraction from homogenate.The tissues were homogenized in 0.1 M phosphate buffer,pH 7.4,to obtain the 10%homogenates using a Potter-Elvehjem homogenizer,6–8 strokes at medium speed.During this operation,the samples were kept under ice.Homogenates were centrifuged at 10,000 rpm for 20 min at 4°C.The sediments consisted of primary mitochondrial pellets and the supernatants were kept at-20°C until further analysis.Post mitochondrial supernatant(PMS)was used for the estimation of various biochemical analyses.Protein content in various samples was estimated by the method described by Lowry et al.[14]with bovine serum albumin(BSA)as a protein standard.
2.5.1.Nonenzymatic antioxidants
GSH was studied as a nonenzymatic antioxidant.Tissue-reduced glutathione level was estimated as total acid soluble sulfhydryl concentrations colorimetrically at 480 nm using Ellman's reagent dithiobis 2-nitrobenzoic acid(DTNB)as per the procedure modified by Jollow et al.[15].PMS was precipitated with sulphosalicylic acid(4.0%)in the ratio of 1:1.The samples were kept at 4°C for 1 h and then subjected to centrifugation at 4000 rpm for 15 min at 4°C.The assay mixture contained 0.4 mL supernatant,2.2 mL of 0.1 M sodium phosphate buffer(pH 7.4)and 0.4 mL DTNB making a total volume of 3 mL.The optical density of reaction product was read immediately at 412nm on a spectrophotometer and results were calculated using a molar extinction coefficient of 1.36 × 104M-1cm-1and were expressed as μmoles/gram tissue.2.6 mL phosphate buffer and 400 μL DTNB were taken as blank.
2.5.2.Enzymatic antioxidants
SOD and CAT together are enzymatic antioxidants providing first line defense against free radicals in tissues.The activities of both the enzymes were calculated by standard techniques.
2.5.2.1.Estimation of SOD activity.SOD activity was measured according to the procedure described by Misra and Fridovich[16].The assay mixture consisted of 0.8 mL of glycine buffer(50mM,pH 10.4),0.2 mL of supernatant(prepared in glycine buffer)and 20 μL of epinephrine in a final volume of 1.02 mL.SOD activity can be measured kinetically at 480 nm.The activity was measured indirectly by the oxidized product of epinephrine,i.e.adrenochrome.SOD activity was expressed as nmol of(-)epinephrine protected from oxidation by the sample compared with the corresponding reading in the blank.The activity was calculated by using its extinction coefficient(?)4.02 × 103M-1cm-1,and expressed as nmoles of epinephrine protected from oxidation/min/mg protein.
2.5.2.2.Determination of CAT activity.CAT activity was estimated by using the method of Clairborne[17]with slight modifications.1.95 mL of phosphate buffer(pH 7.4)was taken,and 1.0 mL of hydrogen peroxide and 50 μL of PMS were added in a 3mL cuvette.The total volume for the assay was 3 mL.Optical density(OD)was taken via kinetic method at 240nm in a spectrophotometer.The activity was calculated by using its extinction coefficient(?)39.6M-1cm-1,and expressed as μmoles of H2O2consumed/min/mg protein.
2.5.3.Oxidative stress
LPO occurs due to tissue exposure to free radicals and this biomarker for oxidative stress was assessed.
LPO was determined by the method of Mihara and Uchiyama[18]with slide modifications.Brie fly,0.25mL of tissue PMS was mixed with 25 μL of 10 mM butylated hydroxytoluene(BHT).3 mL of phosphoric acid(1%)and 1 mL of 0.67%thiobarbituric acid(TBA)were added and the reaction mixture was incubated at 95°C for 1h.The absorbance was measured at 535nm.The level of LPO was expressed as μmoles of thiobarbituric acid reactive substance(TBARS)formed/g tissue using a molar extinction coefficient of 1.56×105M-1cm-1.
2.6.Liver function test
Biochemical indices of liver function determinants include AST,ALT,ALP activities and TBIL level.The liver function test was performed in the 10%rat liver homogenate.Commercially available diagnostic test kits were used for these tests.ALT and AST activities were determined according to the method described by Reitman and Frankel[19].The method was standardized with Kinetic Method(Standard Karmen Unit assay)for accuracy and the absorbance was taken at 505 nm.TBIL level was determined by the method described by Cherian et al.[20].The absorbance of the color was measured at 546 nm(546 and 630 nm in bichromatic mode)which is directly proportional to the concentration of TBIL in the sample.The ALP activity was determined by the method of Kind and King[21].The OD was measured at 510 nm.
2.7.Genotoxic assessments
Free radicals affect the genetic constituents of tissue.Detailed assays,to assess clastogenic(disruption or breakages of chromosomes),aneugenic effects and DNA strand-damaging effects because of oxidative stress,were assessed.
2.7.1.MNT
micronucleus(MN)frequency in erythrocytes was evaluated according to the method described by Schmid[22].Both femurs were extracted from the test animals and cleared off from muscular tissues.The bone was cut open and the marrow was flushed out with 5%fetal bovine serum(FBS).The solution obtained was evenly suspended.The solution was kept for 5min at room temperature,the supernatant was discarded and pellet was saved.The sediment was mixed with a pipette,and a small drop of cell suspension was dropped at the one end of a clean dry slide and evenly smeared and finally air dried.The staining procedure followed a combination of May-Gruenwald and Giemsa staining in succession:the slides were first covered with undiluted May-Gruenwald solution for 3 min and replaced by dilute(1:1)May-Gruenwald solution with distilled water for 2min,followed by Giemsa staining in Giemsa for 5min.The slides were rinsed and dried,treated with xylene and mounted in dibutylphathalate xylene(DPX).All the slides were coded and scored by a single observer for analysable MN.A total number of 2000 erythrocytes were examined for each group under a light microscope(Nikon Eclipse E200),with oil immersion at 400×magnification.Scoring of micronuclei was performed on randomized and coded slides.The MN frequency was determined as follows:
MN frequency=(No.of cells containing MN)(1000)/Total No.of cells counted.
2.7.2.Comet assay
DNA damage was assessed using an alkaline comet assay as per as the method described by Singh et al.[23]with slight modifications.All of the comet assay experiment was performed under dim light conditions to avoid additional DNA damage.Cells were homogenized with PBS-CMF,20 mM EDTA,pH 7.4 and filtered through a 100 μm mesh strainer to get cell suspension.2mL PBSCMF was added to the cell suspension,followed by centrifugation at 2000 rpm at 4°C for 10min.The cell pellet was collected and resuspended in PBS-CMF.The cell viability test was performed using the Trypan blue exclusion method by Anderson et al.[24]and samples showing viability 80%were considered for comet assay.The cells were suspended in 0.5%low melting agarose(LMPA)and overlaid on slides precoated with a fine layer of 1.25%normal melting agarose(NMPA).The third layer of 0.75%LMPA was poured onto the slides.Slides were immersed in lysing solution(25M NaCl,100 mM EDTA,10 mM Trizma base,0.2 mM NaOH,1%Triton X-100 and DMSO,pH 10)for 1h at 4°C to lyse cells.The slides were later immersed in chill electrophoresis buffer(300mM NaOH,1 mM EDTA,pH>13)for 20 min to allow DNA unwinding,followed by electrophoresis at 25 V and 300 mA current in the same buffer for 30 min.Following electrophoresis,slides were neutralized in neutralizing buffer(0.4 M Tris buffer,pH 7.5).The slides were dried and stained with ethidium bromide.Photographs were obtained at 400×.A total number of 300 cells were scored per group,and 50 cells per replicate were analyzed with Cometscore?software (version 1.5,TriTek Corporation,Sumerduck).The degree of DNA damage was represented as percent DNA in the tail.
3.Results
3.1.Non-enzymatic antioxidant
GSH level decreased significantly in the liver,kidney and brain tissues in the treatment groups,when compared with the control.The trend of the decrease in GSH level of liver,kidney and brain tissues of treatment groups compared to the control is given in Fig.1.
Brain(%change 79.71%-40.73%),showed a maximum depletion of GSH level in the treatment groups with respect to the control followed by the liver(%change 73.33%-54.14%).Kidney(%change 80.64%-64.51%)showed the least decline in GSH level in the treatment groups compared to the control.The percent change was calculated as the ratio of decrease in the GSH level between the treatment groups and the control.
3.2.Enzymatic antioxidants
3.2.1.SOD
SOD activity increased significantly in the liver,kidney and brain tissues.Trends of increase in SOD activities of liver,kidney and brain tissues in the treatment groups compared to the control are given in Fig.2.
Brain(%induction 233.43%–317.65%)showed a maximum increase in SOD enzyme activity in the treatment groups with respect to the control followed by the liver(%induction 145.07%–187.111%).Kidney(%induction 149.95%–177.12%),showed the least increased SOD activity in the treatment groups compared to the control.
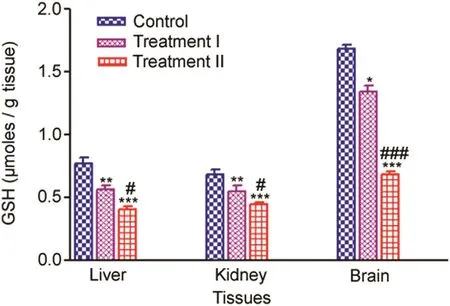
Fig.1.Tissue-specific GSH(μmoles/g tissue)measured after 14 days oral administration of NP in Treatment I(38.91mg/kg b.wt)and Treatment II(65.78 mg/kg b.wt)groups,and the control(75 μL DMSO/kg b.wt).Values are Mean ± SE(n=6).Analysis of variance(One-way ANOVA)followed by Tukey test.Significant differences were indicated by*p<0.05,**p<0.01 and***p<0.001,when compared with the control while#p<0.05 and###p<0.01 were used to show the significant difference between the treatment groups.
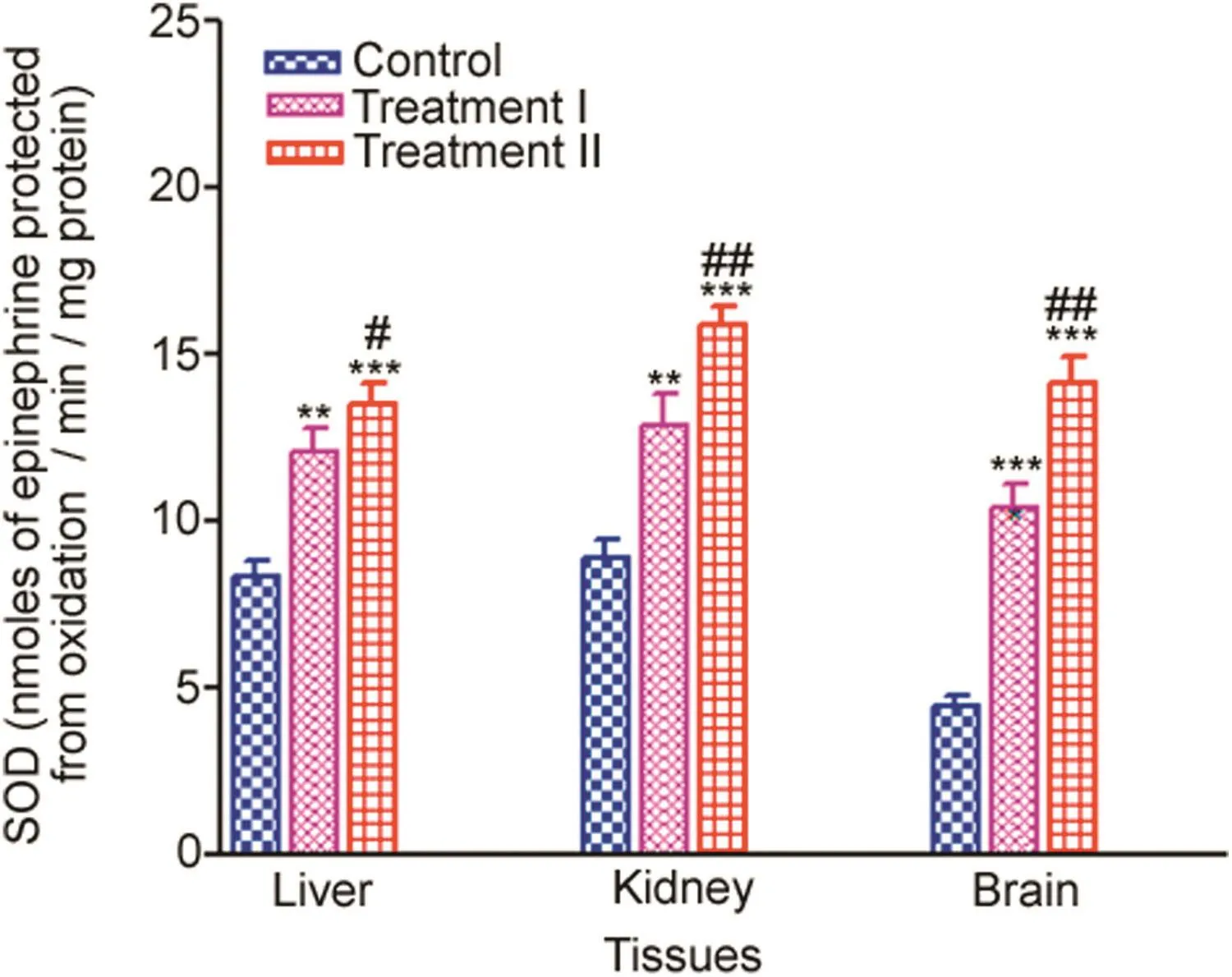
Fig.2.Tissue-specific SOD(nmoles of epinephrine protected from oxidation/min/mg protein)measured after 14 days oral administration of NP in Treatment I(38.91 mg/kg b.wt)and Treatment II(65.78 mg/kg b.wt)groups,and the control(75 μL DMSO/kg b.wt).Values are Mean ± SE(n=6).Analysis of variance(Oneway ANOVA)followed by Tukey test.Significant differences were indicated by**p<0.01 and***p<0.001,when compared with the control values while#p<0.05 and##p<0.01 were used to show the significant difference between the treatment groups.
3.2.2.CAT
CAT activity increased significantly in the liver,kidney and brain tissues in the treatment groups,when compared to the control.Trends of increase in CAT activities of liver,kidney and brain tissues in the treatment groups compared to control are given in Fig.3.
Liver(%induction 132.43%–179.21%)showed a maximum increase in CAT activity in the treatment groups with respect to the control followed by the kidney(%induction 132.86%–172.6518%).Brain(%induction 124.51%–137.78%)showed the least increase in CAT activity in the treatment groups compared to the control.
3.3.Induction of oxidative stress
Tissue-specific disturbances in the antioxidant system were observed in all the experimental rat groups;therefore,this generated oxidative stress,which was evaluated by estimation of LPO in the liver,kidney and brain tissues.
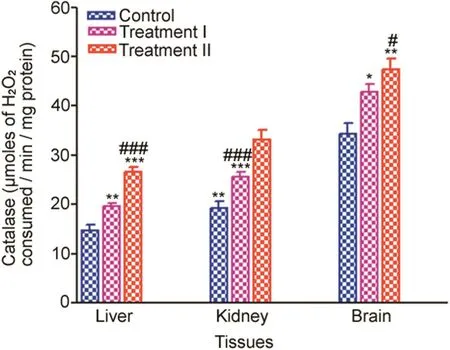
Fig.3.Tissue-specific CAT(μmoles of H2O2consumed/min/mg protein)measured after 14 days oral administration of NP in Treatment I(38.91 mg/kg b.wt)and Treatment II(65.78 mg/kg b.wt)groups,and the control(75 μL DMSO/kg b.wt).Values are Mean±SE(n=6).Analysis of variance(One-way ANOVA)followed by Tukey test.Significant differences were indicated by*p<0.05,**p<0.01 and***p<0.001 when compared with the control values while#p<0.05 and###p<0.001 level were used to show the significant difference between the treatment groups.
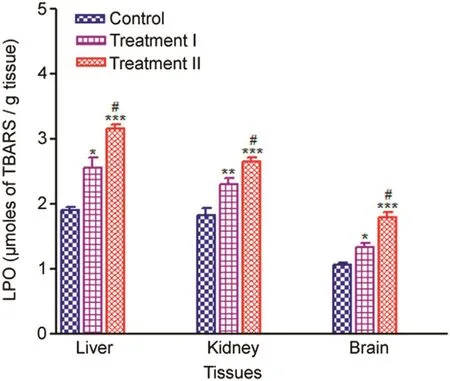
Fig.4.Tissue-specific LPO(μmoles of TBARS/g tissue)measured after 14 days oral administration of NP in Treatment I(38.91 mg/kg b.wt)and Treatment II(65.78 mg/kg b.wt)groups,and the control(75 μL DMSO/kg b.wt).Values are Mean ± SE(n=6).Analysis of variance(One-way ANOVA)followed by Tukey test.Significant differences were indicated by*p<0.05,**p<0.01 and***p<0.001,when compared with the control values while#p<0.05 was used to show the significant difference between the treatment groups.
LPO level increased significantly in the liver,kidney and brain tissues in the treatment groups compared to the control.The trend of increase in LPO of liver,kidney and brain tissues in the treatment groups compared to the control is given in Fig.4.
In the treatment groups,induction of LPO in the treatment groups compared to the control was significantly higher in the brain(%induction 124.91%–169.01%),followed by liver(%induction 135.40%–167.51%),and kidney(%induction 125.69%–145.38%)showed the least increase in LPO level compared to the control.
3.4.Effect of NP administration on liver function
Liver function was measured by liver-specific AST,ALT,ALP activities and TBIL level.AST,ALT,ALP activities and TBIL level significantly increased in all the NP administered rats(treatment groups),when compared to the control.The trend in the increase of ALT,AST,ALP activities and TBIL level in the treatment groups compared to the control is given in Fig.5.
ALT showed the maximum significant increase(%induction 164.59%–208.27%)in the treatment groups compared to the control followed by TBIL(%induction 147.39%–192.17%)and ALP(%induction 164.93%–189.32%).AST activities showed the least increase in the treatment groups compared to the control(%induction 131.90%–164.70%).
3.5.Genotoxic assessments
3.5.1.Effect of NP on MN frequency
Two types of erythrocytes could be distinguished using differential staining by May-Gruenwald-Giemsa in bone marrow cells.The polychromatic erythrocytes with MN(MNPCEs)significantly increased in the treatment groups compared to the control.NP in the treatment groups induced a statistically significant increase in the percentage of PCEs with micronuclei in a dose-dependent manner,with the treatment I group showing a mean±SE value of 1.87±0.15 and treatment II group 2.54±0.20,when compared to the control values(1.09±0.10).
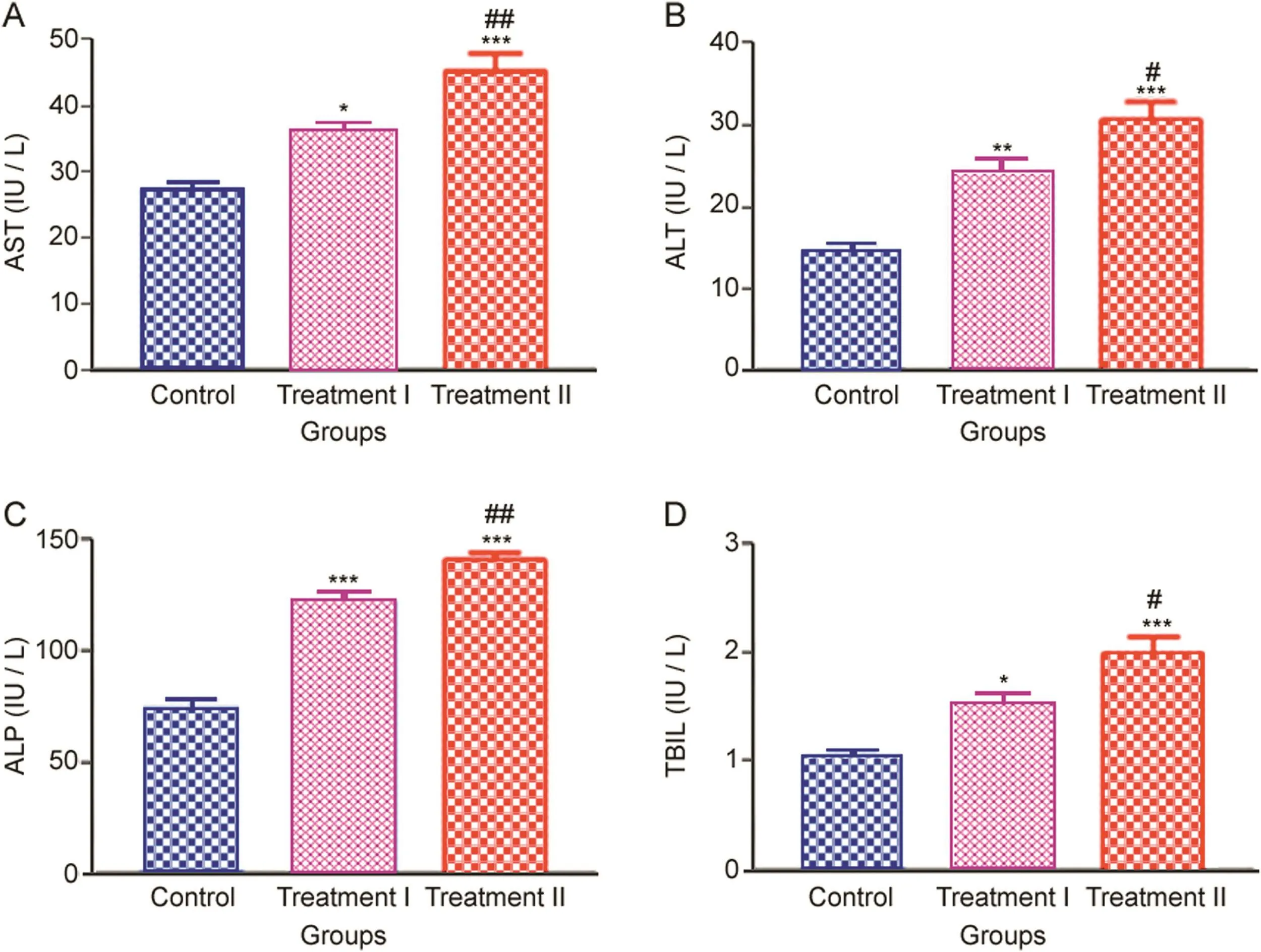
Fig.5.Liver-specific(A)AST,(B)ALT and(C)ALP activities,and(D)TBIL levels measured after 14 days oral administration of NP in Treatment I(38.91 mg/kg b.wt)and Treatment II(65.78 mg/kg b.wt)groups,and the control(75 μL DMSO/kg b.wt).Values are Mean ± SE(n=6).Analysis of variance(One-way ANOVA)followed by Tukey test.Significant differences were indicated by*p<0.05,**p<0.01 and***p<0.001,when compared with the control values while#p<0.05 and##p<0.01 were used to show the significant difference between the treatment groups.
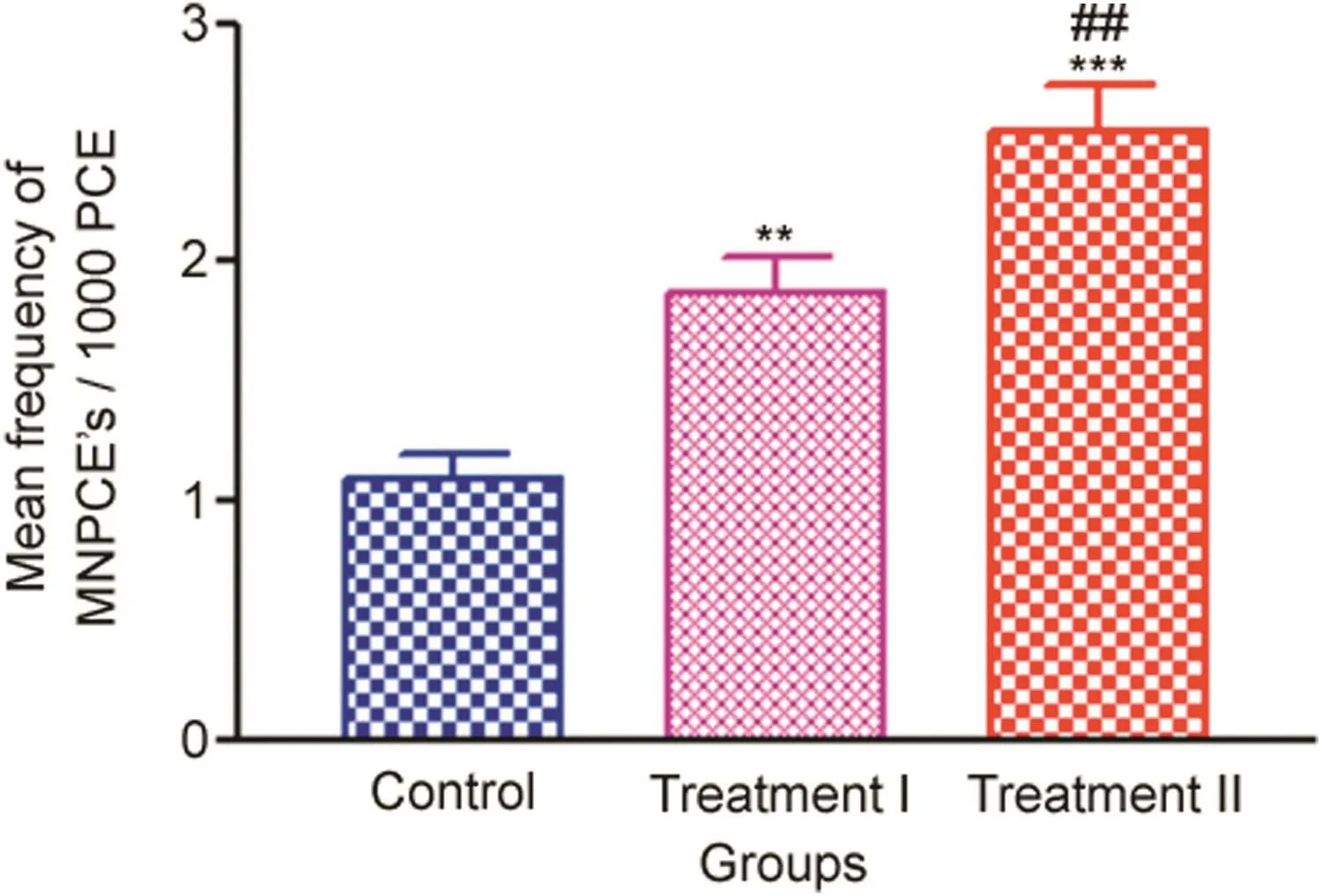
Fig.6.Frequency of bone marrow micronuclei induction measured after 14 days oral administration of NP in Treatment I(38.91 mg/kg b.wt)and Treatment II(65.78 mg/kg b.wt)groups,and the control(75 μL DMSO/kg b.wt).Values are Mean±SE(n=6).Analysis of variance(One-way ANOVA)followed by Tukey test.Significant differences were indicated by**p<0.01 and***p<0.001,when compared with the control group and##p<0.01 was used to show the significant difference between the treatment groups.
The trends in the increase of MN frequencies in the treatment groups compared to the control are given in Fig.6.The representative photomicrographs showing PCEs in the control and PCEs with micronucleated cells in the treatment groups are shown in Fig.7.
3.5.2.Effect of NP administration on DNA damage
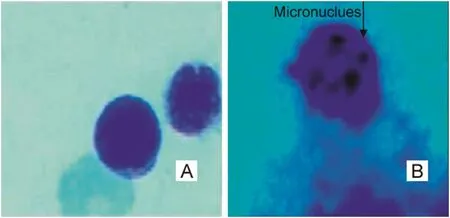
Fig.7.Representative photomicrographs showing(A)PCEs in control and(B)MNPCEs in NP treated rats in bone marrow cells.
Quantification of DNA damage for each cell was calculated as the percent of the total DNA in the tail.The trend of percent DNA tail in the treatment groups compared with the control is given in Fig.8.Results showed significant DNA damage induced by two different doses of NP in leukocytes of in vivo-treated rats as compared to the control(p<0.001).The treatment groups showed clear induction of DNA damage.The Treatment II group showed the highest significant increase in the percent DNA tail(16.789±0.606),followed by the Treatment I group(10.91±0.6490),which were 24-fold and 15-fold higher,respectively,when compared with the control values(0.692±0.090).The degree of damage was directly proportional to the concentrations(p<0.001)used,having the highest induction factor in the Treatment II group(24.26),followed by Treatment I group(15.77)when compared with the control.The representative photomicrograph leukocytes and,percent DNA in the tail of the control,Treatment I and Treatment II groups are given in Fig.9.
4.Discussions
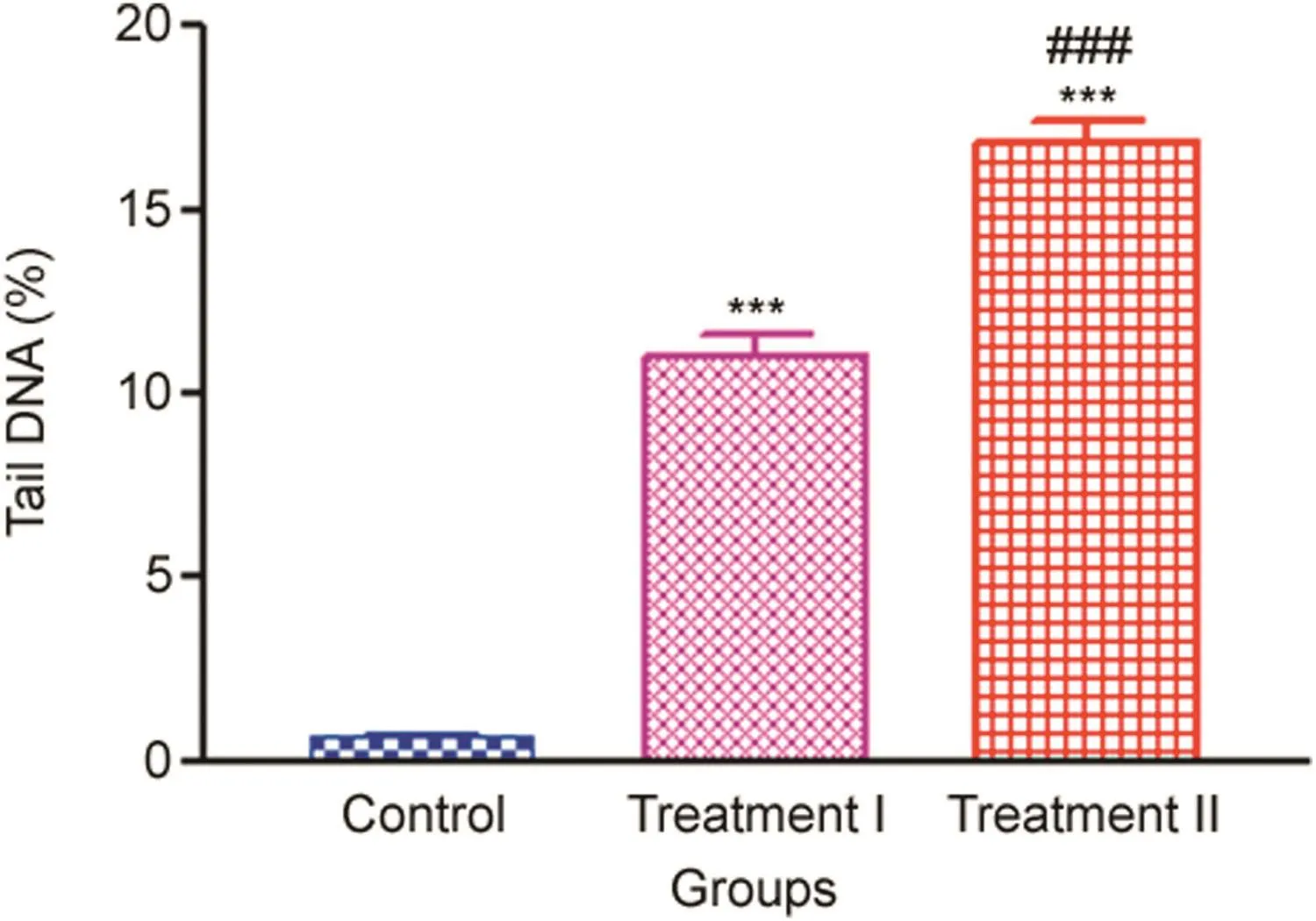
Fig.8.%tail DNA in leukocytes measured after 14 days oral administration of NP in Treatment I(38.91 mg/kg b.wt)and Treatment II(65.78 mg/kg b.wt)groups,and the control(75 μL DMSO/kg b.wt).Values are Mean ± SE(n=3).Analysis of variance(One-way ANOVA)followed by Tukey test.Significant differences were indicated by***p<0.001,when compared with the control and###p<0.001 was used to show the significant difference between the treatment groups.
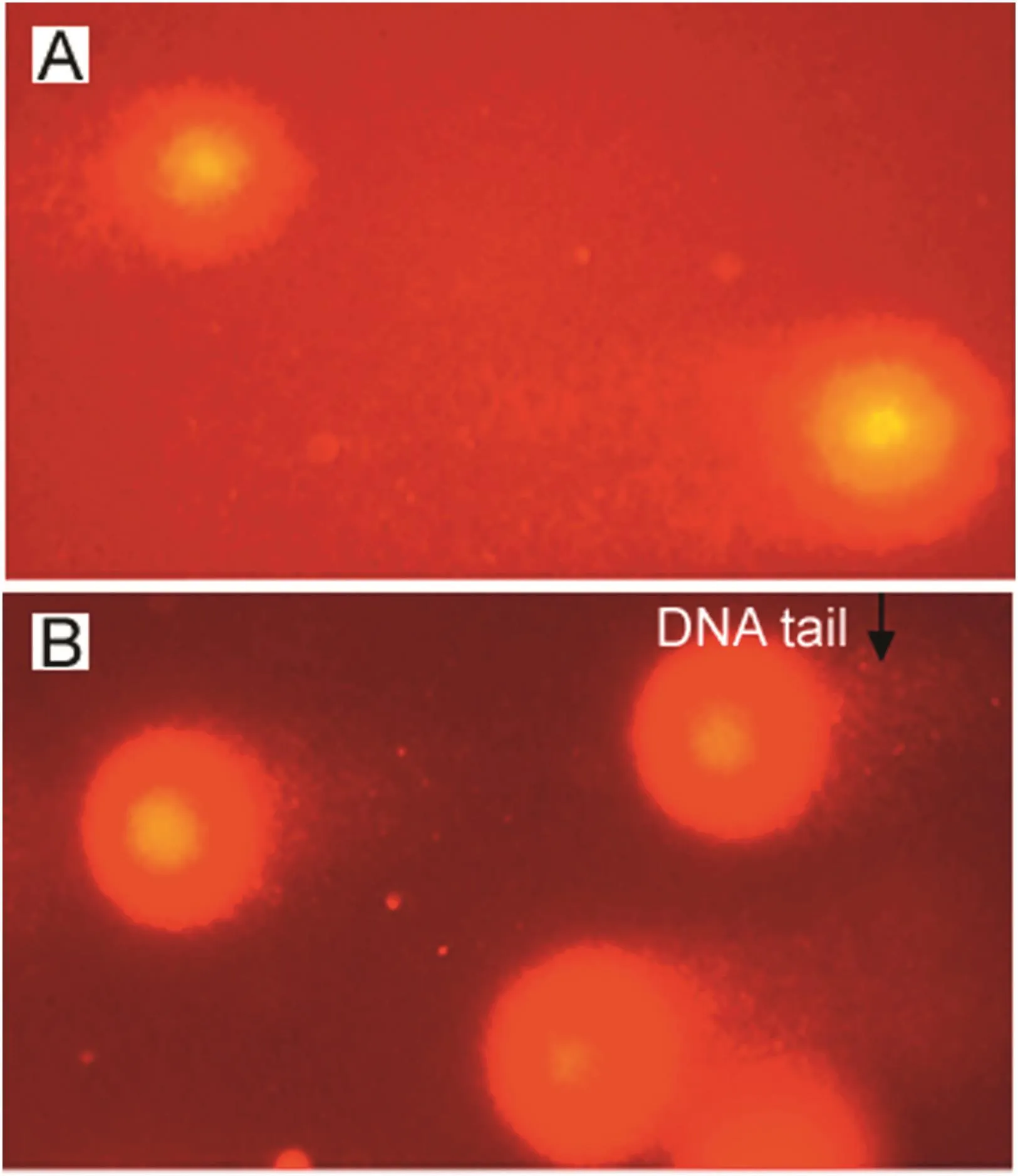
Fig.9.Representative photmicrographs showing%tail DNA in(A)control and(B)NP treated rats in leukocytes.
In the present study,the level of GSH decreased significantly in the liver,kidney and brain tissues of treatment groups compared to the control,which confirms the oxidized state of the cells and indicates an inefficient metabolism of glutathione system in the ROS scavenging induced by NP.Our results are in line with a previous study,where NP administration decreased the level of cardiac tissue glutathione as compared to the normal and toxic control groups in rats[25].In the present study,NP also caused a significant increase in SOD activities in the liver,kidney and brain tissues of treatment groups compared to the control,which indicates an adaptive response to increased oxidative stress.The increased SOD activities in the present study favour the evidence of the pro-oxidant action of NP,and our results are in line with the previous study reported by Gómez-Oliván et al.[13],where NP was reported to cause increased SOD activity over 48 h NP exposures in Daphnia magna.
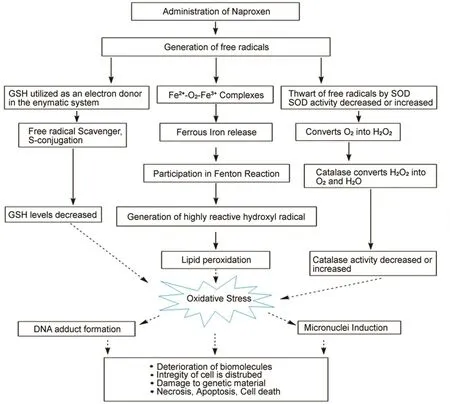
Fig.10.Diagrammatic representation of possible mechanism of NP induced biochemical alterations,oxidative stress,hepatotoxicity and genotoxicity in male Wistar rats.
In the present study,NP treatment resulted in significant increase in CAT activities in the liver,kidney and brain tissues of treatment groups compared to the control.Similar increased CAT activities in the treatment groups were reported by Gómez-Oliván et al.[13]in Daphnia magna over 48h NP exposures.This increase may also be attributed to the increased SOD activities or possibly to an increase in the formation of H2O2in the cells due to the increase of ROS linked to arachidonic acid metabolism via lipoxygenase(LOX)pathway instead of COX pathway blockage by NSAIDs[26].
In this study,NP induced a significant increase in lipid peroxidation in the treatment groups,suggesting the generation of ROS.The results of the present study are in accordance with a study where NP-induced lipid peroxidation in the liver microsomes and the isolated hepatocytes of rats caused by the ROS are produced during NP oxidative metabolism[27].Similarly,NP treatment caused elevated lipid peroxide levels in rat gastric mucosa[28].Increased lipid peroxidation appears to be involved in the upregulation of several antioxidant enzymes.Therefore,the increased lipid peroxidation,decreased glutathione level,increased SOD and CAT activities in the liver,kidney and brain tissues following NP treatment in the present study indicate a possible involvement of NP induced oxidative stress that altered antioxidant level in the treatment groups.
Administration of NSAIDs results in elevated ALT,AST and ALP activities and bilirubin level[9,29,30],which correlates with the present study,where treatment groups showed significant elevated liver-specific AST,ALT,ALP activities and TBIL level compared to the control after NP treatment for 14 days.The results of the present study corroborate with the previous study in rats[31].The increased liver enzymes in the present study may also be possibly due to the fact that NP induced ROS generation since the liver is the major organ attacked by ROS[32].
Micronuclei induction in the PCEs of bone marrow cells has been regarded as one of the most sensitive biomarkers for mutagenic genotoxicity of a compound[33,34].The increased micronuclei induction in the present study correlates with the previous study which demonstrated the cytotoxic effect of NSAIDs concentrations in the blood of Cyprinuscarpio and found a significant increase in micronuclei[9].This significant increase in micronuclei induction in the present study may be due to chromosome missegregation,resulting from aneugenic and clastogenic effects elicited by NP induced ROS.
In the present study,NP caused a significant increase in the%DNA in the tail in cells of treatment groups compared to the control.Similar results were obtained by Gómez-Oliván et al.[13],where 48 and 96 h NP exposure increased the%DNA tail in the cells of Daphnia magna.Similarly,significant DNA damage was present in the MG-63 osteosarcoma cells treated with NP[35].It can be interpreted that the NP-induced DNA damage may be due to the result of ROS generated oxidative stress.Therefore,NP-induced ROS generation in the present study might be the possible mechanism for the damage to the genetic materials in Wistar rats.The possible mechanism of NP induced biochemical alterations,oxidative stress,hepatotoxicity and genotoxicity in male Wistar rats is shown in Fig.10.
5.Conclusion
NP administration resulted in biochemical changes,like alteration of tissue sulfhydryl(GSH)levels,affecting the tissue antioxidants like SOD and CAT activities resulting in the increased oxidative stress.NP also caused elevated liver enzymes in the treatment groups,suggesting that it has some deleterious effect on the basic structure and functions of the liver.NP administration resulted in significant micronuclei inductions and damage to the genetic materials,exhibiting mutagenic activities in the male Wistar rats.From the present study,it can be concluded that NP is a potential genotoxic agent at the doses used that induces oxidative stress,hepatotoxicity and genotoxicity in vivo and thus use of this drug should be restricted.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study was supported by grants from DBT NER(BT/PR16164/NER/95/88/2015),DST PURSE-(Phase-II)(PAC-JNU-DSTPURSE-462),UGC RNW,UGC SAP at the level of DRS-I&II,and UPE-II,JNU(Project Id No.247)to Dr.A.C.Mondal,School of Life Sciences,Jawaharlal Nehru University,New Delhi,India.
 Journal of Pharmaceutical Analysis2018年6期
Journal of Pharmaceutical Analysis2018年6期
- Journal of Pharmaceutical Analysis的其它文章
- Novel ligand-based docking;molecular dynamic simulations;and absorption,distribution,metabolism,and excretion approach to analyzing potential acetylcholinesterase inhibitors for Alzheimer's disease
- Recovery rates of combination antibiotic therapy using in vitro microdialysis simulating in vivo conditions
- Cytotoxic effect of Rosa canina extract on human colon cancer cells through repression of telomerase expression
- Long-term stability of gentamicin sulfate-ethylenediaminetetraacetic acid disodium salt(EDTA-Na2)solution for catheter locks
- Highly sensitive LC–MS/MS method to estimate doxepin and its metabolite nordoxepin in human plasma for a bioequivalence study
- Detection and determination of undeclared synthetic caffeine in weight loss formulations using HPLC-DAD and UHPLC-MS/MS
