Prognostic factors of refractory NSCLC patients receiving anlotinib hydrochloride as the third- or further-line treatment
Jing Wang, Yizhuo Zhao, Qiming Wang, Li Zhang, Jianhua Shi, Zhehai Wang, Ying Cheng, Jianxing He,Yuankai Shi, Hao Yu, Yang Zhao, Weiqiang Chen, Yi Luo, Xiuwen Wang, Kejun Nan, Faguang Jin,Jian Dong, Baolan Li, Zhujun Liu, Baohui Han, Kai Li
1Department of Pulmonary Oncology, Tianjin Medical University Cancer Institute and Hospital, Tianjin 300060, China;2Department of Respiratory Medicine, Shanghai Chest Hospital, Shanghai 230030, China; 3Department of Medical Oncology,Henan Province Tumor Hospital, Zhengzhou 450008, China; 4Department of Respiratory Medicine, Peking Union Medical College Hospital, Beijing 100730 China; 5Department of Medical Oncology, Linyi Cancer Hospital, Linyi 276001, China;6Department of Internal Medicine, Shandong Cancer Hospital, Jinan 250117, China; 7Department of Thoracic Oncology, Jilin Cancer Hospital, Changchun 130012, China; 8Department of Thoracic Surgery, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou 510120, China; 9Department of Medical Oncology, Cancer Hospital Chinese Academy of Medical Sciences, Beijing 100021, China; 10Department of Biostatistics, School of Public Health, Nanjing Medical University,Nanjing 210029, China; 11Department of Respiratory Medicine, Lanzhou Military General Hospital, Lanzhou 730050, China;12Department of Head and Neck Oncology, Hunan Cancer Hospital, Changsha 220633, China; 13Department of Chemotherapy, Qilu Hospital of Shandong University, Jinan 250000, China; 14Department of Medical Oncology, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710061, China; 15Department of Respiratory and Critical Diseases, Tang Du Hospital, Xi'an 710038, China; 16Department of Oncology, Yunnan Cancer Hospital, Kunming 650032, China; 17General Department, Capital Medical University Beijing Chest Hospital, Beijing 101149, China
ABSTRACT Objective: Anlotinib hydrochloride is a multitarget tyrosine kinase inhibitor that targets vascular endothelial growth factor receptor, fibroblast growth factor receptor, platelet-derived growth factor receptor, c-Kit, and c-MET; therefore, it exhibits both antitumor and anti-angiogenetic activities. A phase III trial has shown that anlotinib improved progression-free survival (PFS) and overall survival (OS) in patients with advanced non-small cell lung cancer (NSCLC), who presented with progressive disease or intolerance after standard chemotherapy. This study aimed to analyze the characteristics of patients receiving anlotinib treatment to determine the dominant populations who are fit for the treatment.Methods: Data were collected from March 2015 to January 2017 from a randomized, double-blind, placebo-controlled,multicenter, phase III trial of anlotinib (ALTER0303). A total of 437 patients were enrolled and randomly allocated (2:1) to the anlotinib and placebo groups. Kaplan–Meier analysis and log-rank test were performed to compare PFS and OS. Cox proportional hazards model was adopted for multivariate prognostic analysis.Results: Multivariate analysis indicated that high post-therapeutic peripheral blood granulocyte/lymphocyte ratio and elevated alkaline phosphatase levels were independent risk factors for PFS. Meanwhile, elevated thyroid-stimulating hormone, blood glucose, and triglyceride levels; hypertension; and hand–foot syndrome were independent protective factors of PFS. High posttherapeutic peripheral blood granulocyte/lymphocyte ratio, an Eastern Cooperative Oncology Group (ECOG) score ≥ 2, and the sum of the maximal target lesion length at baseline were independent risk factors of OS, and hypertriglyceridemia was an independent protective factor of OS.Conclusions: This study preliminarily explored the possible factors that affected PFS and OS after anlotinib treatment in patients with advanced refractory NSCLC, and the baseline characteristics of the therapeutically dominant populations were then identified.
KEYWORDS Non-small cell lung cancer; anlotinib; third- or further-line therapy; prognostic factor analysis
Introduction
Non-small cell lung cancer (NSCLC) accounted for the highest morbidity and mortality rates among all other malignancies in recent years. Patients who received chemotherapy have a median survival of < 1 year, and those with driver gene mutations who receive targeted therapies also developed resistance after 1 year of treatment. The National Comprehensive Cancer Network guidelines provided regulations only for the first- and second-line regimens. Therefore, the primary focus of research is to help patients who are resistant to third- or further-line regimens recover and to lengthen their survival. Phase II1and III trials of the novel vascular-targeting agent anlotinib have shown that progression-free survival (PFS) and overall survival (OS)significantly improved in the anlotinib group than in the placebo group. However, this conclusion was only based on the general data of the patients. Thus, we evaluated the major factors that affected both the PFS and OS of the patients from the phase III trial with the hope of determining the therapeutically dominant populations and discovering an optimal therapeutic regimen for individuals with advanced refractory NSCLC.
Materials and methods
General data
Between March 2015 and August 2016, 437 patients were enrolled from 31 research centers in China, and the data of the patients from March 2015 to January 2017 were collected.The major inclusion criteria were as follows: 1) patients who were pathologically diagnosed with stage III B/IV advanced NSCLC and had measurable nidus; 2) those who have progressive disease after receiving at least two standard systematic chemotherapeutic regimens or those who could not tolerate the toxicity of these treatments; 3) those who were negative for epidermal growth factor receptor (EGFR)and anaplastic lymphoma kinase (ALK) and can provide detectable specimens from tumor tissues or the hydrothorax for gene detection before participating in the study or those who were positive for EGFR and ALK and presented with progressive disease (PD) or intolerable adverse effects after treatment with relative targeted drugs; and 4) those with an Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) score of 0–1 points. All patients must sign the informed consent form prior to enrollment. The present trial was approved by the corresponding ethics committees of our institution and is registered in ClinicalTrials.gov (registration number: NCT02388919).
Methods of treatment
Patients were randomized to receive anlotinib or placebo in a 2 : 1 ratio. The medication was administered from days 1 to 14 in a 21-day cycle, and the initial dose of anlotinib was 12 mg oral administered once daily.
Efficacy evaluation
Objective efficacy measures were evaluated using the guidelines of the response evaluation criteria in solid tumors version 1.1.
OS was defined as the duration between randomization and death from any cause. For patients who were considered lost to follow-up, the date of the last follow-up visit was calculated equally as the date of death.
PFS was defined as the duration between randomization and objective tumor progression or death.
For patients who had complete response (CR) or partial response (PR) and stable disease (SD) based on the efficacy evaluation, tumor nidi should be re-examined 4 and 6–8 weeks after the initial efficacy evaluation to identify the efficacy until disease progression (DP).
Disease control rate was defined as the percentage of patients who can be evaluated and achieved CR, PR, and SD for at least 4 weeks.
Record of adverse events
Patients were regularly followed-up as per the trial protocol,and each observation index was recorded accordingly.Adverse events were assessed on the basis of the guidelines of the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE 4.0), and the most severe post-treatment adverse events in each therapeutic cycle were statistically analyzed.
Statistical analysis
All analyses were performed using the SAS 9.4 software (SAS Institute Inc., Cary, NC, the USA). Demographic data and baseline disease characteristics were presented as categorical variables. Univariate and multivariate Cox proportional hazards models were adopted to analyze the influential factors associated with PFS and OS. To establish the final model, a list of potential variables were firstly provided based on prior information regarding their clinical relevance, and the best subset method was then utilized to select the final model according to Chi-square statistics and clinical relevance. Variables that showed significance in the multivariate analysis were stratified, whereas the median OS or PFS were reported. Parameters with obvious clinical reference values, such as blood cholesterol and low-density lipoprotein (LDL) cholesterol level, were classified based on clinical reference values; meanwhile, parameters without obvious clinical reference values, such as pre-randomization maximal target lesion length and post-treatment granulocyte/lymphocyte ratio, were grouped using the cutoff values that were calculated using the receiver operating characteristic (ROC) curves. Cutoff values were obtained using survival status (dead or alive) at the end of follow-up and were considered as outcome variables. The value of the predictive variable with maximal sensitivity + sepecificity-1 was selected as the cutoff point. The between-group comparisons of OS and PFS were analyzed using t-test. For all analyses, a P value < 0.05 was considered statistically significant.
Results
Baseline data and efficacy
In the present study, 437 and 377 patients were included in the full analysis set (FAS) and per protocol set (PPS),respectively, and 256 and 121 patients were classified into the anlotinib and placebo groups, respectively.
By the end of the follow-up period (January 06, 2017) with a median follow-up period of 9.24 months for the anlotinib group and 7.33 months for the placebo group, the median PFS were 5.37 months [95% confidence interval (CI):4.40–5.63] for the anlotinib group and 1.40 months (95% CI:1.07–1.50) for the placebo group, which indicated statistically significant differences (P < 0.0001). Compared to the placebo group, the anlotinib group had an extended duration for the control of advanced NSCLC by 3.97 months and a lower risk for DP by 75%, which indicated statistically significant differences [hazard ratio (HR) = 0.25, 95% CI: 0.19–0.31, P <0.0001]. The OS were 9.63 months (95% CI: 8.17–10.60 ) and 6.30 months (95% CI: 5.00–8.10) in the anlotinib and placebo groups, respectively. Compared to that of the placebo group, the median OS of the anlotinib group improved by 3.33 months and their risk for mortality decreased by 32%. This result showed statistically significant differences (HR = 0.68, 95% CI: 0.54–0.87, P = 0.0018). In addition, after the data cutoff, we continued the follow-up for OS until May 2017, and further survival analysis showed a similar result. That is, a median OS of 9.60 months (95% CI:8.4–10.6) was obtained, and it was significantly (P = 0.0018)longer in the anlotinib group than in the placebo group with an improvement of 3.30 months. The baseline data of the 256 patients from the anlotinib group are listed in Table 1.
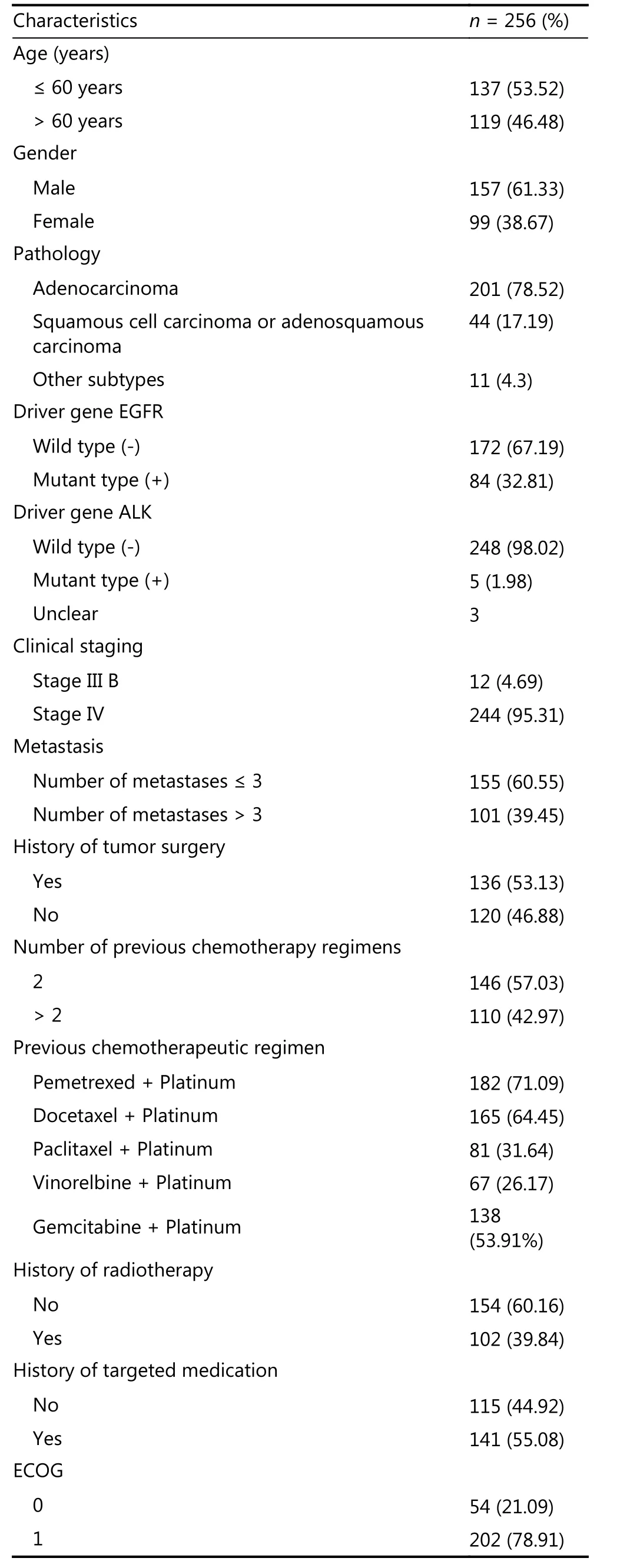
Table 1 Clinicopathological data of 256 patients in the anlotinib group
Univariate and multivariate analyses of efficacy
Based on the primary observation parameters of the current trial and previous reports, we preliminarily selected 43 variables that might affect prognosis, and Kaplan–Meier univariate analysis and log-rank test were then conducted to assess the correlations between the variables and PFS or OS.The factors were categorized as follows: 1) patient factors,including sex, age, ECOG score at the end of the medication,blood granulocyte/lymphocyte ratio, changes in laboratory parameters before and after treatment, and occurrence of the most severe adverse events; 2) therapeutic factors, such as pre-randomization lines and duration of treatment and content of therapeutic regimens; and 3) tumor factors, such as sum of pre-randomized maximal target lesion length,clinical stage, pathological type, etc.
Since the pre-randomization ECOG score of the trial was limited between 0 and 1 point and the post-treatment body performance of some patients worsened, which resulted in a higher ECOG score of 0–3 points in the univariate analysis,the patients were stratified into 0–1-point subgroup and 2–3-point subgroup. The cutoff value for the stratification of age was 60 years. Adverse events were defined on the basis of the definitions of CTCAE 4.0 (hand–foot syndrome, oral mucositis, and rash), and hypertension was defined as blood pressure ≥ 140/90 mmHg.
Classification of the cutoff values of the laboratory parameters was based on their respective clinical reference values: hypertriglyceridemia was defined as a triglyceride level > 1.7 mmol/L, hypercholesterolemia was defined as cholesterol level > 5.17 mmol/L, elevated alkaline phosphatase (ALP) level was defined as an ALP level> 135 U/L, hyperglycemia was defined as blood sugar level> 5.9 mmol/L, hypomagnesemia was defined as blood magnesium level < 0.66 mmol/L, hypocalcemia was defined as serum calcium level < 2.10 mmol/L, hyponatremia was defined as blood sodium level <137 mmol/L, hyper-lowdensity lipoproteinemia was defined as lipoprotein level> 3.1 mmol/L, elevated thyroid-stimulating hormone (TSH)was defined as TSH level > 4.85 mIU/L, and prolonged QT interval on electrocardiogram (ECG) was defined as QT interval > 480 ms.
For variables without a reference value, the ROC curves were used to analyze and determine the cutoff values with significant differences in OS, which is the primary endpoint of the trial. The cutoff value of pre-randomization maximal target lesion length was 72 mm, the sensitivity was 0.5283,the specificity was 0.7368, and the area under the curve was 0.6600. The cutoff value of post-treatment granulocyte/lymphocyte ratio was 3.10, the sensitivity was 0.6689, the specificity was 0.6989, and the area under the curve was 0.7089.
Factors with statistically significant differences in the univariate analysis are listed in Table 2. However, variables without statistically significant correlations with PFS or OS are not shown.
The SAS statistical software was utilized to select 15 combinations of variables using the fitting curve. The Cox model was then used to analyze the combination of variables,and results showed that the following variables had statistically significant differences (Table 3). However, the variables without statistically significant correlations with PFS or OS are not shown.
Results of the multivariate analysis indicated that high post-treatment granulocyte/lymphocyte ratio and elevated ALP levels were considered independent risk factors of PFS,whereas hypertension, hand–foot syndrome (hand–foot skin reaction), hyperglycemia, hypertriglyceridemia, and elevated TSH levels were independent protective factors of PFS. Posttreatment ECOG score ≥ 2, high post-treatment peripheral blood granulocyte/lymphocyte ratio, and the sum of prerandomization maximal target lesion length were considered independent risk factors of OS, whereas hypertriglyceridemia was an independent protective factor of OS.
To further evaluate the clinical significance of the cutoff values, stratified median PFS and OS were reported for each variable, which was statistically significant in the multivariate model according to these cutoff values(Table 4).
Discussion
Anlotinib is a self-developed multitargeted inhibitor designed to inhibit vascular endothelial growth factor receptor-2(VEGFR2), VEGFR1, VEGFR3, fibroblast growth factor receptor-1 (FGFR1), FGFR2, FGFR3, platelet-derived growth factor receptor-α (PDGFRα), PDGFRβ, c-KIT, c-Met, and EGFR in China2,3. Compared with similar target agents, such as sorafenib4, anlotinib targets the tumor vascular microenvironment and the responsible genes and signaling pathways associated with malignant biological behaviors,such as cancer cell growth, invasion, and metastasis.Therefore, it is considered to be a multitargeted, dualdimension, comprehensive anti-tumor drug that can control tumor resistance after a single chemotherapy or targeted drug treatment. In this trial, anlotinib was used in patients with advanced NSCLC who developed resistance after at least two therapeutic regimens. Results revealed a median PFS of 5.37 months and a median OS of 9.63 months, which lower therisks for DP by 75% (HR = 0.25, 95% CI: 0.19–0.31, P <0.0001) and 32% (HR = 0.68, 95% CI: 0.54–0.87, P =0.0018), respectively, compared to the placebo group. The PFS and OS of patients with advanced NSCLC (squamous cell carcinoma and adenocarcinoma) receiving classic second-line chemotherapeutic regimens are approximately 2.5 and 7 months, respectively5. Patients with adenocarcinoma who received second-line therapies based on pemetrexed had a median OS of 8.3 months6, whereas patients with adenocarcinoma who are taking tyrosine kinase inhibitor (TKI; Tarceva) as the second-line therapy had a median OS of 7.8 months7. However, data about the efficacy of third-line therapy that is supported by class II evidence or higher recommendations are still limited. In this trial, we used anlotinib as the third-line salvage regimen for patients who are resistant to treatment or those who cannot tolerate toxicities, and higher PFS and OS rates were observed in patients receiving these regimens than in those receiving
previous standard second-line regimens. This might be due to the simultaneous inhibition of tumor and its angiogenesis and blockage of multiple key receptors, which effectively prevented tumor growth-related signaling. However, all enrolled patients who presented with advance cancer and refractory lung malignancies (including squamous cell cancer, adenocarcinoma, and large cell carcinoma but not limited to adenocarcinoma only) were significantly resistant to all the drugs compared with those with tumors who were receiving second- and inferior-line therapies. Hence, we conducted a stratified analysis to assess the factors that affect efficacy to determine therapeutically dominant populations and prevent the use of ineffective medications.
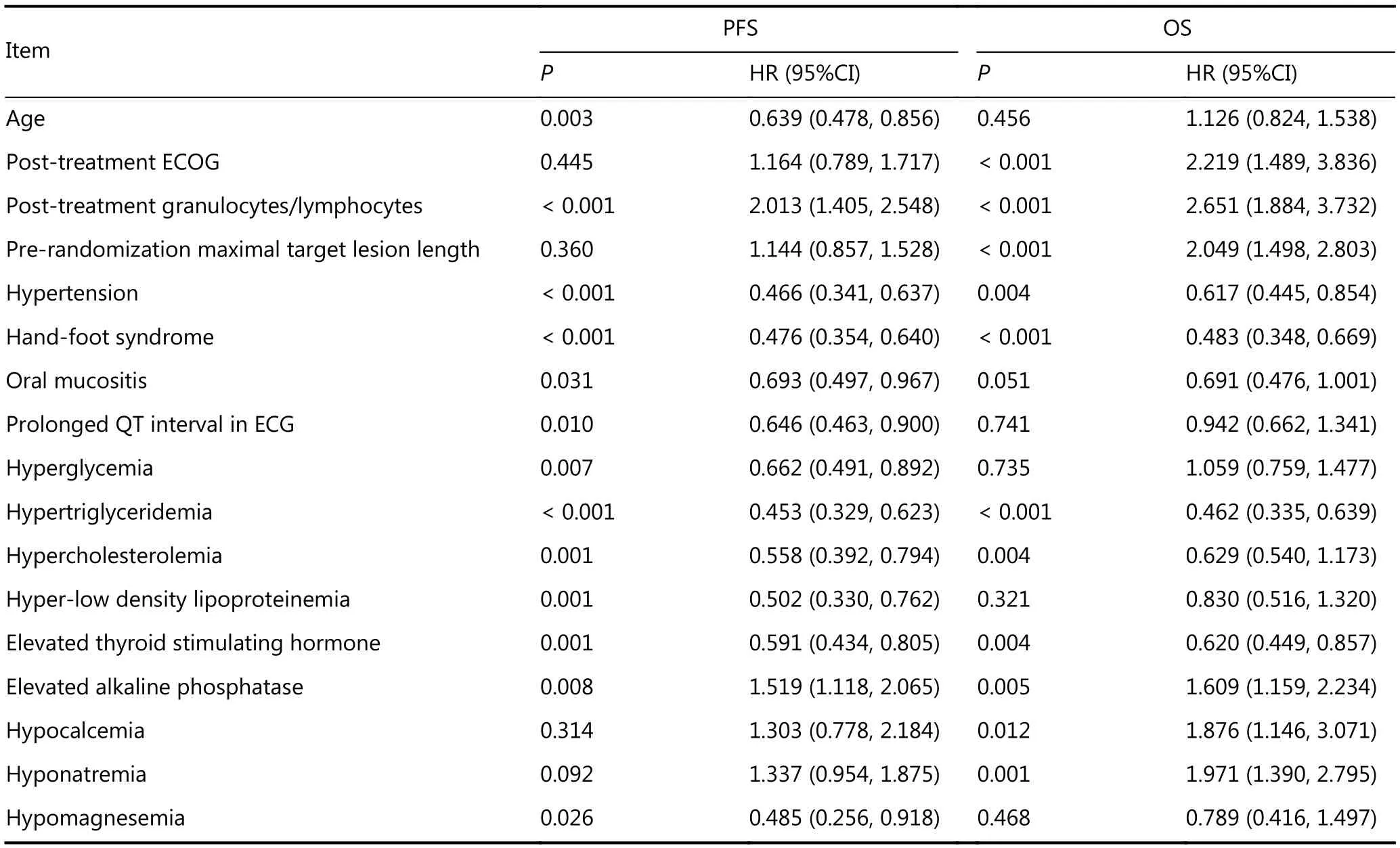
Table 2 Results of the prognostic factor analysis of patients from the anlotinib group (univariate analysis)

Table 3 Results of the prognostic factor analysis of patients in the anlotinib group (multivariate analysis)
Results of the univariate analysis showed that 13 factors affected PFS, and they were primarily patient factors and adverse events (see table 2). No therapeutic factors significantly affected PFS. A total of 11 factors affect OS, and seven factors affected PFS. They were patient factors and tumor factors (Table 2), no therapeutic factors were involved. Based on the abovementioned results and previous reports, several parameters that affect the prognosis were introduced into the multivariate Cox model. The results showed that the post-treatment adverse events of hypertension and hand–foot syndrome, blood glucose level >5.9 mmol/L, triglyceride level > 1.7 mmol/L, and TSH level >4.85 mmol/L were considered independent protective factors of OS, whereas post-treatment granulocyte/lymphocyte ratio> 3.10 and ALP level > 135 U/L were considered independent risk factors of PFS. Post-treatment triglyceride level > 1.7 mmol/L was an independent protective factor for OS,whereas a post-treatment ECOG score of 2–3, post-treatment granulocyte/lymphocyte ratio > 3.10, and maximal target lesion length> 72 mm were independent risk factors for OS. To assess the practical cutoff values clinically, we adopted the ROC curve to analyze the cutoff values showing the most significant differences in PFS and OS. After comparing with the reference values used for clinical practice, the cutoff values presenting the near coincidence in each normal upper laboratory indexes and changes in blood pressure were almost similar to the normal upper limits of the corresponding clinical reference ranges. Therefore, the cutoff values of the factors in the current study, which were estimated via univariate and multivariate analyses, are considered independent factors of PFS or OS, and they should indicate clinical significance. During the univariate and multivariate analyses, we also directly selected the upper limits of the normal clinical reference ranges as the cutoff values for comparisons among populations with different PFS and OS for analysis. For measurement data other than clinical laboratory parameters and without a normal reference range, such as pre-randomization maximal target lesion length and post-treatment granulocyte/lymphocyte ratio, the abovementioned ROC curve analysis was adopted to determine the cutoff values presenting the most significant differences in PFS or OS. To re-confirm the power of the parameters identified via multivariate analysis in predicting PFS or OS, we further divided the groups based on the cutoff values of each index, and the PFS and OS of the subgroups were calculated. The results validated that the differences in PFS or OS between the two subgroups were statistically significant. Therefore, selection of the abovementioned cutoff values was considered reasonable.
Based on the results, the previous treatment has not been considered an independent factor. Previously reported factors that correlated with the development of tumor resistance (pre-treatment lines and types of treatment)showed no statistical significance in the univariate or multivariate analysis. Traditionally, it was believed that increased chemotherapeutic line triggered multidrug resistance (MDR) in the tumor cells6, resulting in resistance to multiple chemotherapy drugs. Therefore, the efficacy of the second-line regimen was considered to be inferior to the first-line regimen. EGFR-TKIs can also trigger T790 mutation and c-Met gene amplification, resulting in resistance to first-generation drugs8,9. However, results of the current study showed that the abovementioned factors did not affect the prognosis after anlotinib treatment. This was probably attributable to the fact that anlotinib has mechanisms of action that are completely independent of the previous therapeutic mechanisms that caused changes in molecules that are susceptible to drug resistance. That is, its efficacy was not affected by MDR induced by chemotherapy,and it still remained effective for c-Met gene amplification2induced by EGFR-TKI. Theoretically, increased therapeutic types and lines increased gene changes, thus indicating the possibility of resistance to any novel drug. However, our present study has not investigated the correlation between the gene mutation load10induced by different previous therapeutic lines and the efficacy of anlotinib or any molecular mechanisms that may induce resistance to anlotinib. Nevertheless, a pre-treatment total tumor load(sum of pre-randomization maximal target lesion length) >72 mm predicted poor PFS and OS in the univariate analysis and poor OS in the multivariate analysis, and this result still indicated that the efficacy of anlotinib may be suboptimal in larger tumors with greater heterogeneity and mutation load.Therefore, efforts should be made to promote anlotinib from third-line therapy to second- or first-line therapy for thetreatment of early tumors.
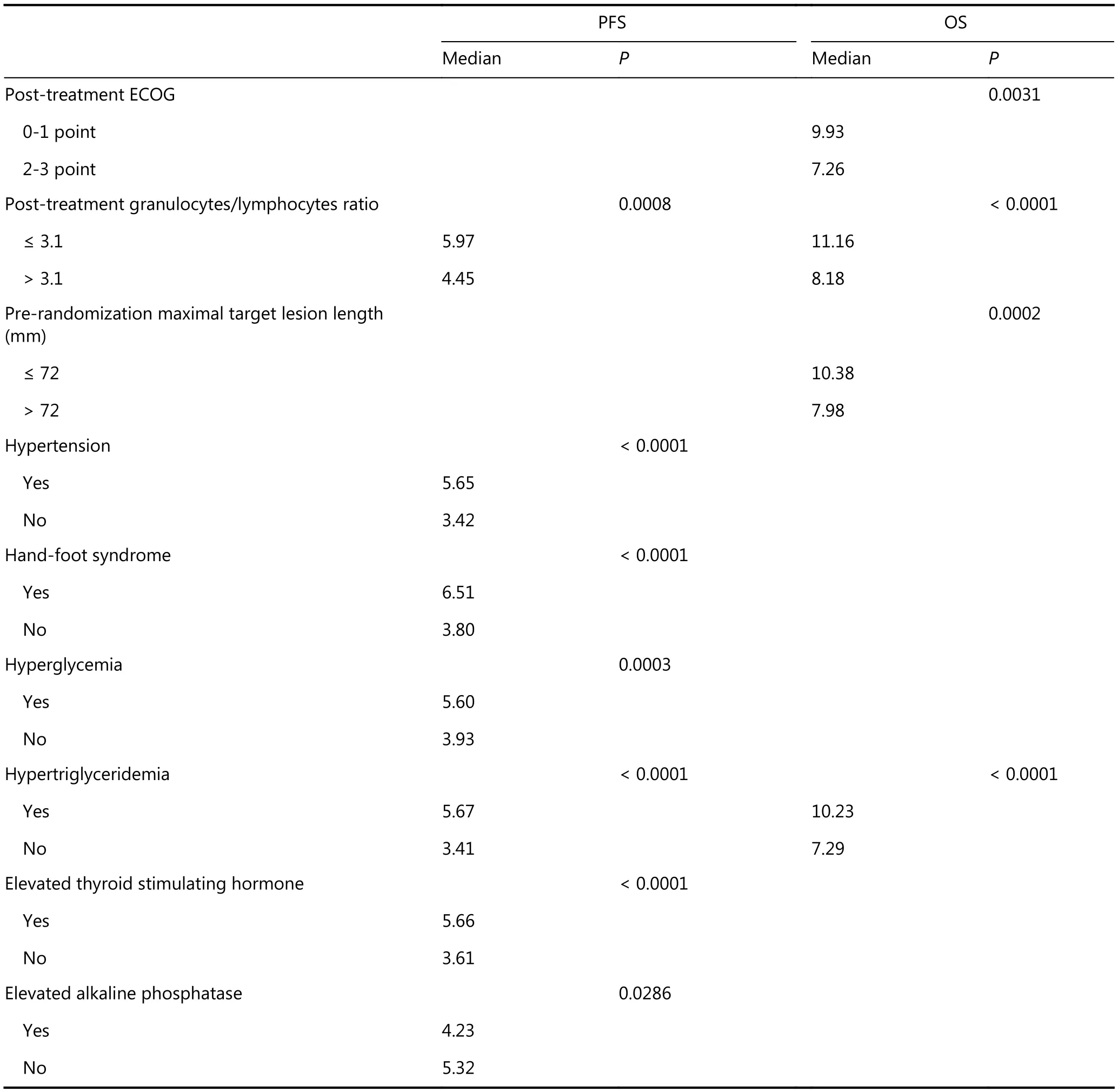
Table 4 PFS and OS of each variable from the subgroups were divided on the basis of the cutoff values
It was noteworthy that factors from the multivariate analysis caused changes in the body and metabolism of patients, and the resultant adverse events (up to seven events,such as post-treatment ECOG score as well as hypertension,hand–foot syndrome, and high blood glucose, triglyceride,TSH, and ALP levels, observed during the trial period)predominately affected efficacy and prognosis. This phenomenon indicated that multiple targets and broad signaling pathways covered by anlotinib could help overcome the acquired resistance induced by previous few-line treatments. However, compared to the mono-target agents, it may cause more adverse reactions in patients and interact with their internal environments. Thus, the level of efficacy of anlotinib was primarily dependent on the individual physical characteristics of the patients.
Similar to EGFR-TKIs11, treatment with anlotinib also resulted in hand–foot syndrome, which was considered to be a predictor of good PFS. Meanwhile, similar to antiangiogenic targeting agents12, anlotinib caused hypertension and prolonged QT intervals, with the former considered a good predictor of PFS. From the perspective of adverse events,although they have been proven tolerable and transient,anlotinib simultaneously functioned as an antiangiogenic targeting and EGFR-TKI agent.
In this study, post-treatment granulocyte/lymphocyte ratio has been considered a risk factor of both PFS and OS; hence,it deserves special attention. Studies conducted in China and other countries have shown that the tumor-free survival rate was shorter in patients with abnormally increased peripheral blood granulocyte levels during relapse after lung surgery than in patients with normal levels. Hence, the concept of“tumor-related leukocytosis” has been proposed13, which is a syndrome that occurs due to the absence of immune function and lack of lymphocytes. Sequential studies have further revealed that the patients’ percentage of CD8+ T cells was usually low, the CD4+/CD8+ T-cell ratio was high, and OS was short14. Although no specific immune-related parameters were detected in this trial, our current results strongly suggested that the efficacy of anlotinib was probably correlated with the post-treatment immune function status and response of the patient, which further indicated the need for special attention to the changes in immune parameters in future studies and the use of anlotinib and immuneenhancing therapy, which is similar to other assessments of TKI in this field15.
Another notable result in our study was the elevated TSH levels and changes in metabolic parameters (including blood triglyceride and blood glucose levels) caused by anlotinib,which may be predictors of good prognosis, and elevated blood triglyceride levels protected both PFS and OS from the risk factors. Although such adverse events in the trial seldom reached grade III (3.06%, 0%), they covered a broad range.That is, the incidence rate of hypertriglyceridemia was 44.56% in the anlotinib group. However, it was only 23.78% in the placebo group. The incidence rates of hypercholesterolemia were 41.84% and 13.99%, respectively,and those of hyper-low density lipoproteinemia were 21.09%and 7.69%, respectively. However, the impact of small molecule targeted therapy on the endocrine system and fat metabolism are less assessed, and the results are usually contradictory. For instance, Ho et al.16have found that the levels of polyunsaturated fatty acids were elevated in patients with EGFR mutations, whereas reduced proportion of polyunsaturated fatty acids in the membrane phospholipids induced by inhibitors also lowered the activity of the EGFR membrane receptors, which shows a synergistic effect with gefitinib17. Other studies have also found that the mammalian target of sirolimus, which is a rapamycin(mTOR) inhibitor, increased the blood levels of total cholesterol and triglycerides18,19. Further studies have revealed that such result might be attributed to the fact that sirolimus reduced the activity of post-heparin lipoprotein lipase, which is the primary enzyme responsible for triglyceride metabolism, and this induces hypertriglyceridemia20. This result indicated that patients may often require adjuvant therapy with lipid-lowering drugs and that several indicators of lipid metabolism may be effective markers of small molecular targeted drugs. Future studies should focus on assessing the effects of anlotinib on the endocrine system and its subsequent potential effect on the immune and hematopoietic systems to identify the possible predictors of efficacy and prognosis. Yang et al.21from the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Science have found that the inhibition of cholesterol esterification could enhance the antitumor activity of CD8+T cells (also known as killer T cells), indicating that such studies are strongly recommended.
In conclusion, anlotinib plays a dual role as an antitumor and antiangiogenic agent. Its broad target range can help overcome the acquired resistance induced by multiple chemotherapies/targeted treatments. However, it is also more susceptible to interactions with the patient’s internal environment than mono-targeted agents. Factors, including tumor burden, gene characteristics, as well as a patient’s immune, endocrine, and metabolic status, may also affect the efficacy and prognosis of anlotinib treatment. Further indepth studies must be conducted to identify the reliable markers of efficacy and prognosis.
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2018年4期
Cancer Biology & Medicine2018年4期
- Cancer Biology & Medicine的其它文章
- 2017 Chinese expert consensus on the clinical application of serum marker for thyroid cancer
- Multidisciplinary team for the diagnosis and treatment of 2 cases of primary intestinal yolk sac tumor
- Comparison of sentinel lymph node detection performances using blue dye in conjunction with indocyanine green or radioisotope in breast cancer patients: a prospective singlecenter randomized study
- PD-L1 expression and its effect on clinical outcomes of EGFR-mutant NSCLC patients treated with EGFR-TKIs
- Five-CpG-based prognostic signature for predicting survival in hepatocellular carcinoma patients
- A new tumor-associated antigen prognostic scoring system for spontaneous ruptured hepatocellular carcinoma after partial hepatectomy
