Synthesis, Crystal Structure and Photoluminescence of a Dinulear Copper Complex①
LI Xio-Ning WU Xio-Yun ZHAO Wn-Wn YANG Ming-Xue YU Rong-Min LU Cn-Zhong②(CAS Key Lortory of Design nd Assemly of Functionl Nnostructures,nd Fujin Provincil Key Lortory of Nnomterils, Fujin Institute of Reserch on the Structure of Mtter, Chinese Acdemy of Sciences, Fuzhou 350002, Chin)(College of Chemistry nd Mterils Science, Fujin Norml University, Fuzhou 350007, Chin)
A novel binuclear cuprous complex [Cu(μ-I)(NPPh)]2(1, NPPh = 1-(2-(diphenylphosphanyl)phenyl)-3-phenyl-4,5-dihydro-1H-pyrazole) was synthesized and characterized.In the solid state, the complex exhibits greenish yellow photoluminescence with a peak maximum of 533 nm, decay time of 34 μs, and a photoluminescence quantum yield of 63.6% at room temperature, respectively.
1 INTRODUCTION
In past twenty years, phosphorescence complexes have been widely used in organic light-emitting diodes (OLEDs) for lighting and display[1-4].However, most of them are noble metals, such as Ir and Pt[5].So developing low-cost, efficient materials for OLEDs is an excited challenge to science researcher.As much cheaper alternative materials[6-10], luminescent cuprous complexes have attracted much attention because of their interesting and promising emissive properties.Many cuprous materials containing P and N ligands with excellent electroluminescence performance have been found[11-16].However,chelating ligands containing both P and N coordinating sites have been seldom studied[17-19].Herein, we report a new binuclear cuprous complex[Cu(μ-I)(NPPh)]2with a chelating N, P ligand(NPPh = 1-(2-(diphenylphosphanyl)phenyl) 3-phenyl-4,5-dihydro-1H-pyrazole).The complex was characterized structurally by single-crystal X-ray diffraction.Its photophysical properties were also studied particularly.
2 EXPERIMENTAL
2.1 Materials and instruments
All reactions were performed under air atmosphere unless specified.1H NMR and31P NMR spectra were recorded on a Bruker Avance III 400 MHz NMR spectrometer.UV-Vis absorption spectra were recorded with a Perkin-Elmer Lambda 45 UV/Vis spectrophotometer.Photoluminescence spectra were measured by a HORIBA Jobin-Yvon FluoroMax-4 spectrometer.Emission decay time was recorded on the same fluorimeter with a multichannel scaling (MCS) peripheral equipment and a spectraLED pulsed source (373 nm).Signals were collected with a FluoroHub module and analyzed by the DAS6 Decay Analysis software (HORIBA Jobin-Yvon).The variable-temperature measurements (73~313 K) of emission decay time and spectra were performed using a LINKAM THMS600 system with a variable-temperature range of 77~873 K.The powder photoluminescence quantum yield was defined as the number of photons emitted per photon absorbed by the systems and measured by FluoroMax-4 equipped with an integrating sphere.
2.2 Synthesis of the NP ligand
The NP ligand of 1-(2-(diphenylphosphanyl)phenyl)-3-phenyl-4,5-dihydro-1H-pyrazole was synthesized by the following steps.Scheme 1 shows the synthetic route of the NP ligand.
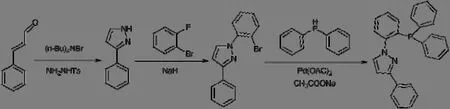
Scheme 1.Synthetic route of the NP ligand
2.2.1 Synthesis of 3-phenyl-1H-pyrazole
To a Schlenk tube with a magnetic bar was added cinnamaldehyde (50 mmol), p-toluenesulfonyl hydrazide (60 mmol, 1.2 equiv), NaOH (1.5 equiv),(n-Bu)4NBr (1.5 equiv) and distilled water (200 mL).The reaction mixture was refluxed for 10 h in an oil bath.The mixture would be layered after the reaction finished and was cooled to room temperature.And then the mixture was extracted and washed with CH2Cl2and brine.After being dried over sodium sulphate, the crude product was purified by column chromatography on silica gel to afford a white solid (6.4 g, 89%).1H NMR (400 MHz, CDCl3) δ 7.69 (d, J = 7.4 Hz, 2H), 7.54 (s,1H), 7.34 (s, 2H), 7.26 (t, J = 7.1 Hz, 1H), 6.55 (s,1H).
2.2.2 Synthesis of 1-(2-bromophenyl)-3-phenyl-4,5-dihydro-1H-pyrazole
The mixture of 3-phenyl-1H-pyrazole (5.01 g, 26 mmol), 2-bromofluorobenzene (2.64 g, 21.6 mmol),and NaH (4.14 g, 51.8 mmol) in 50 mL of anhydrous DMF was refluxed 1 h at 150 ℃ under nitrogen atmosphere.After being cooled to room temperature, the solvent was removed by vacuum-rotary evaporation.And then the mixture was extracted and washed with CH2Cl2and brine.After being dried over sodium sulphate, the crude product was purified by column chromatography on silica gel to afford oily products (5.17 g, 80%).
2.2.3 Synthesis of 1-(2-(diphenylphosphanyl)phenyl)-3-phenyl-4,5-dihydro-1H-pyrazole
The mixture of 1-(2-bromophenyl)-3-phenyl-4,5-dihydro-1H-pyrazole (10 mmol), diphenylphosphine (10 mmol), sodium acetate trihydrate (10 mmol), palladium(II) acetate (0.05 mmol) in 10 mL of anhydrous DMF was refluxed 12 h at 130 ℃under nitrogen atmosphere.After cooling to room temperature, the solvent was removed by vacuumrotary evaporation.And then the mixture was extracted and washed with CH2Cl2and brine.After being dried over sodium sulphate, the crude product was purified by column chromatography on silica gel to afford a white solid (2.91 g, 72%).
2.3 Synthesis of [Cu(μ-I)(NPPh)]2 (1)
CuI (1 mmol) was stirred for 10 min in CH2Cl2(10 mL) at room temperature, and then the NP ligand (1 mmol) was added.After stirring for another 2 hours, the mixture was filtered and the crude products of1were obtained by the solvent evaporating under vacuum.The single crystals of1were crystallized by diffusion of diethyl ether into CH2Cl2solution (405 mg, 68%).1H NMR (400 MHz, CDCl3): δ 8.06 (d, J = 1.8 Hz, 2H), 7.59 (dd,J = 9.5, 7.9 Hz, 8H), 7.51~7.17 (m, 28H), 6.92 (t, J= 7.6 Hz, 2H), 6.07 (t, J = 2.2 Hz, 2H).31P NMR:–10.05 (s).Anal.Calcd.for C54H42Cu2I2N4P2: C,54.45; H, 3.53; N, 4.71%.Found: C, 54.21; H, 3.71;N, 4.69%.
2.4 Structure determination
A colourless crystal of complex1with dimensions of 0.30mm × 0.30mm × 0.30mm was used for X-ray diffraction analysis.Diffraction data of the complex were collected on a SuperNova, Dual, Cu at zero, Atlas diffractometer equipped with graphitemonochromated CuKα radiation (λ = 1.54184 ?).A total of 8140 reflections were collected at 100 K in the range of 4.45≤θ≤68.24o by using an ω-scan mode, of which 4222 were unique with Rint=0.0290 and 3787 were observed with I > 2σ(I).The structure was solved by direct methods with SHELXS-97 and refined by full-matrix leastsquares methods with SHELXL-97 program package[20].All of the non-hydrogen atoms were located with successive difference Fourier synthesis.Hydrogen atoms were added in idealized positions.The non-hydrogen atoms were refined anisotropically.The final R = 0.0289, wR = 0.0670 (w =1/[σ2(Fo2) + (0.0333P)2+ 0.0412P], where P = (Fo2+ 2Fc2)/3), S = 1.048, (Δ/σ)max= 0.002, (Δρ)max=0.549 and (Δρ)min= –1.066 e/?3.Selected bond lengths and bond angles from X-ray structure analysis are listed in Table 1.
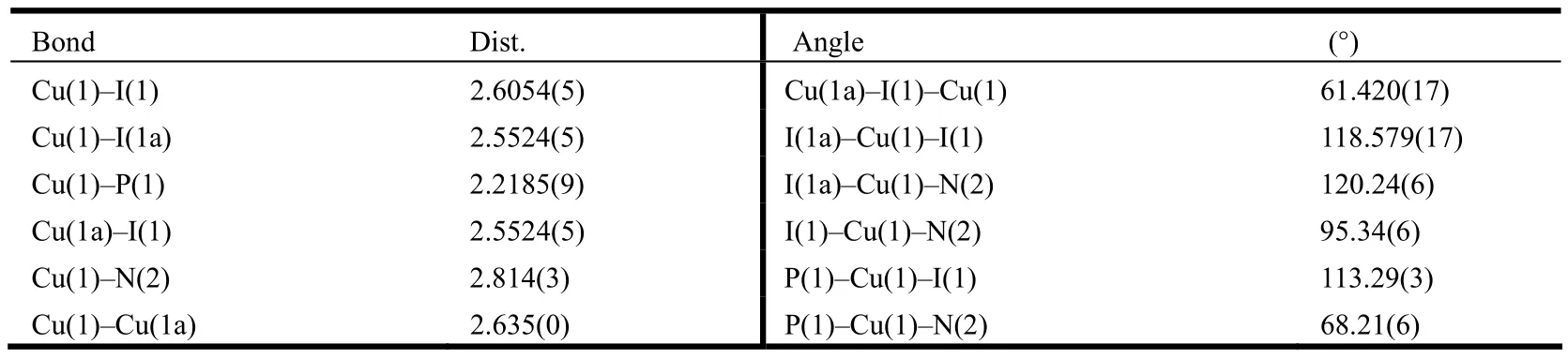
Table 1.Selected Bond Lengths (?) and Bond Angles (°)
3 RESULTS AND DISCUSSION
Complex1was synthesized from the reaction of equivalent amounts of copper iodide and NP ligand in dichloromethane.Single crystals of the complex were obtained by diffusion of diethyl ether into the CH2Cl2solution.The complex is air stable both in solution and in the solid state.It has been characterized by1H and31P NMR as well as by X-ray structure analysis.Its ORTEP diagram and molecular structure are shown in Fig.1.
Single-crystal analysis reveals that1crystallizes in the triclinic space group P21/n with Z = 4.As shown in Fig.1, each copper atom is bridged by two iodide anions, and the ends of the molecule are chelated by a bidentate chelating NP ligand.The coordinate geometry of the copper atom can be described as a tetrahedral geometry.The molecule of [Cu(μ-I)(NP)]2resides at an inversion center.The[Cu2I2] core is planar.The Cu–N (2.814(3) ?),Cu–P (2.2185(9) ?) and Cu–I (2.6054(5), 2.5524(5)?)) distances are in the normal ranges found in those Cu–N, Cu–P and Cu–I distances in similar Cu complexes.The Cu··Cu distance in the complex is 2.635(0) ?, which is smaller than the sum of van der Waals radii (about 2.8 ?), indicating obvious d10-d10interaction in this complex.The angle of I–Cu–I (118.579(17)°) is close to the tetrahedral value of 109.5o[7], while N–Cu–P (68.21(6)°)deviates obviously from the tetrahedral value due to the small bite angle of the NP ligand.The dihedral angle of N(2)–P(1)–Cu(1)–I(1)–I(1a) is 100.28(0)°,deviating slightly from 90o as a perfect tetrahedral geometry.
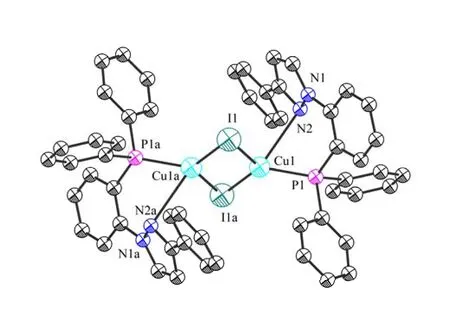
Fig.1.Molecular structure (left) and ORTEP diagram (right) of complex 1.Thermal ellipsoids are drawn at 50% probability.Hydrogen atoms are not displayed for clarity
Fig.2 demonstrates the UV-vis absorption spectrum of complex1in CH2Cl2at ambient temperature.Complex1exhibits an intense absorption (ε >104M–1cm–1) at 275 nm located in high-energy region (< 300 nm), which is similar to that of NP ligand at 273 nm.The intense absorption band with an obvious shoulder is assigned to spin-allowed π-π* ligand centered (LC) transitions of NP ligand.The low-energy section absorption band located at the 324~390 nm region that is inconspicuously observed in the spectrum of the ligand is assigned to metal-ligand charge transfer (MLCT) and halogen-ligand charge transfer (XLCT) affected by the Cu(I) and halide atoms.
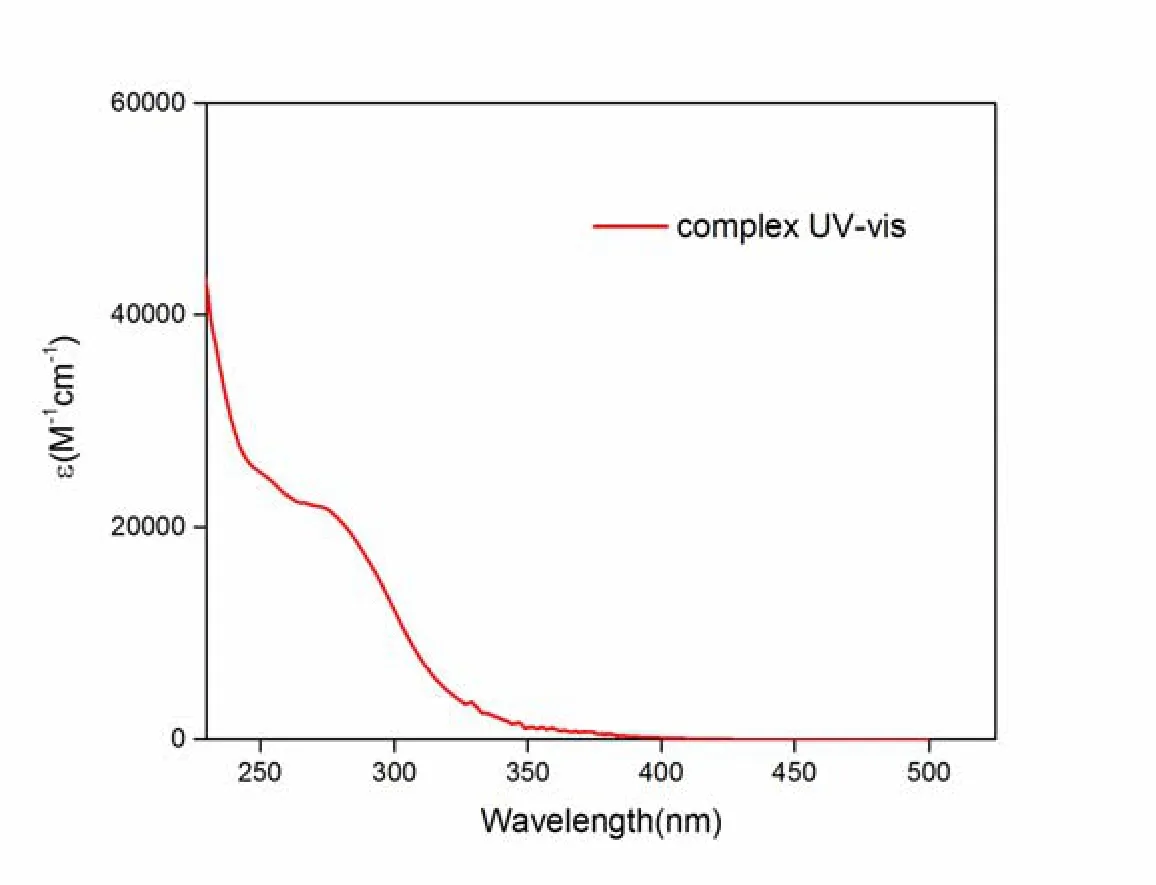
Fig.2.UV-vis absorption spectra of complex 1 in CH2Cl2
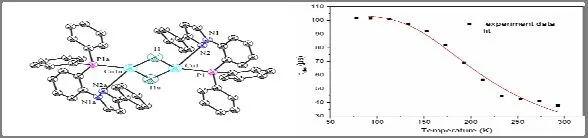
The emission spectra in the solid state at 293 and 77 K are shown in Fig.3.Both of the two emission spectra were recorded under excitation at λexc= 370 nm.At 298 K, complex1shows greenlish yellow emission and the photoluminescence quantum yield of 63.6%.Along with temperature going down, the red-shift was observed from the emission spectra with peak maxima 533 to 548 nm.For further understanding the nature of emission, the decay time at various temperature between 77 and 293 K is measured, and the results are depicted in Fig.4.The black points represent the experiment data, and the red curve is fitted according to equation (1):
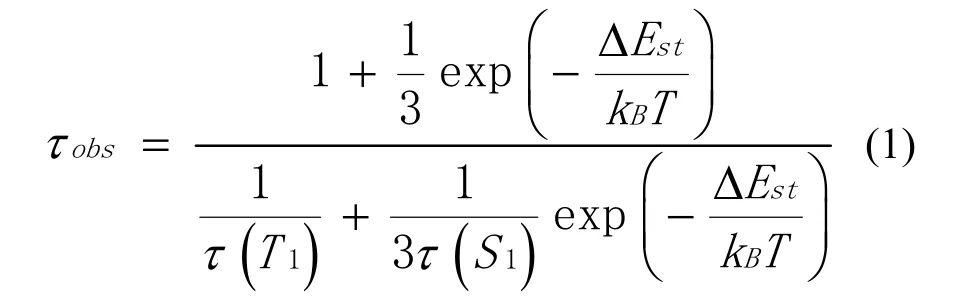
In this equation, T and kBare the absolute temperature and Boltzmann constant, respectively.ΔESTis the energy gap between S1and T1states.τ(S1) and τ(T1) represent the individual decay time of the lowest singlet excited state (S1) and the lowest triplet excited state (T1).The parameters are well fitted with values of τ(S1) = 912 ns, τ(T1) = 103 μs, and ΔEST= 0.07 eV into1.Lifetime of the complex is lengthened with the reduction of temperature from 37.8 μs at 293 K to 101.7 μs at 77 K.This is due to the fact that the lower triplet excitons cannot be converted to singlet states via reverse intersystem crossing by absorbing surroundding environmental heat energy in low temperature.The observed changes of the emission decay time and the emission peak upon changing of the temperature indicate that complex1is a TADF material[21].
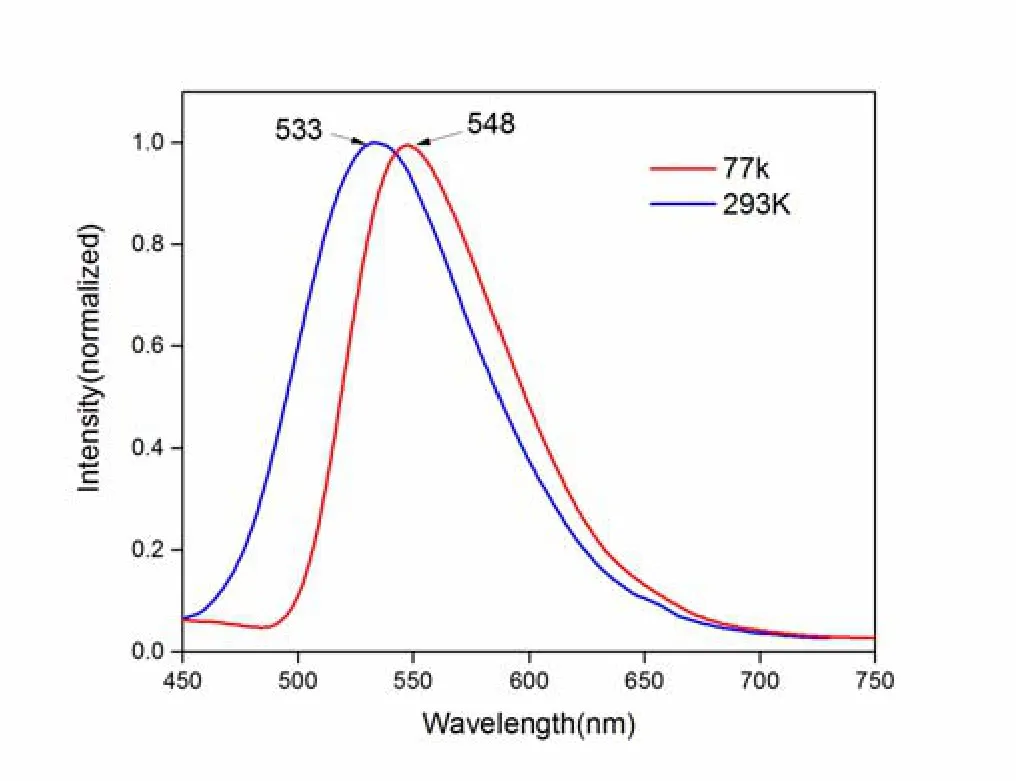
Fig.3.Emission spectra of complex 1 in the solid state at 77 and 293 K
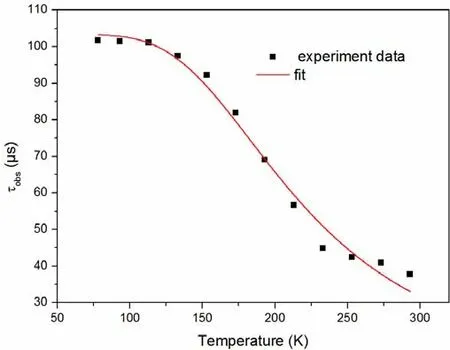
Fig.4.Temperature dependence of the decay time for complex 1 in powder.The solid line represents a fit curve according to Eq.(1)
In summary, a novel halide-bridged Cu(I) dinuclear complex with chelating aminophosphane ligand was obtained.It is rarely that high photoluminescence quantum yield of 63.6% could be obtained in copper complexes.This efficient emitter can be used as emitting dopant in OLEDs.
REFERENCES
(1) Baldo, M.A.; O'Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R.Highly efficient phosphorescent emission from organic electroluminescent devices.Nature1998, 395, 151–154.
(2) Baldo, M.A.; Lamansky, S.; Burrows, P.E.; Thompson, M.E.; Forrest, S.R.Very high-efficiency green organic light-emitting devices based on electrophosphorescence.Appl.Phys.Lett.1999, 75, 4–6.
(3) Yam, V.W.W.; Wong, K.M.C.Luminescent metal complexes of d6, d8and d10transition metal centres.Chem.Commun.2011, 47, 11579–11592.
(4) Che, C.M.; Lai, S.W.Structural and spectroscopic evidence for weak metal-metal interactions and metal-substrate exciplex formations in d10metal complexes.Coord.Chem.Rev.2005, 249, 1296–1309.
(5) Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X.Recent progress in metal-organic complexes for optoelectronic applications.Chem.Soc.Rev.2014, 43, 3259–3302.
(6) Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C.Highly efficient organic light-emitting diodes from delayed fluorescence.Nature2012, 492, 234–238.
(7) Czerwieniec, R.; Yu, J.; Yersin, H.Blue-light emission of Cu(I) complexes and singlet harvesting.Inorg.Chem.2011, 50, 8293–8301.
(8) Hofbeck, T.; Monkowius, U.; Yersin, H.Highly efficient luminescence of Cu(I) compounds-TADF combined with short-lived phosphorescence.J.Am.Chem.Soc.2014, 137, 399–404.
(9) Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H.; Chen, R.; Zheng, C.; Zhang, L.; Huang, W.Thermally activated delayed fluorescence materials towards the breakthrough of organoelectronics.Adv.Mater.2014, 26, 7931–7958.
(10) Kaeser, A.; Delavaux-Nico, B.; Duhayon, C.; Duhayon, J.E.Heteroleptic silver(I) complexes prepared from phenanthroline and bis-phosphine ligands.Inorg.Chem.2013, 52, 14343–54.
(11) Kuang, S.M.; Cuttell, D.G.; McMillin, D.R.; Fanwick, P.E.; Walton, R.A.Synthesis and structural characterization of Cu(I) and Ni(II)complexes that contain the [2-(diphenylphosphino)phenyl]ether ligand.Novel emission properties for the Cu(I) species.Inorg.Chem.2002, 41,3313–3322.
(12) Zhang, Q.S.; Komino, T.; Huang, S.P.; Matsunami, S.; Goushi, K.; Adachi, C.Triplet exciton confinement in green organic light-emitting diodes containing luminescent charge-transfer Cu(I) complexes.Adv.Funct.Mater.2012, 22, 2327–2336.
(13) Chen, X.L.; Yu, R.; Zhang, Q.K.; Zhou, L.J.; Wu, X.Y.; Zhang, Q.; Lu, C.Z.Rational design of strongly blue-emitting cuprous complexes with thermally activated delayed fluorescence and application in solution-processed OLEDs.Chem.Mater.2013, 25, 3910–3920.
(14) Kang, L.J.; Chen, J.; Teng, T.; Chen, X.L.; Yu, R.M.; Lu, C.Z.Synthesis, crystal structure and photoluminescence of a dimer with tetrakis(pyrazol-i-yl) borate linker.Chin.J.Struct.Chem.2015, 334, 1761–1767.
(15) Elena, C.; Elena, L.; Chiara, B.; Umberto, G.; Daniele, M.; Stefania, R.Cu(I) hybrid inorganic-organic materials with intriguing stimuli responsive and optoelectronic properties.Coordination Chemistry Reviews2016, 306, 566–614.
(16) Huang, C.H.; Wen, M.; Wang, C.Y.; Lu, Y.F.; Huang, X.H.; Li, H.H.; Wu, S.T.; Zhuang, N.F.; Hu, X.L.A series of pure-blue-light emitting Cu(I)complexes with thermally activated delayed fluorescence: structural, photophysical, and computational studies.Dalton Trans.2017, 46, 1413–1419.
(17) Volz, D.; Chen, Y.; Wallesch, M.; Liu, R.; Steininger, R.; Weinhardt, L.; So, F.; Baumann, T.Bridging the efficiency gap: fully bridged dinuclear Cu(I)-complexes for singlet harvesting in high-efficiency OLEDs.Adv.Mater.2015, 27, 2538–2543.
(18) Chen, X.L.; Yu, R.M.; Wu, X.Y.; Liang, D.; Jia, J.H.; Lu, C.Z.A strongly greenish-blue emitting Cu4Cl4cluster with efficient spin orbit couping(SOC): fast phosphorescence versus thermally activated delayed fluorescence.Chem.Commun.2016, 52, 6288–6291.
(19) Kobayashi, A.; Hasegawa, T.; Yoshida, M.; Kato, M.Environmentally friendly mechanochemical syntheses and conversions of highly luminescent Cu(I) dinuclear complexes.Inorg.Chem.2016, 55, 1978?1985.
(20) Sheldrick, G.M.SHELXS-97 and SHELXL-97.Program for X-ray Crystal Structures Solution and Refinement.University of G?ttingen: G?ttingen,Germany1997.
(21) Liang, D.; Chen, X.L.; Liao, J.Z.; Hu, J.Y.; Jia, J.H.; Lu, C.Z.Highly efficient cuprous complexes with thermally activated delayed fluorescence for solution-processed organic light-emitting devices.Inorg.Chem.2016, 55, 7467–7475.
- 結構化學的其它文章
- Synthesis and Characterization of a Palladium Complex Supported by Bidentate Ligand and Catalysis of the Vinyl Polymerization of Norbornene①
- Structural, Electronic, Optical and Thermodynamic Properties of Nanolaminated Boride Cr4AlB6①
- Synthesis, Structure and Photochromism of a New Diarylethene and Its Ag(I) Complex①
- Ionothermal Synthesis, Structure and Luminescent Properties of a New 2-D Bismuth(III) Coordination Polymer with (6,5)-Connected Topological Sheet①
- Synthesis, Crystal Structure and Properties of a 1D Heteronuclear Cobalt-sodium Polymer with Bridging Ligand 2-(2-Hydroxy-3-methoxybenzylidene)Hydrazinecarbothioamide①
- Iodoplumbate(II)-based Hybrid Templated by 1,4-Diazabicyclo[2.2.2]octane Derivative: Structure,Photocurrent Response Behavior and Photocatalytic Activity for the Degradation of Organic Dye①

