Iodoplumbate(II)-based Hybrid Templated by 1,4-Diazabicyclo[2.2.2]octane Derivative: Structure,Photocurrent Response Behavior and Photocatalytic Activity for the Degradation of Organic Dye①
LI Jin-Wei JIANG Cheng-Chun
(School of Construction and Environment Engineering,Shenzhen Polytechnic, Shenzhen, Guangdong 518055, China)
A new iodoplumbate/organic hybrid has been synthesized, whose unique (Pb3I11)n5n-chain is constructed from face- and edge-sharing PbI6octahedra.C–H…I hydrogen bonds contribute to the structural extending from 1D chains to a 3D network.The energy band gap of 2.64 eV indicates its broad-gap semiconductor nature, and it exhibits both photocurrent response property and photocatalytic activity for the degradation of rhodamine B.
1 INTRODUCTION
The main group element (Bi(III), Pb(II), Sn(II),Ge(II), et al) halometallates have captured the interests of chemists for their fascinated functional properties[1–3].Among them, lead-based halometallates are special because of their unique photoconductivity, ionic-conductivity, electrical-conductivity, and photo/electro-luminescence properties[4–6].And recently, they have been extensively studied due to their fascinated applications in thin film transistors(TFTs)[7]and solar energy conversion[8,9].When concerning about their structures, lead iodides/organic hybrids can present an enormous number of intriguing structural topologies, where lead centers exhibit a wide variety of coordination numbers and stereochemistries with or without the suggestion of a‘lone pair’ in their coordination sphere[10,11].Therefore, their anion structures range from isolated anions to infinite chains, layered perovskites, and threedimensional polymeric networks based on face-,edge-, or vertex-sharing PbI6octahedra[12].Organic ammoniums countercations with tunable charge, size and shape have been proved to be good templates during the design and synthesis of different functional hybrids[13,14].So far, the organic ammonium countercations have ranged from short to long chain alkylammonium cations (4 to 18 carbon atoms),cyclic alkylammonium cations (3 to 8 carbon atoms)or chiral aromatic cations[12,15], which exhibit structural flexibilities or rigidities in the process of assembly of functional hybrids.But the usage of templates possessing both flexibility and rigidity in this system is still in its infancy[16-19].In this work,we used template possessing both rigidity (1,4-diazabicyclo[2.2.2] octane) and flexibility (substitutes lengths of ethyl) to produce a new lead iodide/organic hybrid, [(Et2DABCO)2(Pb3I11)(H3O)]n(1,Et2DABCO = N,N?-diethyl-1,4-diazabicyclo[2.2.2]octane).Its band gap, photocurrent response property and photocatalytic activity were also discussed.
2 EXPERIMENTAL
2.1 Materials and methods
All chemicals except Et2DABCO·I2of regent grade were obtained from commercial sources and used without further purification.Elemental analyses for C, H and N were performed on a Vario MICRO elemental analyzer.IR spectra were recorded on a Perkin-Elmer Spectrum-2000 FTIR spectrophotometer (4000~2400 cm-1).UV-Vis spectrum was measured on a Perkin-Elmer lambda 900 UV/Vis spectrophotometer equipped with an integrating sphere at 293 K, and a BaSO4plate was used as reference.TG data were collected under argon atmosphere using a Mettler Toledo TGA/DSC 3+thermal analyzer (thermal ramp 5 ℃/min, temperature range 25~2800 ℃).
2.2 Synthesis
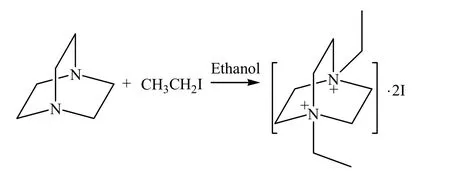
2.2.1 Synthesis of Et2DABCO·I2
Et2DABCO·I2was synthesized by a one step N-alkylated reaction of 1,4-diazabicyclo[2.2.2]octane with iodoethane according to the literature method[20]:
2.2.2 Synthesis of
[(Et2DABCO)2(Pb3I11)(H3O)]n(1)1was prepared by the solution method.PbI2(0.0461 g, 0.1 mmol) and Et2DABCO·I2(0.0424 g,0.1 mmol) were dissolved in 15 mL DMF and stirred for 30 minutes.Then KI (0.0166 g, 0.1 mmol) and I2(0.0253 g, 0.1 mmol) were added into the above suspension followed by continuous stirring for 1 h.Afterwards, 1 mL HI (10%) was used to adjust the pH to 2.5.After that the solution was stirred to be homogeneous yellow and then filtered.The filtrate was kept in an undisturbed surrounding for 15 days with yellow block crystals obtained (0.0276 g, yield 34.8% based on Pb.).We want to introduce polyiodine ions into hybrid system by using I2as the starting material, but out of our expectation, no poly-iodine ion was present in the product.Anal.Calcd.for C20H47I11N4OPb3(2377.11): C, 10.10; H,1.97; N, 2.36.Found: C, 10.28; H, 2.06; N, 2.25.IR(cm-1): 3228(m), 2996(w), 2960(w), 1556(s),1439(s), 1110(s), 850(s), 1388(s), 803(m), 580(w).
2.3 Structure determination
The intensity data of1were collected on a Bruker APEX II diffractometer using graphite-monochromated MoKα radiation (λ = 0.71073?) at room temperature.The empirical absorption correction was based on equivalent reflections.Structure was solved by direct methods followed by successive difference Fourier method.Computations were performed using SHELXTL and final full-matrix least-squares refinements were against F2[21].All non-hydrogen atoms were refined anisotropically.Hydrogen atoms of C–H were generated geometrically.Crystal data of1:monoclinic, space group P21/c with Mr= 2377.11, a= 10.3899(13), b =18.366(2), c = 15.3630(14) ?, β =121.433(6)°, V = 2501.4(5) ?3, Z = 2, Dc=3.155g/cm3, F(000) = 2062, μ(MoKα) = 16.879 mm–1,the final R = 0.0478 and wR = 0.1320 (w = 1/[σ2(Fo2)+ (0.0885P)2+ 0.0000P], where P = (Fo2+ 2Fc2)/3), S= 1.044, (Δ/σ)max= 0.000, (Δρ)max= 3.796 and(Δρ)min= –3.170 e/?3.Selected bond lengths and bond angles are given in Table 1.Hydrogen bond lengths and bond angles are shown in Table 2.
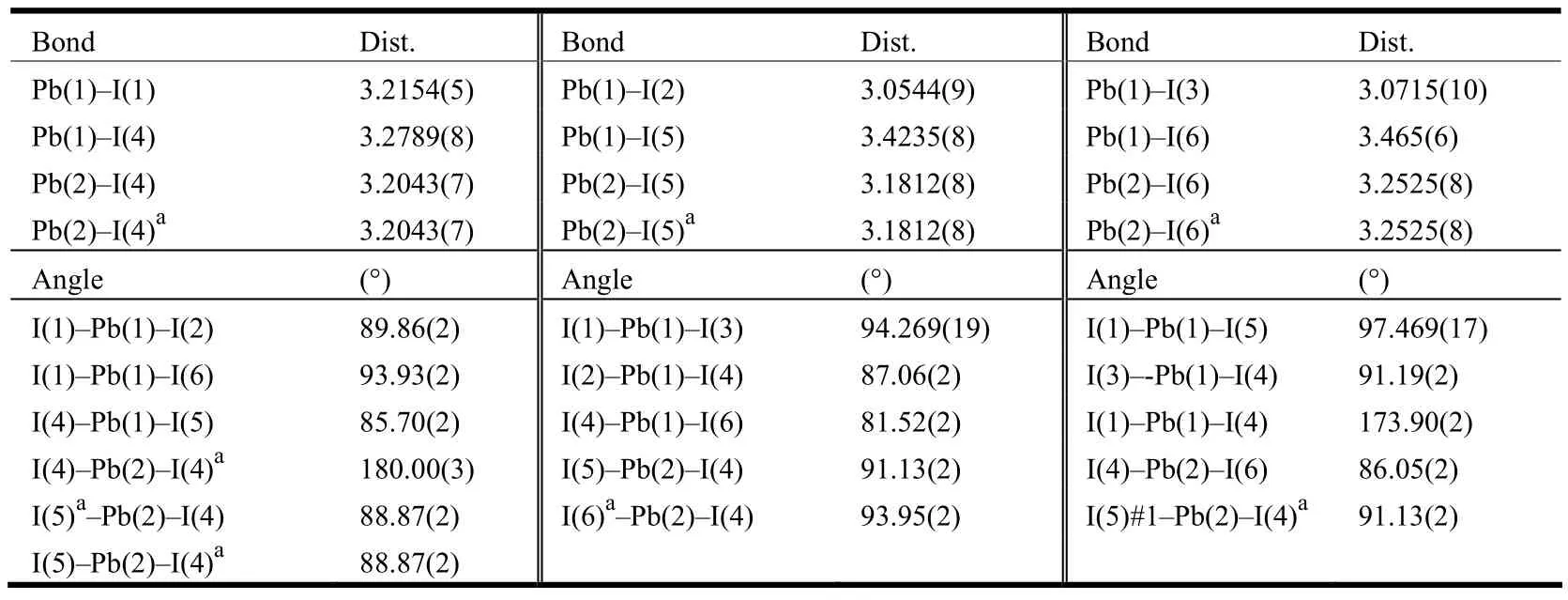
Table 1.Selected Bond Lengths (?) and Bond Angles (°)

Table 2.Hydrogen Bridging Details of 1
2.4 Electrode preparation and photocurrent measurement
Film of1was prepared using the solution coating method.0.5 mg of the new prepared compound1was dissolved in 1.5 mL DMF, and the solution was coated on the ITO glass (10.6 × 0.6 cm).The coating film was obtained after the solvent was carefully removed under reduced pressure.A 150 W highpressure xenon lamp, located 10 cm away from the surface of the ITO electrode, was employed as a fullwavelength light source.The photocurrent experiment was carried out on a CHI650E electro-chemistry workstation using a three electrodes system, in which the sample-coated ITO glass was used as the working electrode, Pt wire as auxiliary electrode and a saturated calomel electrode (SCE) as the reference electrode.The supporting electrolyte solution was a 0.1 mol·L?1sodium sulfate aqueous solution.The applied potential was 0.5 V for all measurements.The lamp was kept on continuously, and a manual shutter was used to block exposure of the sample to thelight.The sample was typically irradiated at an interval of 10 s.
2.4 Photocatalytic testing
The visible light source was set as a 300 W Xe arc lamp equipped with a λ ≥ 420 nm cutoff filter and an IR filter, and the output light intensity was measured as 110 mw/cm2.During the photodegradation experiment of RhB, 40 mg catalyst powder was suspended in 80 mL RhB solutions (concentration:10 ppm).Before irradiation, the suspensions were magnetically stirred in the dark for 2 h to achieve adsorption-desorption equilibrium of the organic contaminants on the catalyst surfaces.3 mL of the sample solutions was taken out at given time intervals and separated through sample filtration.The residual concentrations of pollutants in solution were analyzed by recording variations of the organics at the absorption band maximum in the UV-Vis spectra using a UV-Vis spectrophotometer.The percentage of degradation is reported as C/C0, where C is the absorption of RhB at each irradiated time interval of the main peak of the absorption spectrum at 553 nm,and C0is the absorption of the starting concentration when adsorption-desorption equilibrium is achieved.
3 RESULTS AND DISCUSSION
3.1 Structure description
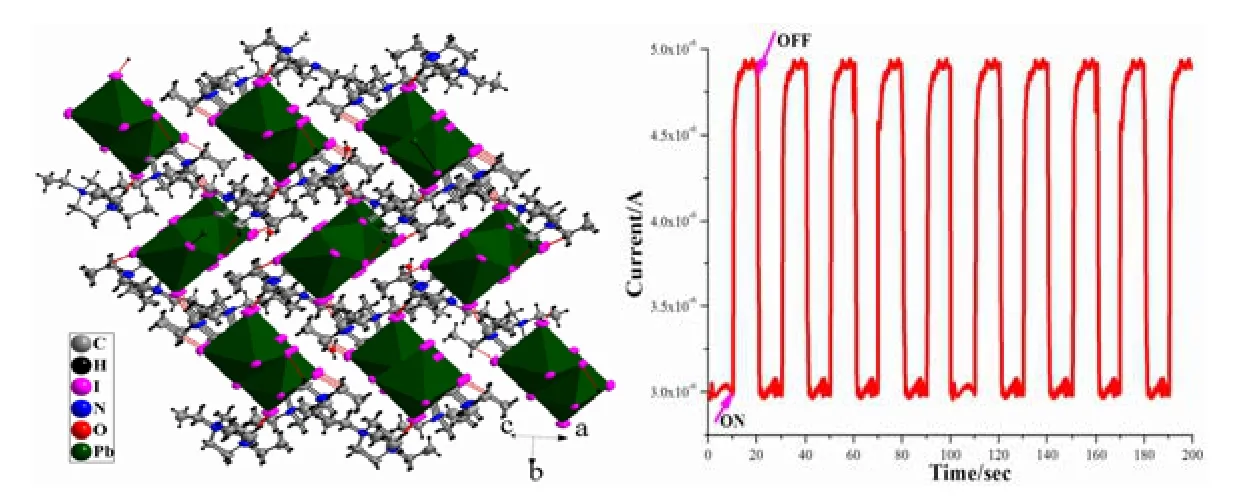
1crystallizes in monoclinic space group P21/c,which is composed of 1D (Pb3I11)n5n-chain,(Et2DABCO)2+dications and one protonized water.C–H··I hydrogen bonds contribute to the formation of a 3D network.As shown in Fig.1, there are two crystallographically independent Pb centers (Pb(1),Pb(2)), whose coordination environments are both slightly distorted octahedra.The Pb–I bond distances range among 3.0544(9)~3.465(6) ? (for Pb(1)I6octahedron) and 3.1812(8)~3.2525(8) ? (for Pb(2)I6octahedron, Table 1).The I–Pb–I bond angles fall in the ranges of 81.52(2)~97.469(17)° (for Pb(1)I6octahedron) and 88.87(2)~93.95(2)° (for Pb(2)I6octahedron).Therefore, the distortion degree of Pb(1)I6octahedron is greater than that of Pb(2)I6.In addition, the bond lengths and bond angles are similar to those observed in the literature[12,22].The Pb(1), Pb(2) and Pb(1)#2-centered PbI6octahedra(#2: –x–1, –y, –z–2) are connected into a linear Pb3I12trinuclear cluster via face-sharing model (Fig.1),which further vertex-share with each other through the μ2-I(1) to give an infinite staircase chain.The Pb(1)–Pb(2) and Pb(2)–Pb(1)#2separations are 4.133(20) ?.The 1D (Pb3I10)n4n-staircase chain shaped by edge-sharing of Pb3I12trinuclear clusters has been previously reported[12], but the case of vertex-sharing Pb3I12trinuclear cluster in1has never been observed.The bond lengths of (Et2DABCO)2+are normal.The N(1)–C(1)–C(2) and N(2)–C(9)–C(10) angles are 115.39° and 117.14° respectively,which are larger than ideal sp3-hybrid carbon angle of 109.28o.So, a slight unfolding of ethyl groups has occurred.This unfolding might be driven by the hydrogen bonds between (Et2DABCO)2+dicaiton and (Pb3I11)n5n-chain.As shown in Fig.2, one(Et2DABCO)2+dication links three neighboring(Pb3I11)n5n-chains through C–H··I hydrogen bonding interactions to extend the 1D chains into a 3D network (Fig.2), and the mean C··I separation is 3.831(8) ? and the mean C–H··I angle is 140°(Table 2).One protonized water molecule is also stacked in the lattice, whose protonation is judged from the charge balance.In all, weak interactions including hydrogen bonding and electrostatic interacttions stabilize the packing of crystal of1.
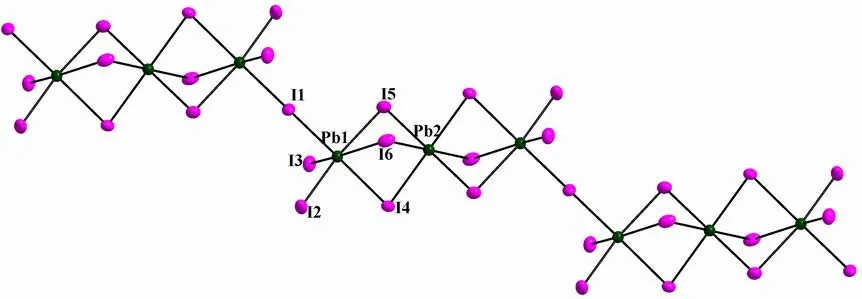
Fig.1.1D (Pb3I10)n4n- staircase chain of 1 constructed from vertex-sharing Pb3I12 trinuclear clusters
3.2 Adsorption spectra and linear absorption optical property
The stability of compound1has been investigated by thermogravimetry measurement, which was measured under argon atmosphere in the 25~800 °C temperature range (TGA curve is shown in Fig.3a).The result implies that compound1exhibits good thermal stability up to 200 oC.The weigh loss from 200 to 400 °C corresponds to the loss of lattice protonized water, (Et2DABCO)2+dications and I2vapor (weight loss: theoretical 64.1%, observed 64.5%).The phase purity of bulk compound1has been verified by powder X-ray diffraction (PXRD).Obviously, the peaks in the experimental patterns are consistent with the corresponding simulated ones,suggesting its good phase purity (Fig.3b).
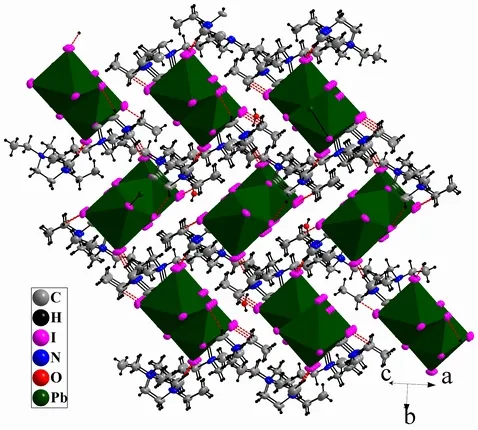
Fig.2.3D network based on C–H…I hydrogen bonds in 1
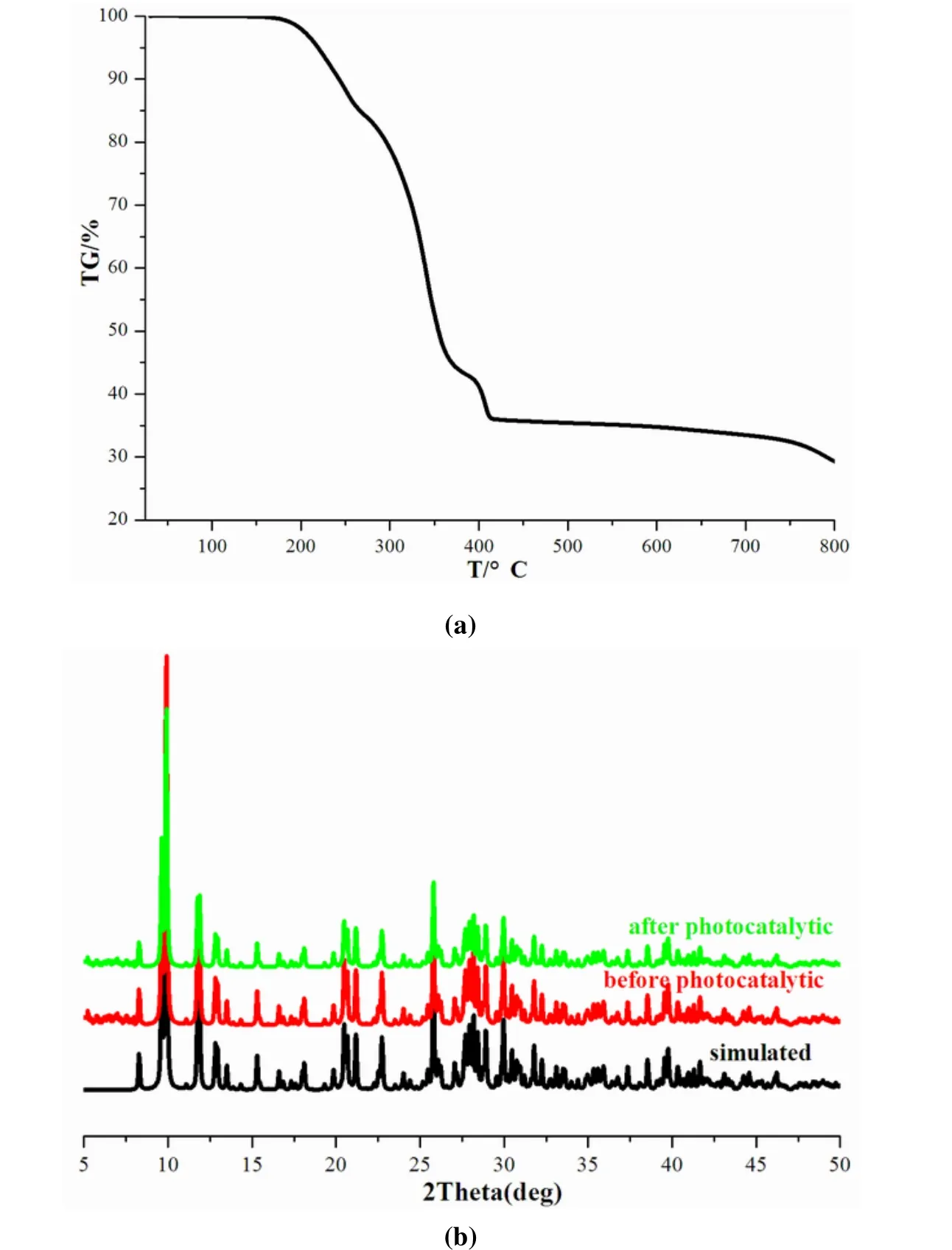
Fig.3.(a) Thermogrevimetric curve for 1; (b) PXRD patterns of 1 before and after photocatalysis
Fig.4a shows the diffuse reflectance UV-Vis absorption spectra of bulk PbI2and1.Compound1exhibits broad adsorption ranging from 250 to 485 nm, and absorption peak at 383 nm can be observed.Compared the UV-Vis absorption spectra of bulk PbI2and relative compounds, the peak at 383 nm can be assigned to the characteristic of the corner, edge-,or face-sharing PbI6octahedra[23-25].Due to the presence of absorption peak around the visible region,its photocatalytic activity was further driven by visible light excitation.The optical gap of1was assessed from its optical diffuse reflectance data, and the Kubelka-Munk functions converted from the diffuse reflectance data are plotted in Fig.4b[26,27].From Fig.4b, the optical gap of 2.64 eV was calculated, illustrating its semiconductor property and the existence of direct transitions.Compared with that of bulk PbI2(2.47~2.49eV)[28], the gap of1is broadened clearly,which might be led by the introduction of nonconjugated organic cations.Together with the decreased ban gaps of iodoplum-bate(II)-based hybrids incorporated with conjugated organic ligand-containing[25], we can control the band gaps of hybrids by using different organic templates.
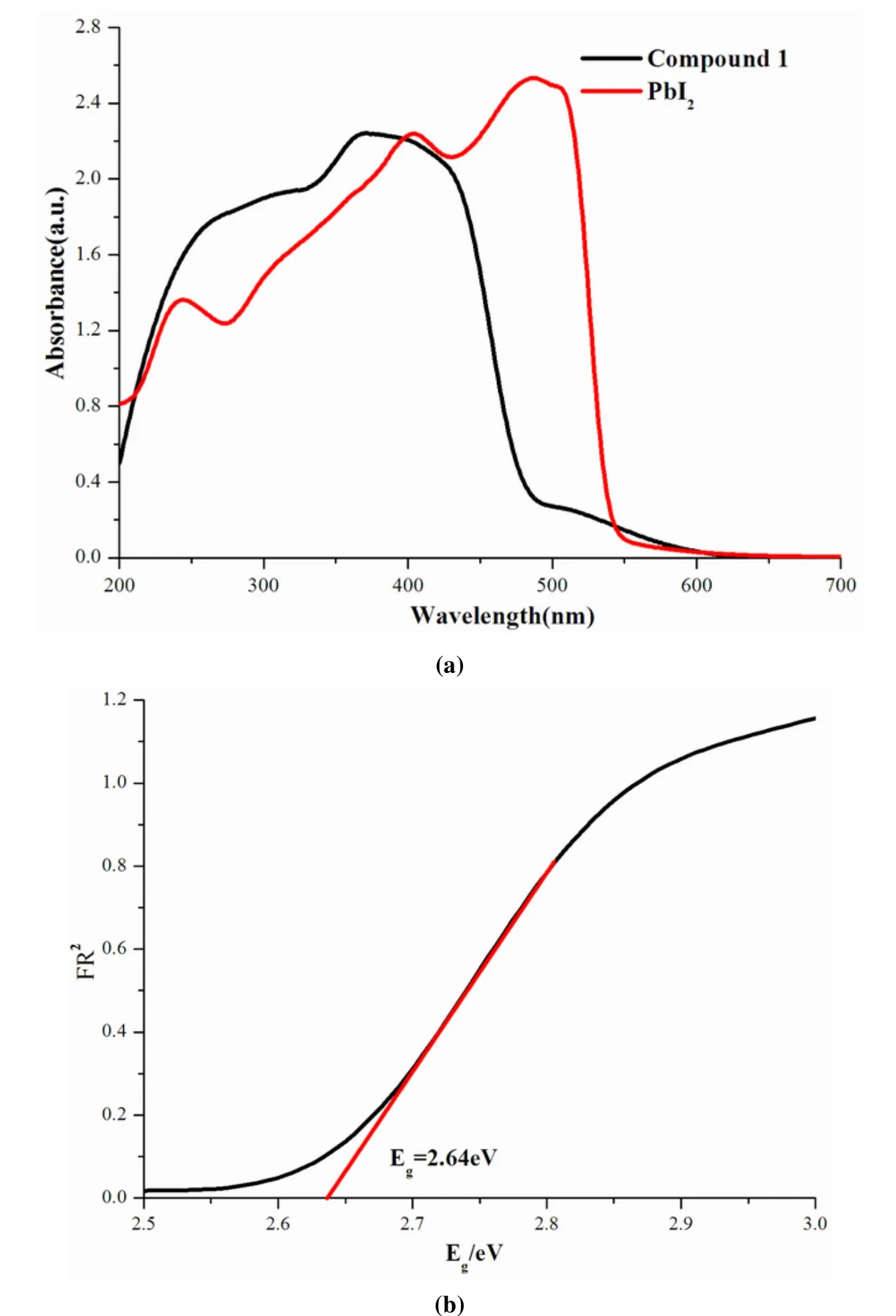
Fig.4.(a) UV-Vis spectrum of 1, (b) Optical adsorption spectrum of 1
3.3 Photocurrent response behavior
The photocurrent response experiment of1was recorded with a three-electrode system in order to investigate the photoelectric conversion behavior of1(the detailed description is given in the experimental section), and the result can be seen in Fig.5.Upon repetitive irradiation with xenon light on and off (interval 10 s), repeatable and steady photocurrents with rapid responses can be achieved, and there are not any decay after ten on/off cycles of illumination.The photocurrent reaches 4.8 μA, which is much larger than that of Zn4L2(bpca)4·4DMF·9H2O(0.014 μA)[29].Inorganic PbI2possesses good carrier mobility, but its hole-electron recombination is high,which leads to its low light-induced current[30].The photocurrent response mechanism can be explained as the electron-transfer among the [(Et2DABCO)2-(Pb3I11)(H3O)]n/ITO electrodes in solution: upon irradiation, electron transfers can occur from the(Pb3I11)n5n-donors to the (Et2DABCO)2+cations to give (Et2DABCO)?+species, then the (Et2DABCO)?+radicals transfer their electrons to the ITO electrodes to produce the effective electron flow.
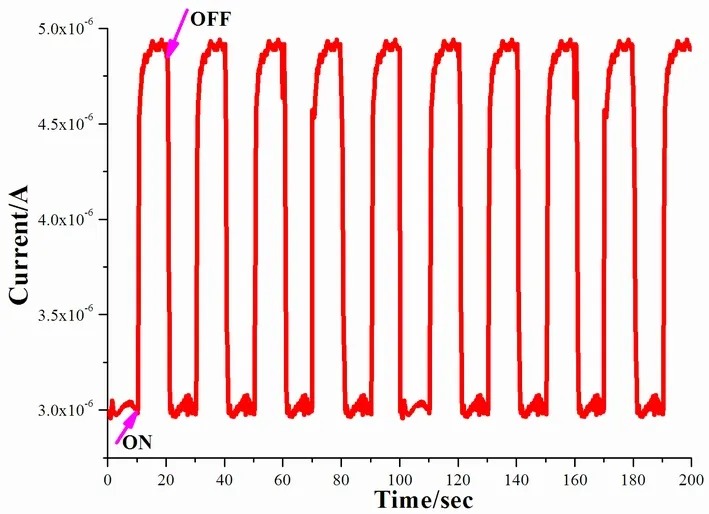
Fig.5.Photocurrent responses of 1 irradiated by a full-wavelength band high-pressure xenon lamp
3.4 Photocatalytic degradation of organic pollutant
Viologen-containing compounds have been proved to possess good ability of photocatalytic degradation organic pollutant[31,32,33].In this work, N-containing dye, rhodamine B (RhB) was selected as a model pollutant for degradation experiment.The wavelength and absorption intensity changes of RhB under the irradiation of xenon-lamp with the presence of catalysts are revealed in Fig.6a.The degradation experiments without the presence of catalyst and with PbI2were also conducted in order to verify the activity of catalyst1.The reference experiments show that the adsorption peaks change little.But with the presence of1, the adsorption spectra of RhB decrease to different extents with the lengthening of irradiation time, suggesting that the degeneration reactions on RhB have occurred.The peak shifts at 552 nm can not be observed, indicating that only deethylation of RhB has happened.Fig.6b shows the rates of RhB degradation (measured as RhB concentration versus irradiation time) in aqueous solutions with the presence of1, PbI2and without catalyst.After irradiation for 210 min, the degradation ratio is about 82.1% (in the presence of1), 8.9% (PbI2) and 4.1% (no catalyst).These results verify the degradation activity of1, and the degradation ratio of PbI2is consistent with the literature data (about 10%)[30].In order to verify the stability of1as photocatalyst, the sample was recovered from the reaction systems with filtration method.The PXRD pattern of recovered samples nearly identical with that of the as-prepared sample(Fig.3b), indicating its good stability as photo-catalyst.The pohtocatalystic degradation reaction is a heterocatalystic process, in which the catalysts can retain their patterns.
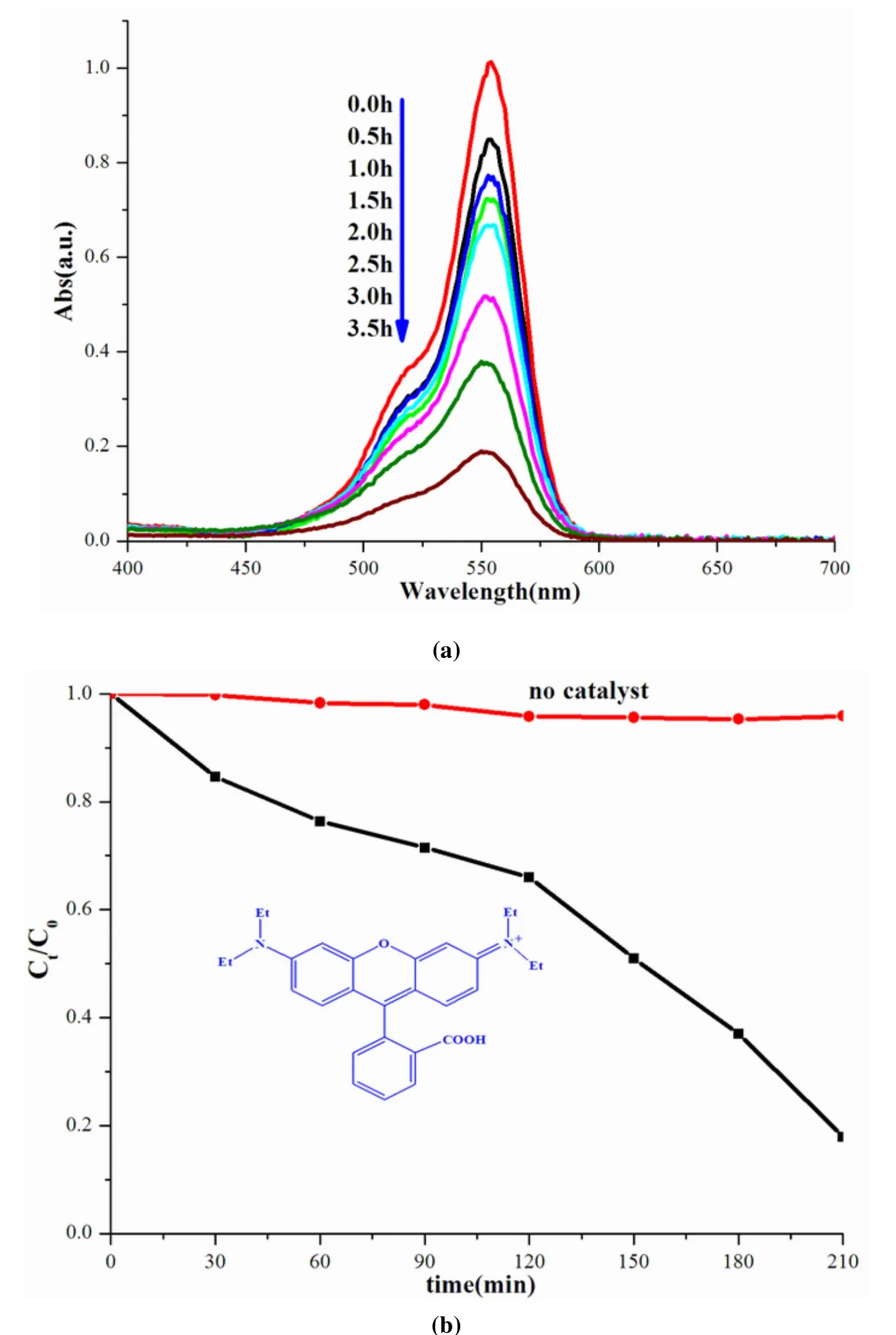
Fig.6.(a) Time-dependent UV-Vis spectra of RhB in the presence of 1 under the irradiation of xenon-lamp;(b) Concentration change of RhB irradiated under xenon-lamp as a function of irradiation time with or without the presence of 1.Ct and C0 stand for the RhB concentrations after and before irradiation
4 CONCLUSION
A new lead iodide/organic hybrid has been synthesized, whose (Pb3I11)n5n-chain was templated by(Et2DABCO)2+dication.Its (Pb3I11)n5n-chain is constructed from face- and edge-sharing PbI6octahedra,which has never been observed in haloplumbate polymeric chains.C–H··I hydrogen bonds contribute to the structural extending from 1D chains to a 3D network.Its energy band gap of 2.10 eV indicates semiconductor nature.Interestingly, it exhibits both photocurrent response property and photocatalytic activity for the degradation of rhodamine B.
REFERENCES
(1) Leblanc, N.; Mercier, N.; Zorina, L.; Simonov, S;.Auban-Senzier, P.; Pasquier, C.Large spontaneous polarization and clear hysteresis loop of a room-temperature hybrid ferroelectric based on mixed-halide [BiI3Cl2] polar chains and methylviologen dication.J.Am.Chem.Soc.2011, 133,14924–14927.
(2) Dang, Y.Y.; Liu, Y.; Sun, Y.X.; Yuan, D.S.; Liu, X.L.; Lu, W.Q.; Liu, G.F.; Xia, H.B.; Tao, X.T.Bulk crystal growth of hybrid perovskite material CH3NH3PbI3.CrystEngComm.2015, 17, 665–670.
(3) Stoumpos, C.C.; Frazer, L.; Clark, D.J.; Kim, Y.S.; Rhim, S.H.; Freeman, A.J.; Ketterson, J.B.; Jang, J.I.; Kanatzidis, M.G.Hybrid germanium iodide perovskite semiconductors: active lone pairs, structural distortions, direct and indirect energy gaps, and strong nonlinear optical properties.J.Am.Chem.Soc.2015, 137, 6804?6819.
(4) Chung, I.; Song, J.H.; Im, J.; Androulakis, J.; Malliakas, C.D.; Li, H.; Freeman, A.J.; Kenney, J.T.; Kanatzidis, M.G.CsSnI3: semiconductor or metal? High electrical conductivity and strong near-infrared photoluminescence from a single material: high hole mobility and phase-transitions.J.Am.Chem.Soc.2012, 134, 8579–8587.
(5) Mitzi, D.B.; Feild, C.A.; Harrison, W.T.A.; Guloy, A.M.Conducting tin halides with a layered organic-based perovskite structure.Nature1994,369, 467–469.
(6) Mitzi, D.B.; Wang, S.; Feild, C.A.; Chess, C.A.; Guloy, A.M.Conducting layered organic-inorganic halides containing <110>- oriented perovskite sheets.Science1995, 267, 1473–1476.
(7) Kagan, C.R.; Mitzi, D.B.; Dimitrakopoulos, C.D.Organic-inorganic hybrid materials as semiconducting channels in thin-film field-effect transistors.Science1999, 286, 945–947.
(8) Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T.Organometal halide perovskites as visible-light sensitizers for photovoltaic cells.J.Am.Chem.Soc.2009, 131, 6050–6051.
(9) Wang, L.; Cleese, C.M.; Kovalsky, A.; Zhao, Y.; Burda, C.Femtosecond time-resolved transient absorption spectroscopy of CH3NH3PbI3perovskite films: evidence for passivation effect of PbI2.J.Am.Chem.Soc.2014, 136, 12205–12208.
(10) Zhang, Z.J.; Guo, G.C.; Xu, G.; Fu, M.L.; Zou, J.P.; Huang, J.S.[(H2en)7(C2O4)2]n(Pb4I18)n·4nH2O, a new type of perovskite co-templated by both organic cations and anions.Inorg.Chem.2006, 45, 10028–10030.
(11) Aragoni, M.C.; Arca, M.; Caltagirone, C.; Devillanova, F.A.; Demartin, F.; Garau, A.; Isaia, F.; Lippolis, V.Inorganic-organic hybrid materials:construction of the first polymeric channelled halometallate(II) system.CrystEngComm.2005, 7, 544–547.
(12) Wu, L.M.; Wu, X.T.; Chen, L.Structural overview and structure-property relationships of iodoplumbate and iodobismuthate.Coord.Chem.Rev.2009,253, 2787–2804.
(13) Mitzi, D.B.; Dimitrakopoulos, C.D.; Kosbar, L.L.Structurally tailored organic-inorganic perovskites: optical properties and solution-processed channel materials for thin-film transistors.Chem.Mater.2001, 13, 3728–3740.
(14) Mercier, N.; Poiroux, S.; Riou, A.; Batail, P.Unique hydrogen bonding correlating with a reduced band gap and phase transition in the hybrid perovskites (HO(CH2)2NH3)2PbX4(X = I, Br).Inorg.Chem.2004, 43, 8361–8366.
(15) Lemmerer, A.; Billing, D.G.Effect of heteroatoms in the inorganic-organic layered perovskite-type hybrids [(ZCnH2nNH3)2PbI4], n = 2, 3, 4, 5, 6; Z= OH, Br and I; and [(H3NC2H4S2C2H4NH3)PbI4].CrystEngComm.2010, 12, 1290–1301.
(16) Wang, G.E.; Xu, G.; Wang, M.S.; Cai, L.Z.; Li, W.H.; Guo, G.C.Semiconductive 3-D haloplumbate framework hybrids with high color rendering index white-light emission.Chem.Sci.2015, 6, 7222–7226.
(17) Wang, G.E.; Xu, G.; Liu, B.W.; Wang, M.S.; Yao, M.S.; Guo, G.C.Semiconductive nanotube array constructed from giant [PbII18I54(I2)9] wheel clusters.Angew.Chem.Int.Ed.2016, 55, 514–518.
(18) Krautscheid, H.; Vielsack, F.[BuN(CH2CH2)3NBu]3[Pb5I16]·4DMF -ein Iodoplumbat mit nahezu D5h-symmetrischem anion.Z.Anorg.Allg.Chem.2000, 626, 3–5.
(19) Krautscheid, H.; Lode, C.; Vielsack.F.; Vollmer, H.Synthesis and crystal structures of iodoplumbate chains, ribbons and rods with new structural types.J.Chem.Soc., Dalton Trans.2001, 1099–1104
(20) Huang, S.; Xie, R.G.Preparation Manual of Organic Synthesis Reagent.Sichuan University Press, Chengdu1988, p252–253.
(21) Sheldrick, G.M.SHELXL-97, Program for X-ray Crystal Structure Refinement.University of G?ttingen, Germany1997.
(22) Wang, G.E.; Wang, M.S.; Jiang, X.M.; Liu, Z.F.; Lin, R.G.; Cai, L.Z.; Guo, G.C.; Huang, J.S.Crystal structures and optical properties of 1-D iodoplumbates templated by in situ synthesized p-phenylenediamine derivatives.Inorg.Chem.Commun.2011, 14, 1957–1961.
(23) Sourisseau, S.; Louvain, N.; Bi, W.; Mercier, N.; Rondeau, D.; Boucher, F.; Buzare, J.Y.; Legein, C.Reduced band gap hybrid perovskites resulting from combined hydrogen and halogen bonding at the organic-inorganic interface.Chem.Mater.2007, 19, 600–607.
(24) Zhu, X.H.; Mercier, N.; Frere, P.; Blanchard, P.; Roncali, J.; Allain, M.; Pasquier, C.; Riou, A.Effect of mono- versus di-ammonium cation of 2,2?-bithiophene derivatives on the structure of organic-inorganic hybrid materials based on iodo metallates.Inorg.Chem.2003, 42, 5330–5339.
(25) Zeng, X.H.; He, X.; Chen, J.Y.; Zhang, J.W.; Li, H.H.; Chen, Z.R.Polymeric iodoplumbate templated by photochemically active coordination cation [Ru(phen)3]2+: structure and properties of a bimetallic inorganic-organic hybrid.J.Clust.Sci.2014, 25, 979–988.
(26) Wendlandt, W.W.; Hecht, H.G.Reflectance Spectroscopy.Interscience Publishers: New York1966, p298–230.
(27) Kotiim, G.Reflectance Spectroscopy.Springer-Verlag: New York1969, p72–102.
(28) Baibarac, M.; Preda, N.; Mihut, L.; Baltog, I.; Lefrant, S.; Mevellec, J.Y.On the optical properties of micro- and nanometric size PbI2particles.J.Phys.Condens.Matter.2004, 16, 2345–2356.
(29) Meng, J.P.; Gong, Y.; Lin, J.H.Enhanced photocurrent response on a CdTe incorporated coordination polymer based on 3-(3-(1H-imidazol-1-yl)phenyl)-5-(4-(1H-imidazol-1-yl)phenyl)-1-methyl-1H-1,2,4-triazole.RSC Adv.2016,6, 73869–73878.
(30) Ma, W.L.; Yang, F.; Wang, Y.S.; Chen, J.R.; Yuan, L.; Xie, D.; Zhao, Y.; Zhang, Y.; Peng, J.F.Surface photovoltage inversion and photocatalytic properties of PbI2 microcrystals under sub-bandgap illumination.J.Mater.Sci.2017, 52, 9696–9708.
(31) Li, H.H.; Zeng, X.H.; Wu, H.Y.; Jie, X.; Zheng, S.T.; Chen, Z.R.Incorporating guest molecules into honeycomb structures constructed from uranium(VI)-polycarboxylates: structural diversities and photocatalytic activities for the degradation of organic dye.Cryst.Growth Des.2015, 15,10–13.
(32) Wang, D.H.; Lin, X.Y.; Wang, Y.K.; Zhang, W.T.; Song, K.Y.; Lin, H.; Li, H.H.; Chen, Z.R.A new iodiplumbate-based hybrid constructed from asymmetric viologen and polyiodides: structure, properties and photocatalytic activity for the degradation of organic dye.Chin.J.Struct.Chem.2017, 36, 2000–2006.
(33) Li, X.K.; Jiang, Y.Q.A new iodocuprate/methyl viologen-based hybird: structure, properties and photocatalytic activity for the degradation of organic dye.Chin.J.Struct.Chem.2017, 36, 1020–1026.
- 結(jié)構(gòu)化學(xué)的其它文章
- Synthesis and Characterization of a Palladium Complex Supported by Bidentate Ligand and Catalysis of the Vinyl Polymerization of Norbornene①
- Structural, Electronic, Optical and Thermodynamic Properties of Nanolaminated Boride Cr4AlB6①
- Synthesis, Structure and Photochromism of a New Diarylethene and Its Ag(I) Complex①
- Ionothermal Synthesis, Structure and Luminescent Properties of a New 2-D Bismuth(III) Coordination Polymer with (6,5)-Connected Topological Sheet①
- Synthesis, Crystal Structure and Properties of a 1D Heteronuclear Cobalt-sodium Polymer with Bridging Ligand 2-(2-Hydroxy-3-methoxybenzylidene)Hydrazinecarbothioamide①
- Synthesis, Crystal Structure and Photoluminescence of a Dinulear Copper Complex①

