Construction and NIR Luminescence Properties of Zn-Ln Rectangular Nanoclusters
JIANG Dongmei, BO Le, ZHU Ting, TAO Junbin, YANG Xiaoping
College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou 325035, Zhejiang Province, P. R. China.
Abstract: Heterometallic d-4f nanoclusters are currently of interest due to their potential use in material science and as probes in biology. Self-assembly by metalligand coordination is one of the most efficient processes that organize individual molecular components into nanosized species. However, for lanthanide-based selfassemblies, their stoichiometries and structures are difficult to control during synthesis, because the Ln(III) ions often display high and variable coordination numbers. As a result, the structures of lanthanide complexes are commonly influenced by a variety of factors, such as the type of metal ions, the formation of ligands, and the nature of counter anions. In this article, two Zn-Ln nanoclusters[Ln2Zn2L2(OAc)6] (Ln = Yb (1) and Er (2)) were prepared using a new long Schiff base ligand with a Ph(CH2)Ph backbone. These nanoclusters show interesting rectangular-like structures. The long Schiff base ligand displays a “stretched” configuration and is bound to the metal ions through its N and phenoxide and methoxy O atoms. As a result, large clusters (0.7 nm × 1.1 nm × 2.2 nm for 1) were formed. In the crystal structures of 1 and 2, each Ln3+ ion and its closer Zn2+ ion are linked by one OAc- anion and phenolic oxygen atoms of two long Schiff base ligands,forming a ZnLn unit. Two such ZnLn units are then bridged by two Schiff base ligands to form the nano-rectangular structures. Energy dispersive X-ray spectroscopy (EDX) analyses of the clusters indicate that the molar ratio of Zn : Ln is about 1 : 1, in agreement with their crystal structures. Thermogravimetric analyses show that the clusters lose about 5%of the weight when heated to below 100 °C. Melting point measurements show that the clusters are thermodynamically stable. Upon excitation of the ligand-centered absorption bands, 1 and 2 exhibit the NIR luminescence of Yb3+ and Er3+,respectively. The clusters show two excitation bands from 250 to 500 nm, in agreement with their absorption spectra,confirming that energy transfer occurs from the Zn/L centers to Ln3+ ions. These results indicate that the chromogenic Zn/L components in these nanoclusters can act as efficient sensitizers for lanthanide luminescence. The efficiencies of the energy transfer from Zn/L-centers to Yb3+ is higher than that to Er3+, being 75.71% and 25.00% for 1 and 2, respectively.These results provide new insights into the design of polynuclear nanoclusters with interesting luminescence properties.
Key Words: Construction; Zn-Ln nanocluster; Schiff base ligand; Structure; Luminescence
1 Introduction
Metal nanoclusters have emerged as a new class of nanomaterials and have attracted considerable interest in the recent decade1–5. Heterometallic d-4f nanoclusters are currently of interest due to the remarkable physical and chemical properties associated with this class of materials6,7. Recently,attention has focused on the clusters of Yb(III), Nd(III) and Er(III) with the near-infrared (NIR) emission around 900–1600 nm, which is highly transparent to biological systems and fibre media8. The d-block metal ions introduced into the clusters may conceivably play two different roles in the luminescence properties of Ln3+ions. They may enhance the luminescence via d→4f energy transfer9,10, or they may quench the luminescence via 4f→d energy transfer11,12. For the Zn2+ion, the saturated d10electronic configuration prevents the quenching of lanthanide luminescence through a d-d transition (4f→d energy transfer)13,which favors the use of a light-absorbing Zn(II) chromophore as the sensitizer for lanthanide luminescence.
Schiff base ligands are classical ligands to synthesize d-4f heteronuclear complexes. Our recent studies have focused on the synthesis of 4f homometallic and d-4f heterometallic clusters with Schiff-base ligands14,15. We have employed essentially two kinds of “salen” style Schiff-base ligands in which one is a rigid conjugated ligand with a phenylene backbone H2La(Scheme 1a),and the other is exemplified by the flexible Schiff-base ligands H2Lb–d(Scheme 1b). In past studies, we discovered that “multidecker” 4f and d-4f complexes with lanthanide ions sandwiched between alternating layers of the rigid Schiff-base ligand Lacould be isolated16,17. When the more flexible Schiff base ligands H2Lb–dwere used in the synthesis, a variety of 4f and d-4f polynuclear complexes (d = Ni2+, Cu2+, Zn2+and Cd2+) were formed18–20. In these polynuclear complexes, the ligands exhibit classical “salen” type of coordination modes with the d-metal ions bound in the N2O2cavities and the 4f-metal ions in the O2O2cavities (Scheme 1a, “bending” configurations). Obviously, the backbone structures of the Schiff base ligands may affect the formation of the polynuclear d-4f complexes. As part of our continuing studies focused on the construction of luminescent polynuclear lanthanide-based frameworks, we report here two Zn-Ln nanocluster [Ln2Zn2L2(OAc)6] (Ln = Yb (1) and Er (2))with a new Schiff base ligand 6,6′-((1E,1′E)-((methylenebis(4,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(2-methoxyphenol) (H2L), which has a Ph(CH2)Ph backbone(Scheme 1b). Differing from most other salen-type Schiff base ligands used in the synthesis of d-4f complexes (i.e., H2La–d,Scheme 1a), H2L has a longer backbones and exhibits a“stretched” coordination mode with metal ions in 1 and 2(Scheme 1b). In a linear configuration the length of H2L is approximately 2.5 nm. This appears to aid in the formation of large metal clusters. For example, 1 and 2 have nanorectangular-like structures with sizes of approximately 0.7 nm × 1.1 nm × 2.2 nm, which are much larger than those lanthanide- based complexes formed by H2La–d16–20. Interestingly, 1 and 2 display the typical emission spectra of lanthanide ions.
2 Experimental
2.1 Materials and General Methods
All reactions were performed under dry oxygen-free dinitrogen atmospheres using standard Schlenk techniques. The Schiff-base ligand H2L was prepared according to wellestablished procedures21. Physical measurements: NMR:AVANCE III AV500. 500 spectrometer (1H, 500 MHz) at 298 K;IR: Nicolet IS10 spectrometer; Powder XRD: D8ADVANCE.Elemental analyses (C, H, N) of compounds were carried out on a EURO EA3000 elemental analysis after dried in an oven at 100 °C for 2 h. Melting points were obtained in sealed glass capillaries under dinitrogen and are uncorrected. The thermogravimetric analyses (TA) were carried out on a TA Instruments Q600. Absorption spectra were obtained on a UV-3600 spectrophotometer, and excitation and emission spectra on a FLS 980 fluorimeter.
2.2 Preparation of H2L

Scheme 1 Coordination modes of Schiff base ligands: (a) “bending”configuration (H2La–d); (b) “stretched” configuration (H2L).
2-Hydroxy-3-methoxybenzaldehyde (20.0 mmol, 3.0430 g)was dissolved in 15 mL EtOH, and a solution of 4,4′-methylenedianiline (10.0 mmol, 1.9826 g) in 20 mL EtOH was then added drop by drop. The resulting solution was stirred and heated under reflux for 2.5 h. It was allowed to cool and was then filtered. The solid was washed with EtOH (3 × 5 mL) and then dried in the air at room temperature to give yellow product. Yield(based on 4,4′-methylenedianiline): 4.5251 g (97%). Elemental analysis: Found: C, 75.64%; H, 6.43%; N, 6.09%; Calc. for C29H26N2O4: C, 74.66%; H, 5.62%; N, 6.00%.1H NMR (500 MHz, CDCl3): 8.63 (2H), 7.24 (8H), 7.00 (4H), 6.88 (2H), 3.94(6H), 1.55 (2H). IR (CH3CN, cm-1): 2972(w), 1598 (m), 1470(m), 1260 (s), 1200 (m), 1082 (s), 974 (s), 791 (m), 731(s).
2.3 Preparation of [Yb2Zn2L2(OAc)6] (1)
Zn(OAc)2·2H2O (0.30 mmol, 0.0659 g), Yb(OAc)3·4H2O(0.30 mmol, 0.1267 g) and H2L (0.40 mmol, 0.1866 g) were dissolved in 50 mL MeOH at room temperature, and a solution of triethylamine in MeOH (7.19 mol·L-1, 1 mL) was then added.The resulting solution was stirred and heated under reflux for 30 min. It was allowed to cool and was then filtered. Diethyl ether was allowed to diffuse slowly into the filtrate at room temperature and pale yellow crystals were obtained after one week. The crystals were filtered off, washed with MeOH (5 mL)and dried in the air for one week. Yield (based on Yb(OAc)3·4H2O): 0.1031 g (35%). m. p. > 187.6 °C (dec.).Elemental analysis: Found: C, 44.21%; H, 4.83%; N, 3.20%.Calc. for C70H66Zn2N4Yb2O20·3MeOH·6H2O: C, 44.63%; H,4.62%; N, 2.85%. IR (CH3CN, cm-1): 1652 (s), 1594 (s), 1438(s), 1232 (s), 1192 (s), 1070 (w), 970 (s), 854 (m), 736 (s), 668(s).
2.4 Preparation of [Er2Zn2L2(OAc)6] (2)
The procedure was the same as that for 1 using Zn(OAc)2·2H2O (0.35 mmol, 0.0768 g), Er(OAc)3·4H2O (0.35 mmol, 0.1458 g) and H2L (0.45 mmol, 0.2099 g). Pale yellow single crystals of 2 were formed after one week. Yield (based on Er(OAc)3·4H2O): 0.1264 g (37%). m. p. > 188.5 °C (dec.).Elemental analysis: Found: C, 43.67%; H, 4.89%; N, 3.02%.Calc. For C70H66Zn2N4Er2O20·3MeOH·6H2O: C, 44.90%; H,4.65%; N, 2.87%. IR (CH3CN, cm-1): 1652 (s), 1596 (w), 1390(s), 1228 (s), 1188 (s), 968 (m), 854 (s), 736 (s).
2.5 Photophysical Studies
The UV-visible absorption spectra were recorded at RT using an UV-3600 spectrophotometer. The solvent employed was of HPLC grade. The wavelength range was set from 200 to 600 nm.Luminescence spectra in the visible and NIR regions were recorded on a FLS 980 fluorimeter. The light source for excitation and emission spectra was a 450 W xenon arc lamp with continuous spectral distribution from 190 to 2600 nm.Liquid nitrogen cooled Ge PIN diode detector was used to detect the NIR emissions from 800 to 1700 nm. The temporal decay curves of the fluorescence signals were stored by using the attached storage digital oscilloscope. The overall quantum yields(Φem) were obtained by using an integrating sphere, according to eqn Φem= Nem/Nabs, where Nemand Nabsare the numbers of emitted and absorbed photons, respectively. Besides, systematic errors have been deducted through the standard instrument corrections. All the measurements were carried out at room temperature.
2.6 Crystallography
Data were collected on a Smart APEX CCD diffractometer with graphite monochromated Mo-Kαradiation (λ = 0.071073 nm) at 190 K. The data set was corrected for absorption based on multiple scans and reduced using standard methods. Data reduction was performed using DENZO-SMN. The structures were solved by direct methods and refined anisotropically using full-matrix least-squares methods with the SHELX 97 program package. Coordinates of the non-hydrogen atoms were refined anisotropically, while hydrogen atoms were included in the calculation isotropically but not refined. Neutral atom scattering factors were taken from Cromer and Waber. Crystallographic data for 1 and 2 have been deposited with the Cambridge Crystallographic Data (CCDC reference numbers 1590241 and 1590242). These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. Selected bond lengths and angles of 1 and 2 are given in Tables S1 and S2 (Supporting Information).
1: C70H66Zn2N4Yb2O20, monoclinic, space group P2(1)/n, a =19.0913(18) nm, b = 20.7436(19) nm, c = 2.2387(2) nm, α = 90°,β = 100.947(2)°, γ = 90°, V = 8.7043(14) nm3, Z = 4, Dc= 1.447 g cm-3, μ(Mo-Kα) = 2.745 mm-1, F(000) = 3788, T = 190 K. R1= 0.0681, wR2= 0.1958 for 13750 independent reflections with a goodness-of-fit of 1.039.
2: C70H66Zn2N4Er2O20, monoclinic, space group P2(1)/n, a =18.900(4) nm, b = 20.622(4) nm, c = 2.2531(4) nm, α = 90°, β =100.983(2)°, γ = 90°, V = 8.620(3) nm3, Z = 4, Dc= 1.464 g cm-3,μ(Mo-Kα) = 2.550 mm-1, F(000) = 3808, T = 190 K. R1=0.1056, wR2 = 0.2618 for 15087 independent reflections with a goodness-of-fit of 1.111.
3 Results and discussion
3.1 Preparation and crystal structures of the nanoclusters
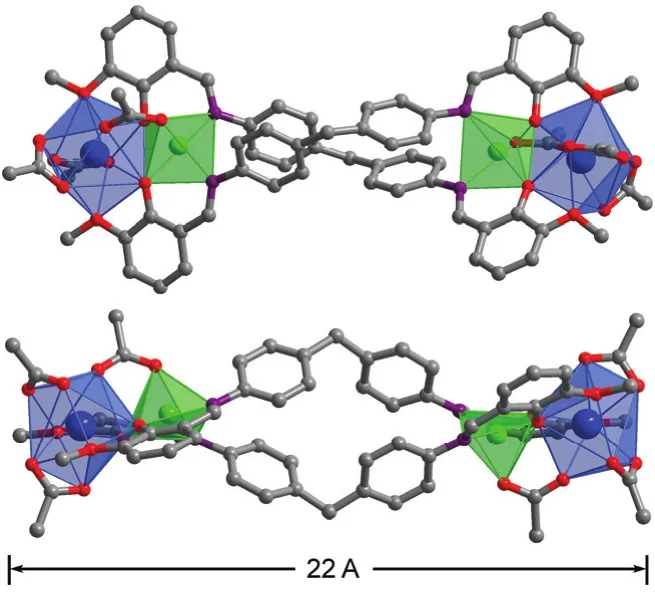
Fig. 1 Two views of the square-like structure of 1: viewed along the ac-axis (top) and b-axis (lower).Yb3+: blue; Zn2+: green. Color online.
The new Schiff base ligand H2L was synthesized from the reaction of 2-Hydroxy-3-methoxybenzaldehyde with 4,4′-methylenedianiline in refluxing ethanol with yields of 97%21.The1H NMR spectrum of H2L show signals for imino protons(―CH=N―) at 8.63 (Fig. S1 in the Supporting Information),while the signal for the aldehyde proton (Ar-CHO) of the reactant at 10.0 disappears. In the presence of Et3N, reactions of H2L with Zn(OAc)2·2H2O and Ln(OAc)3·4H2O in refluxing methanol produced yellow solutions from which isomorphous 1 and 2 were isolated as pale yellow crystalline solids. The IR spectra of 1 and 2 show one absorption band of C=N stretching at 1652 cm-1, which blue shifts as compared to that of the free Schiff base ligand (1598 cm-1for H2L, Fig. S2 (Supporting Information)). Two views of the crystal structure of 1 are shown in Fig. 1. Each Yb3+ion and its closer Zn2+ion are linked by one OAc-anion and phenolic oxygen atoms of two L ligands with an average separation of 0.3406 nm, forming a ZnYb unit. Two such ZnYb units are bridged together by two long Schiff base ligands, forming a nano-rectangular structure of 1 (Fig. 1). The metric dimensions of 1 measure approximately 0.7 nm × 1.1 nm× 2.2 nm. In 1, both Yb3+ions have similar coordination environment. They have nine-coordinate deformed three prism geometries, surrounded by nine oxygen atoms from two L ligands and three OAc-anions. Two Zn2+ions show square pyramidal geometries, coordinated with three oxygen and two nitrogen atoms from two L ligands and one OAc-anion. The Schiff base ligands exhibit “stretched” coordination modes,bound to the metal ions through their N and phenoxide and methoxy O atoms. For the OAc-anions, four bind to two Yb3+ions (μ2-bridging modes), and two bind to one Yb3+and one Zn2+ions (μ2-bridging mode). For 1 and 2, the Zn―O and Zn―N bond lengths range from 0.1978 to 0.2094 nm and 0.2054 to 0.2097 nm, respectively. While, the Yb―O and Er―O bond lengths range from 0.2283 to 0.2610 nm and 0.2272 to 0.2596 nm, respectively.
A panoramic scanning electron microscopy (SEM) image shows the crystalline nature of 1 (Fig. 2a). Energy dispersive X-ray spectroscopy (EDX) analysis of 1 indicates that the molar ratio of Zn : Yb is about 1 : 1, in agreement with the crystal structure (Fig. 2b). The powder XRD patterns of 1 and 2 show large background peaks (Fig. S3 (Supporting Information)),indicating that they are predominantly amorphous. This may be due to the fact that the uncoordinated solvent molecules in 1 and 2 can easily escape from the structures of clusters, and the crystalline products become amorphous. Thermogravimetric analyses show that on heating 1 and 2 undergo weight losses of about 5%–8% before 100 °C (Fig. S4), assigned to the loss of uncoordinated solvent molecules such as MeOH and H2O.Melting point measurements show that 1 and 2 are thermodynamically stable, starting to decompose from 187.6 and 188.5 °C, respectively (Supporting Information).
3.2 Photophysical properties of the nanoclusters

Fig. 3 UV-Vis spectra of the free H2L and clusters 1 and 2 in CH3CN.

Fig. 4 Excitation and emission spectra of the free ligand H2L.

Fig. 2 SEM image (a) and EDX spectrum (b) of 1.
The photophysical properties of 1 and 2 were studied in CH3CN solution and the solid state. The free ligand H2L exhibits absorption bands at 230, 275 and 323 nm which are red-shifted upon co-ordination to metal ions in the clusters (Fig. 3). For the free ligand H2L, excitations at the absorption wavelengths produce broad emission bands at 515 nm (Fig. 4). Upon excitation of the ligand-centered absorption bands, 1 and 2 exhibit the NIR luminescence of Yb3+(2F5/2→2F7/2transition)and Er3+(4I13/2→4I15/2transition), respectively (Figs. 5 and 6). 1 and 2 show two excitation bands from 250 to 500 nm (i.e., λex=302, 421 nm for 1), in agreement with their absorption spectra,confirming that the energy transfers from the Zn/L centers to Ln3+ions occur. These results indicate that the Zn/L centers can act as efficient sensitizers for Ln(III) ions in 1 and 2 (Scheme 2).For each Zn-Ln cluster, the luminescence spectrum in the solid state is similar to that in the solution. The excitation and emission wavelengths (λexand λem), quantum yields (Φem), emission lifetimes (τ) and energy transfer efficiencies (ηsens) of H2L and 1–2 in solution are given in Table 1.
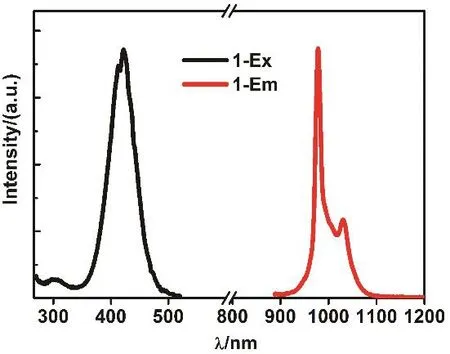
Fig. 5 Excitation and NIR emission spectra of 1 in CH3CN.

Fig. 6 Excitation and NIR emission spectra of 2 in CH3CN.

Scheme 2 Relevant energy levels in 1–6.Those marked with * can act as energy acceptors by either F?rster or Dexter mechanism 25.

Table 1 The excitation and emission wavelengths, quantum yields(Φem), emission lifetimes (τ) and energy transfer efficiencies (ηsens) of H2L and 1–2 in solution.

Fig. 7 NIR emission lifetime of 1 in CH3CN.
As shown in Figs. 5 and 6, 1 and 2 exhibit NIR emission bands of Yb3+and Er3+at 978 and 1532 nm, respectively. The emission lifetimes (τ) of 1 and 2 in CH3CN are 14.05 and 6.05 μs,respectively (Figs. 7 and S5 (Supporting Information)). The intrinsic quantum yields (ΦLn) of Yb3+and Er3+emissions in 1 and 2 are calculated as 0.70% and 0.04%, respectively, using ΦLn= τ/τ0(τ0= 2000 μs22and 14000 μs23for the natural lifetimes of Yb3+and Er3+, respectively). The overall emission quantum yields (Φem) of 1 and 2 are 0.53% and 0.01%, respectively. Thus,the efficiencies (ηsens) of the energy transfer from Zn/L-centers to Yb3+and Er3+in 1 and 2 are calculated as 75.71% and 25.00%,respectively, using ηsens= Φem/ΦLn24, indicating that the Zn/L center in 1 shows higher energy transfer efficiency than that in 2. This difference may be due to the fact that 1 and 2 can show different energy transfer mechanisms from the Zn/L-centers to Ln3+ions. As shown in Scheme 2, compared with Er3+ion, the Yb3+ion has only a single excited state2F5/2at 10200 cm-1which is much lower than those of Zn/L center. The energy-transfer process in 1 may perhaps be described as electron transfer mechanism and/or phonon-assisted energy-transfer mechanisms26,27.The former mechanism is based on the fact that among the lanthanides, Yb(III) does not possess a very negative reduction potential (-1.05 V vs the NHE) and can be transiently reduced to Yb(II) when the sensitizer acts as electron donor in its excited state.
4 Conclusions
In summary, we describe the successful synthesis of two Zn-Ln (Ln = Yb and Er) rectangular clusters from a Schiff base ligand featuring a Ph(CH2)Ph backbone. The long Schiff base ligand displays a “l(fā)inear” configuration in these clusters,resulting in the formation of molecules with nanoparticle like dimensions (0.7 nm × 1.1 nm × 2.2 nm). The Zn/L chromophores of the clusters can sensitize the lanthanide luminescence following Zn/L-center→4f energy transfer. 1 and 2 exhibit interesting NIR luminescence properties. The study of luminescence properties shows that the Zn-Yb cluster 1 has higher energy transfer efficiency than the Zn-Er cluster 2.Further studies focused on the construction of luminescent d-f nanoclusters with higher nuclearity are in progress.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
- 物理化學學報的其它文章
- Electronic Stability of Eight-electron Tetrahedral Pd4 Clusters
- Synthesis of High Yield Au21(SR)15 Nanoclusters
- Luminescence Emission of Copper Nanoclusters by Ethanol-induced Aggregation and Aluminum Ion-induced Aggregation
- Ag7(MBISA)6 Nanoclusters Conjugated with Quinacrine for FRETEnhanced Photodynamic Activity under Visible Light Irradiation
- Synthesis and Structure Determination of Ag-Ni Alloy Nanocluster Ag4Ni2(SPhMe2)8 (SPhMe2 = 2,4-dimethylbenzenethiol)
- PPh3: Converts Thiolated Gold Nanoparticles to [Au25(PPh3)10(SR)5Cl2]2+

