Comparison ofintestinal microbiota and activities of digestive and immune-related enzymes of sea cucumberApostichopus japonicusin two habitats*
WANG Qi (王琦) ZHANG Xiumei (張秀梅) 2 CHEN Muyan (陳慕雁)LI Wentao (李文濤) ZHANG Peidong (張沛東)
1Key Laboratory of Mariculture,Ministry of Education,Ocean University of China,Qingdao 266003,China
2Laboratory for Marine Fisheries and Aquaculture,Qingdao National Laboratory for Marine Science and Technology,Qingdao 266072,China
AbstractSea cucumberApostichopus japonicusstock enhancement by releasing hatchery-produced seeds is a management tool used to recover its population under natural environmental conditions. To assess the suitability of releasing sites, we examined the microbiota of the gut contents ofA.japonicusfrom two populations (one in sandy-muddy seagrass beds and one in rocky intertidal reefs) and the microbiota in their surrounding sediments. The activities of digestive and immune-related enzymes in theA.japonicuswere also examined. The results indicated that higher bacterial richness and Shannon diversity index were observed in all the seagrass-bed samples. There were significant differences in intestinal and sediment microorganisms between the two habitats, with a 2.87 times higher abundance of Firmicutes in the seagrass bed sediments than that in the reefs. Meanwhile, Bacteroidetes and Actinobacteria were significantly higher abundant in the gut content ofA.japonicusfrom seagrass bed than those from the reefs. In addition, the seagrass-bed samples exhibited a relatively higher abundance of potential probiotics. Principal coordinates analysis and heatmap showed the bacterial communities were classified into two groups corresponding to the two habitat types. Moreover, compared toA.japonicusobtained from rocky intertidal habitat, those obtained from the seagrass bed showed higher lysozyme, superoxide dismutase and protease activities. Our results suggest that bacterial communities present in seagrass beds might enhance the digestive function and immunity ofA.japonicus. Therefore, compared with the rocky intertidal reef, seagrass bed seems to be more beneficial for the survival ofA.japonicus.
Key word:Apostichopus japonicus; gut content microf l ora; seagrass bed; rocky intertidal habitat; high throughput sequencing; enzyme activity
1 INTRODUCTION
Sea cucumberApostichopusjaponicusis an economically important holothurian species due to its great medicinal and nutritional value (Bordbar et al.,2011; Purcell et al., 2012). However, an increasing demand for holothurians led to a sharp decline of holothurian natural resources (Reyes-Bonilla and Herrero-Pérezrul, 2003), suggesting that theA.japonicusstock enhancement is urgently required.Stock enhancement by releasing hatchery-produced seeds is a management tool used to recover the declining sea cucumber populations as a result of overfishing or habitat loss. When planning stock enhancement programs, it is necessary to assess if the releasing sites suitable forA.japonicusfarming.Sandy-muddy seagrass (Zosteramarina) bed is one natural habitats due to its optimal diet resources forA.japonicus, such as bacteria, organic debris of seagrass, and other prokaryotes living on seagrass leaves, rhizomes, and rhizospheres (Zhang et al.,2011b). On the other hand, the rocky intertidal habitat is characterized by reefs or gritty substrates on which tidal currents are small, and also by abundant benthic algae and organic debris, and therefore is another appropriate candidate forA.japonicusfarming(Zhang et al., 2011a).
Owing to their high nutritional value,microorganisms in sediments are a direct food source for holothurian, providing them with essential nutrients (Moriarty et al., 1982). Mutual interactions that occur between bacteria in the breeding environment and those in the intestinal fl ora of animals are found, and these interactions play a critical regulatory role in the nutrition, digestion, and immunity of their hosts (O’Hara and Shanahan,2006). Therefore, investigating the association between intestinal microbiota and the Specific habitats of the host is of great signifi cance. At present,composition and diversity ofintestinal microbiota ofA.japonicushave been investigated. Traditional culture-dependent methods had been used to analyze microorganism composition in the digestive tract ofA.japonicus(Zhang et al., 2011c). Denaturing gradient gel electrophoresis (DGGE) has also been used to assess the intestinal microbiota diversity ofA.japonicus(Gao et al., 2010; Li et al., 2010).However, these methods tend to underestimate the composition and diversity ofA.japonicusintestinal microbiota (Muyzer et al., 1993; Rosselló-Mora and Amann, 2001). In recent years, high-throughput sequencing (and Illumina Miseq 454-pyrosequencing platform) can provide a more comprehensive exploration of microbial community composition and diversity and therefore has been widely used to study the diversity of microbial communities in the intestinal microf l ora ofA.japonicusin China (Gao et al., 2014a,b; Dou et al., 2016). However, to our knowledge, no reports ofillumina-Miseq sequencing technology research on the comparison ofintestinal microbial diversity between holothurians inhabiting different habitats have been reported.
Holothurian lacks Specific immune tissues and organs; instead, the coelomic fluid and cells of the organisms participate in the regulation ofimmunity(Eliseikina and Magarlamov, 2002). Accordingly, the enzyme activity of coelomic fluid can reveal the immunological status of organisms. Intestinal enzymes of holothurian including amylase, protease,lipase, the digestive enzyme activity could be inf l uenced by food nutrition, surrounding environment and physiological state of organism. Therefore,digestive enzymes can be used as indicators to determine digestion and absorption ability of diet and metabolism state of sea cucumber (Liu et al., 2013).In the present study, the immune and digestive enzyme activities ofA.japonicuswere investigated to evaluate the health status of sea cucumbers in the two habitats.
Numbers of studies on the analyses of bacterial communities within gut microf l ora, sediment, water have been limited to a single habitat (Gao et al.,2014a, b; Dou et al., 2016). In the present study,Illumina Miseq technology was employed to investigate the composition ofA.japonicusgut content microf l ora and the microbial communities in the ambient sediment of two habitats, seagrass bed and rocky intertidal reefs. Additionally, we investigated the interaction betweenA.japonicusgut content microf l ora and environmental microbial communities, and conducted a comparative analysis of the immune and digestive enzyme activities ofA.japonicusin the two habitats. The aim of this study was to evaluate the suitabilities of seagrass bed and rocky reefs forA.japonicusfarming.
2 MATERIAL AND METHOD
2.1 Sample collection
Healthy adultA.japonicuswere collected in a seagrass (Zosteramarina) bed area of Swan Lake, in Weihai, Shandong Province, China (body weight 136.61±2.76 g,n=10) and a rocky intertidal habitat in Qingdao, Shandong Province, China (body weight 133.62±4.33 g,n=10), respectively. Surface sediments(depth 0-5 cm) were also collected separately from the two sampling areas from the four diagonal locations centered on the sampled (distance 20 cm)A.japonicus. Animal and sediment collection was carried out from both habitats in November of 2014 when the water temperature was 15°C and theA.japonicushad been recovered and fed normally after aestivation in typical active phase. All samples were stored at -20°C prior to analysis within two hours of collection.
2.2 Sequencing sample preparation
Three individualA.japonicusfrom each habitat were randomly selected as replicates for sequencing.The three selected individuals were fi rst surface sterilized with 70% ethanol to avoid bacterial contamination during removal of the intestinal tract.The intestinal contents of each sampled animal were then collected after dissection of the intestinal tract using a sterile scalpel. The content of foregut and hindgut contents was squeezed out separately and gathered in sterile freezing tubes. Careful attention should be paid to avoid collection ofintestinal tract tissues and adherent cells. The sampled intestinal contents and surrounding sediment were labelled and stored at -80°C before use (Table 1).
2.3 High-throughput Illumina Miseq sequencing
Foregut content and hindgut content samples from the three individuals and the ambient sediment samples were compared between the two habitats.DNA was extracted from sample using a FastDNA Soil DNA Kit (Omega Bio-Tek, Norcross, GA, USA),according to the manufacturer’s protocol. The Microbial DNA was amplified of the V3-V4 region of the 16S rRNA gene with barcoded primers, 338F:5′-ACTCCTACGGGAGGCAGCA-3′ and 806R: 5′-GGACTACHVGGGTWTCTAAT-3′. Reactions were carried out in 20 μL volumes containing 0.4 μL of FastPfu polymerase, 4 μL of 5× FastPfu Buffer, 2 μL of 2.5 mmol/L dNTPs, 0.4 μL of each primer(5 μmol/L), and 10 ng of template DNA. The thermocycling were as following conditions: 95°C for 2 min, followed by 25 cycles at 95°C for 30 s, 55°C for 30 s, 72°C for 45 s, and a fi nal extension step at 72°C for 10 min. PCR products were purified with AxyPrep DNA Gel Extraction kit (Axygen scientific,CA, USA). After electrophoresis in 2% agarose gel,purified products were performed quantitative with the QuantiFluor?-ST blue fluorescence quantitative system (Promega, USA). Following quantitation,barcode sequencing of amplicons was sequenced simultaneously on Illumina Miseq PE250 platform at Majorbio Bio-pharm Technology Co., Ltd, Shanghai,China.
The original data derived from Illumina Miseq high-throughput sequencing have been deposited to the NCBI Short Reads Archive database under accession number SRP081041.
2.4 Tissue preparation and enzyme assays
Three individualA.japonicusfrom each habitat were randomly selected as replicates for enzyme activity assay. After drying the skin surface, the coelomic fluid of the three sea cucumber individuals was sucked out with a syringe and injected into a 10-mL centrifuge tube separately. Careful attentionshould also be paid to avoid collection of other tissues.The tubes containing the coelomic fluid samples were stored at -80°C after snap freezing in liquid nitrogen.Following dissection, the respiratory apparatus connected to the gut and the mesenteric adipose tissues were carefully removed. PBS was then used to wash the contents of the gut. The whole gut samples obtained were stored at -20°C prior to use.
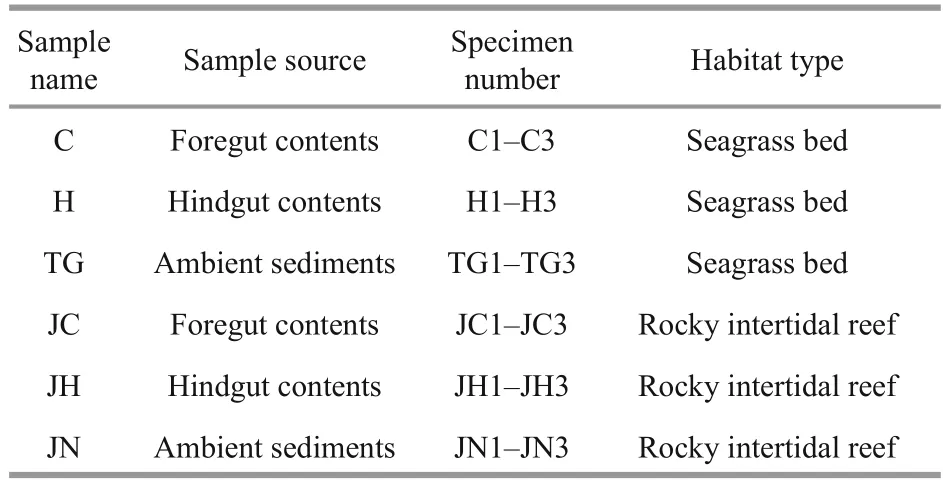
Table 1 Sample collection details for sequencing
For enzyme activity assays, the levels ofintestinal amylase, lipase, and protease activity within digestive tract samples, as well as the levels of activity ofimmune system-related enzymes, including superoxide dismutase, catalase, and lysozyme in the coelomic fluid samples, were analyzed using kits from Nanjing Jiancheng Biotechnology Institute(Nanjing, China) and a microplate reader (Molecular Devices, USA), according to the manufacturer’s protocol. Each sample was analyzed in triplicate. A Molecular Devices microplate reader system was used in the measurement.
2.5 Bioinformatics and statistics
Paired-end reads obtained from Miseq sequencing were merged according to the overlap relationships.Low-quality sequences with a length shorter than 150 bp, with mismatches to the primers and with ambiguous bases were removed. The primers and barcodes were then trimmed from the resulting sequences. The trimmed sequences were assigned to the same operational taxonomic units (OTUs) with 97% similarity using Quantitative Insights into Microbial Ecology (QIIME) (Caporaso et al., 2010),then they were classified taxonomically using the Ribosomal Data-base Project (RDP) classifi er. Good’s method was used to estimate the coverage percentage(Good, 1953). Alpha-diversity including the Shannon diversities, the Chao1 (Chao, 1984), the ACE and Simpson (Belcher and Consultant, 1997) richness estimations were calculated using software R(Jurasinski et al., 2009). Heatmaps were generatedwith the R-package gplots, the bacterial phylogenetic tree was calculated using the unweighted pair group method with arithmetic mean (UPGMAM) method and the relationship among samples was determined by Bray-Curtis distance and the complete clustering method. In order to estimate the visualized microbial community diversity and the β-diversity of all samples, a principal coordinate analysis (PCoA) was performed by R package and QIIME (Peiffer et al.,2013).
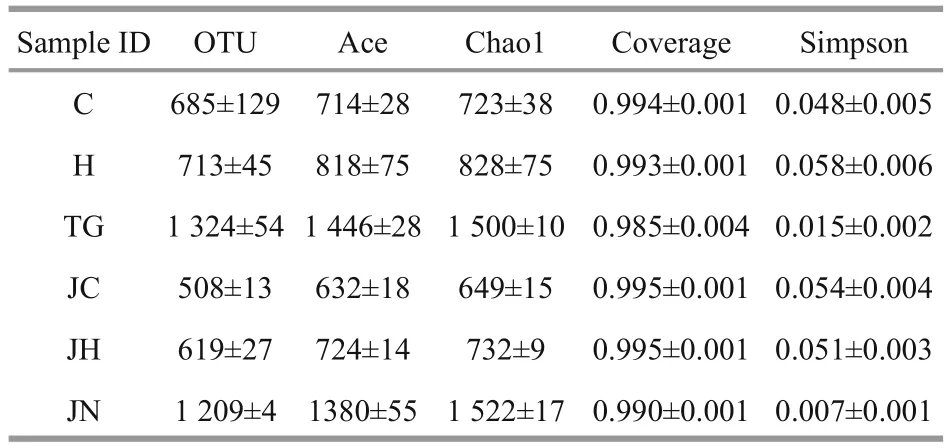
Table 2 Species richness estimates obtained at genetic distances of 3%
Data are presented as means±standard deviations(SD). Differences between results were subjected to one-way analysis of variance followed by Duncan’s multiple range test ort-test, and the statistical signifi cance was set atα=0.05. Data analysis was carried out using Excel 2016 and SPSS 19.0 software.
3 RESULT
3.1 Microbial diversity
A total of 662 996 reads were obtained from samples representing theA.japonicusgut content and ambient sediment samples from two habitats. To get the optimized reads, low-quality sequences were removed from the data set by eliminating those without an exact match to the forward primer, those without a recognizable match to the reverse primer,those with length shorter than 150 bp, and those containing any ambiguous base calls (Ns). The mean average of the optimized read numbers was 41 170±830 for each sample, and the optimized sequences of 18 samples were classified into 12 864 OTUs. The six gut content samples from seagrass bed(C1-C3, H1-H3) comprised 699±151 OTUs, while the six gut content samples from rock intertidal habitat(JC1-JC3, JH1-JH3) comprised 563±69 OTUs.Conversely, the six sediment samples (TG1-TG3,JN1-JN3) comprised 1 267±86 OTUs (Table 2).
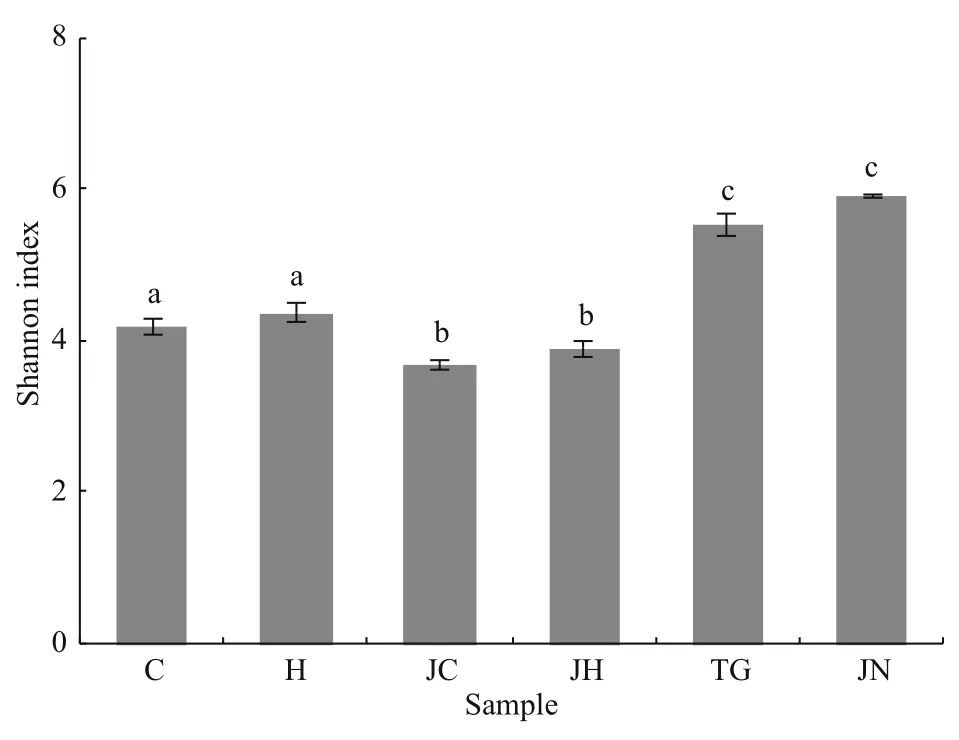
Fig.1 Shannon index of the different samplesDifferent letters indicate significant differences (one-way ANOVA and Duncan’s multiple range test,P<0.05) and bars represent standard errors of means. Abbreviations are as in Table 1.
The Good’s coverage values for all intestinal contents and sediment samples were 99.2%-9.7%and 98.1%-99.3%, respectively (Table 2). Richness diversity was calculated according to the number of OTUs at the 3% dissimilarity levels. Higher bacterial richness was observed in the seagrass-bed samples than in the rocky intertidal habitat samples by the ACE and Chao1 values. Meanwhile, the seagrass-bed samples had significantly higher Shannon diversity index than the rocky intertidal habitat samples(P<0.05, Fig.1). The results indicated that microbial diversity in the gut contents samples from the seagrass bed was higher than in the gut contents samples from rocky intertidal habitat (Fig.1).
3.2 Taxonomic assignment of sequenced samples
We characterized the taxonomic assignment of sequenced samples at the phylum, class, and genus levels. An average of 24±3, 25±1, and 36±1 phyla were detected in the foregut and hindgut contents of theA.japonicusand sediment from seagrass beds,respectively, while in the samples collected from the rocky intertidal habitats, 21±2, 23±1, and 34±1 phyla were identified, respectively. Therefore, richer phyla were identified in seagrass-bed samples than in rocky intertidal habitat samples.
The main phyla detected within the microbial communities of the intestinal and sediment samples from the two habitats were similar to each other, both including Proteobacteria, Actinobacteria, Firmicutes,Chlorof l exi, Bacteroidetes, Verrucomicrobia,Acidobacteria and Cyanobacteria (Table 3).Proteobacteria was the predominant phylum in allsamples, comprising 77.8%±3.1% and 56.2%±4.8%of the reads in the gut content and sediment libraries,respectively. There were significant differences in intestinal and sediment microorganisms between the two habitats in terms of the abundance of bacteria phyla, with 2.87-fold higher abundance of Firmicutes in the seagrass sediments than in the rocky intertidal habitat sediments (P<0.05). Meanwhile, Bacteroidetes and Actinobacteria were significantly more abundant within the guts ofA.japonicusfrom seagrass bed than those from rocky intertidal habitat (P<0.05).
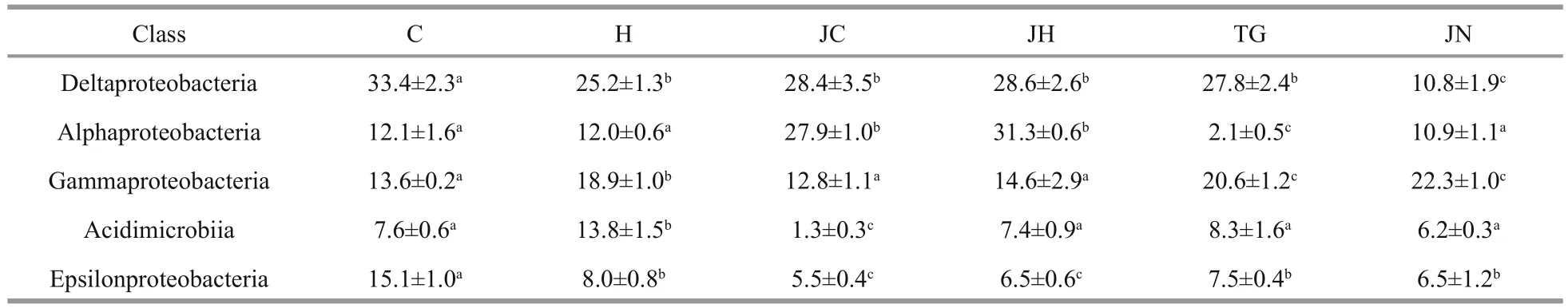
Table 4 Relative abundance (%) of top 5 class bacterial communities in different samples
The foregut, hindgut, and ambient sediment samples obtained from seagrass beds (56±2, 62±4, and 84±4)harbored more bacterial classes than the corresponding samples collected from rocky intertidal habitats (52±1,54±2, and 79±2), respectively. Deltaproteobacteria(36.0%±2.9%) and Alphaproteobacteria (27.8%±9.4%)comprised the two most abundant classes detected within all the gut contents sample libraries; While Gammaproteobacteria was the predominant class(21.4%±4.3%) in all the sediment sample libraries(Table 4).
At genus level, the dominant bacteria present in the intestinal content and ambient sediment samples from the two habitats were also different (Fig.2). The most abundant genera in sediment sample libraries from seagrass bed based on an abundance cutoff of 1%,were sequences related toSva1033_norank(10.4%±1.2%),Sulfurovum(6.5%±1.2%),Anaerolineaceae_uncultured (4.3%±1.9%),Haliea(4.0%±0.9%), Desulfobulbaceae_uncultured(3.6%±3.2%) andAcidiferrobacter(3.6%±0.9%);While in sediment sample libraries from rocky intertidal habitat, the most abundant genera wereJTB255_marine_benthic_group_norank(7.4%±0.3%),Flavobacteriaceae_unclassified(6.2%±0.5%),Sulfurovum(5.4%±0.4%),Saprospiraceae_uncultured (4.4%±0.5%),OM1_clade_norank(3.1%±0.5%), andRhodobacteraceae_unclassified (3.0%±0.8%). The most abundant genera in the gut content sample libraries from seagrass bed,based on an abundance cut off of 1%, were found to have sequences related to Desulfobulbaceae_uncultured (47.0%±1.2%),Sulfurovum(23.1%±4.3%),OM1_clade_norank(9.5%±0.4%),Roseobacter(7.8%±0.6%),Rhodobacteraceae_unclassified(7.7%±1.5%) andMilano-WF1B-44_norank(7.1%±0.2%); in rocky intertidal habitat intestinal contents libraries, Desulfobulbaceae_uncultured(40.6%±1.0%),Roseobacter(20.9%±2.8%),Rhodobacteraceae_unclassified (14.7%±1.2%),Sulfurovum(12.1%±2.3%),Halioglobus(5.9%±1.1%)andHaliea(5.1%±0.5%) were the dominant genera.
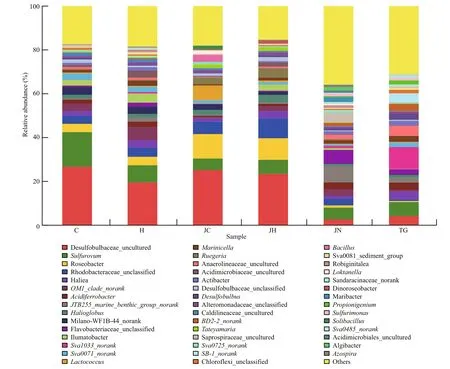
Fig.2 Relative abundance of genus level bacterial communities in different samplesAbbreviations are as in Table 1.
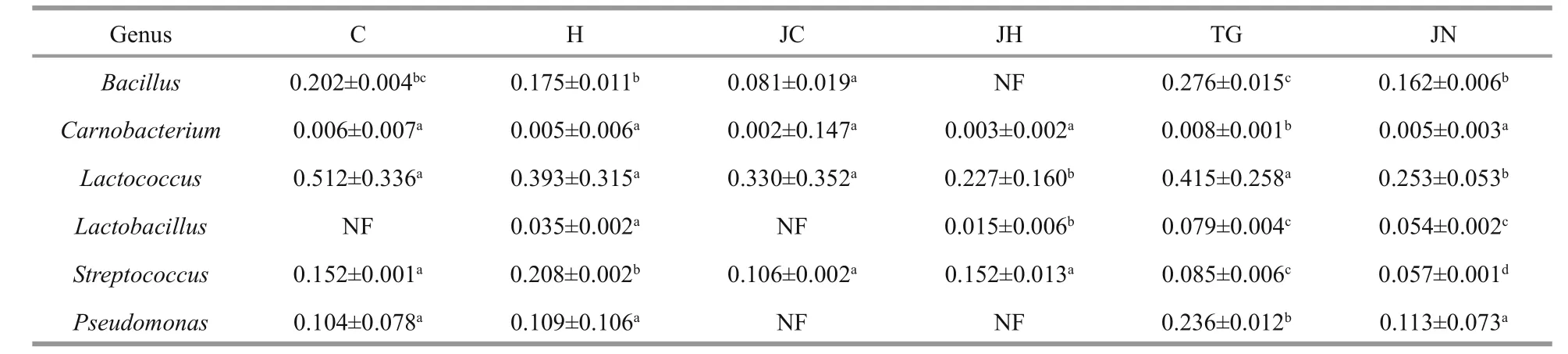
Table 5 Relative abundance (%) of candidate probiotics in the gut content and sediment samples
In this study, OTUs relevant to the generaBacillus,Carnobacterium,Lactobacillus,Lactococcus,PseudomonasandStreptococcus, which have been successfully used as probiotics in aquaculture, were detected in the sequencing libraries of both habitats.However, a greater abundance of such probiotics was detected in the gut microbiota ofA.japonicusobtained from seagrass beds than from rocky intertidal habitats(Table 5).
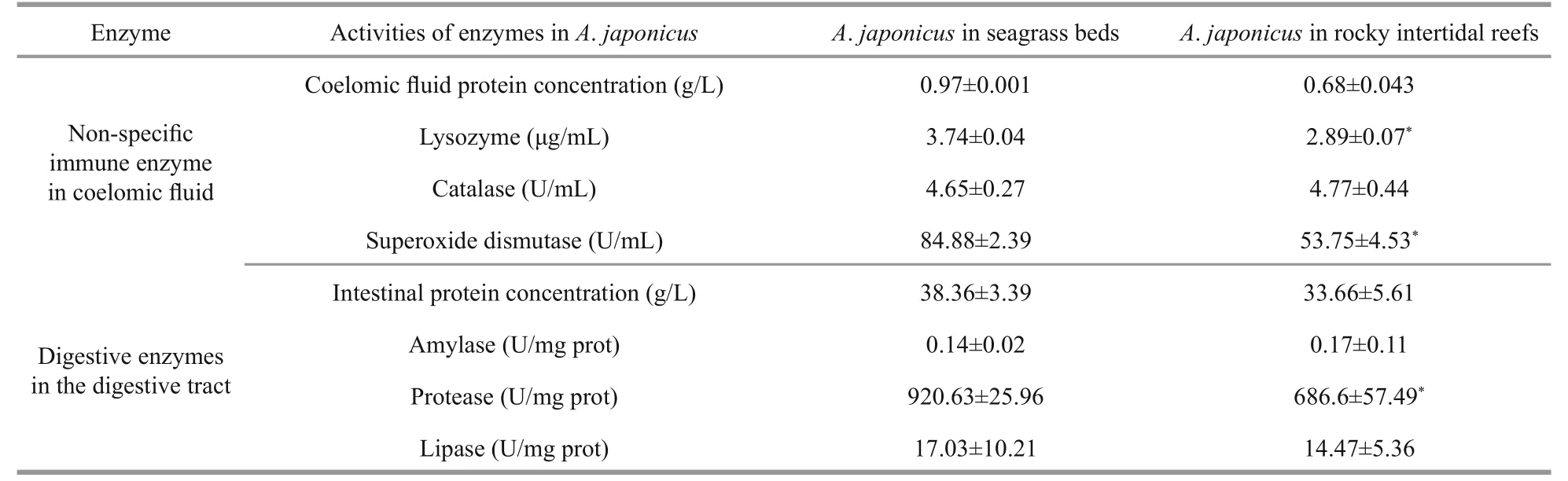
Table 6 Activities of digestive and immune-related enzymes of Apostichopus japonicus in different habitat
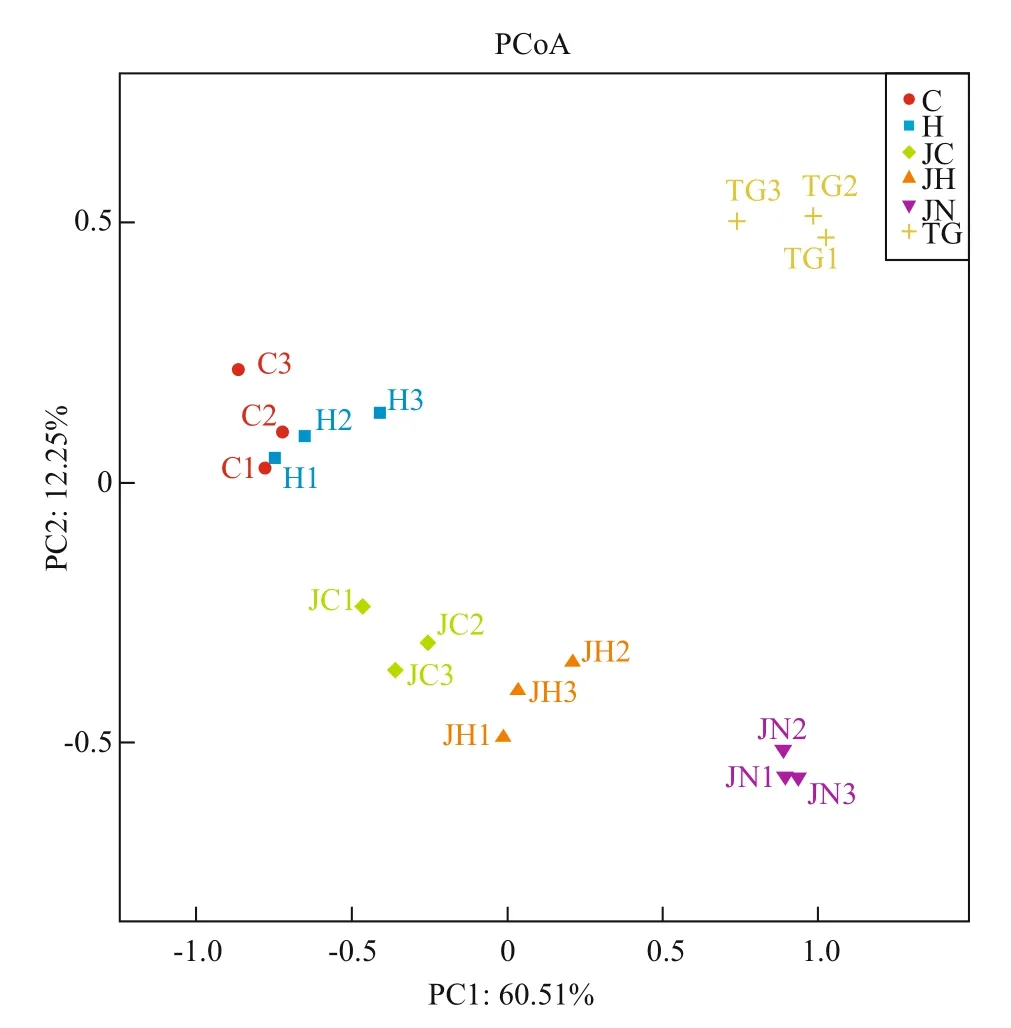
Fig.3 Principal coordinate analysis of the composition of microbial communitiesAbbreviations are as in Table 1.
3.3 Bacterial community comparison
Principal coordinates analysis (PCoA) was performed to compare the bacterial community composition of gut content and sediment samples from the two habitats. All the sequenced samples were segregated into three groups: one group consisted of the gut content samples from seagrassbed (C1-C3, H1-H3), the second group consisted of gut content samples from rocky intertidal samples(JC1-JC3, JH1-JH3) and the third group consisted of sediment samples (TG1-TG3, JN1-JN3) from the two habitats (Fig.3). The gut content samples (C, H,JC and JH) were grouped on the left-hand side of the fi gure along the fi rst principal component axis (PC1),which accounted for 60.51% of the variance in the bacterial communities. The sediment samples (TG and JN) were separated distinctly by the second principal component (PC2) axis, accounting for 12.25% of the total variance. Overall, the two axes on the plot explained 72.76% of the total variance among microbiota samples (Fig.3). The UPGMAN dendrogram showed by heatmap fi gure indicated that all samples generally segregated into two groups: the fi rst group contained six sediment samples; the second group contained all the intestinal content samples(Fig.4). In both groups, samples were clustered based on habitats types. In the second group, the intestinal content samples clustered into different branches based on the microbial contents of the foregut or hindgut of theA.japonicus(Fig.4). The results of PCoA and Heatmap plot were consistent, both indicating that the foregut and hindgut contents and sediments ofA.japonicusfrom different habitats have different characteristic bacterial communities.
3.4 Comparison of the activities ofimmune and digestive system-related enzymes in A. japonicus from two habitats
significantly higher activities of lysozyme and superoxide dismutase were observed in the coelomic fluid samples ofA.japonicusobtained from seagrass beds than those fromA.japonicusinhabited the rocky intertidal habitats (P<0.05, Table 6). Likewise, the digestive tract ofA.japonicusobtained from seagrass beds exhibited 1.34-fold higher protease activity than that of animals obtained from the rocky intertidal habitats (P<0.05, Table 6). There were no significant differences in the activities of other enzymes betweenA.japonicusfrom the two habitats.
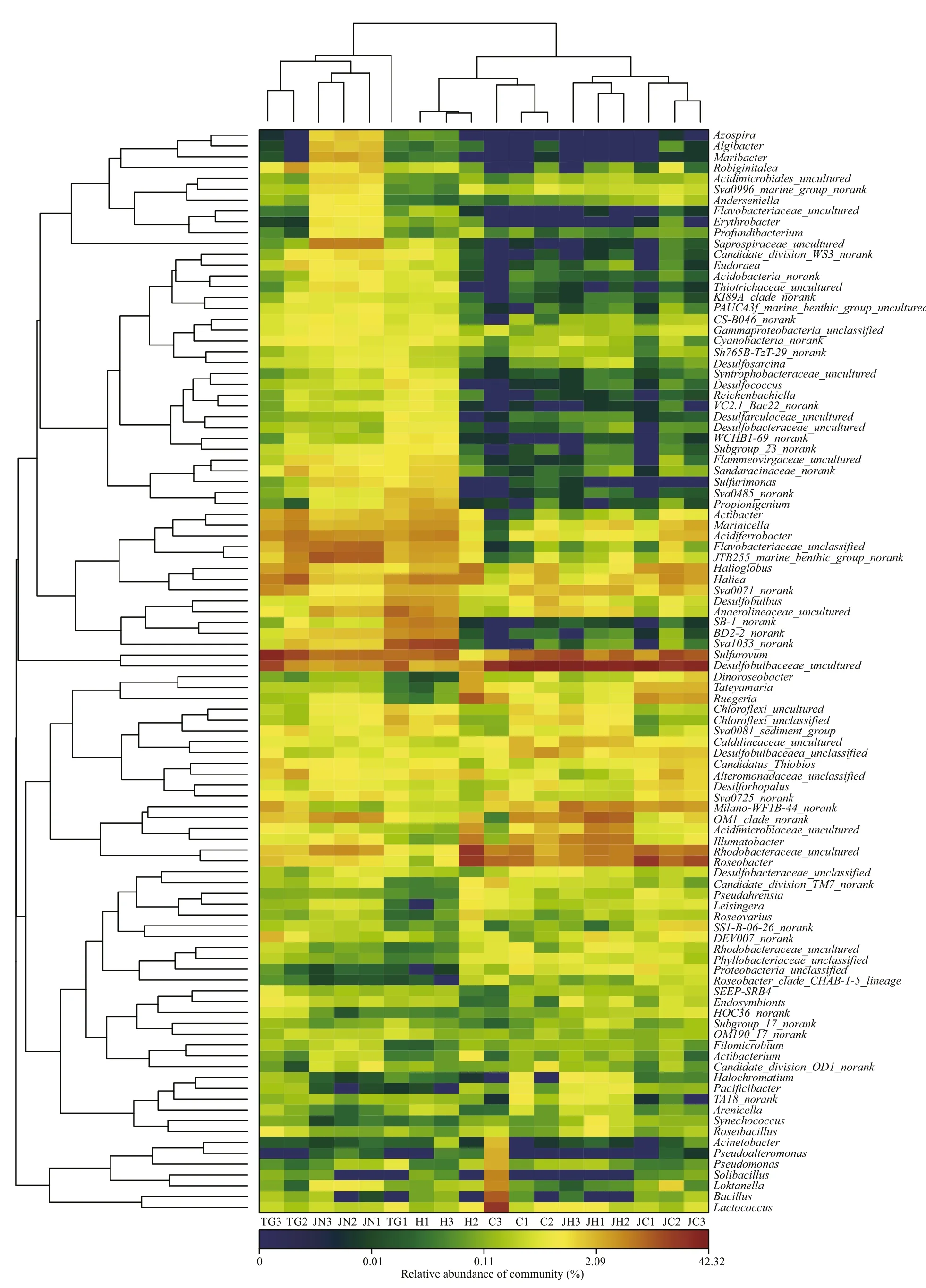
Fig.4 Hierarchically clustered heatmap of the bacterial distribution of different communitiesAbbreviations are as in Table 1.
4 DISCUSSION
4.1 Application of high-throughput sequencing for analyzing the bacterial communities present in the intestinal contents and habitat sediments of A. japonicus
In the present study, we used Illumina Miseq sequencing to analyze the microbial diversity of the gut contents ofA.japonicusand the ambient sediments. Compared with traditional culture-based methods and DGGE techniques, sequence analysis of 16s rRNA has proved to be more effective for the elucidation of microbial diversity (Vaz-Moreira et al.,2011). Previous analyses of the of holothurian gut microbiota via utilizing traditional culture (Sun and Chen, 1989) and DGGE methods (Gao et al., 2010)demonstrated that Gammaproteobacteria were the predominant class in the gut of cultured holothurian.In addition, 454-pyrosequencing-based studies revealed that Holophagae and Deltaproteobacteria are the dominant classes, while Gammaproteobacteria is the second most prevalent class, present in the foregut and hindgut contents of marine bottom-sowedA.japonicus(Gao et al., 2014a). In this study, the predominant class in the gut content libraries was found to be Deltaproteobacteria, while Alphaproteobacteria was the second most prevalent class. In contrast, Gammaproteobacteria was the predominant class in sediment libraries. This discrepancy might be attributable to the lower coverage of microbial diversity, in terms of microbial diversity, associated with traditional culture methods(Vaz-Moreira et al., 2011), differences in OTUs via different sequencing platforms with varying sequencing depths, or variations in physiological and biochemical conditions during sample collection(Jeon et al., 2015).
4.2 Comparison of the microbiota present in A. japonicus gut contents and ambient sediment samples from two different habitats, and analysis of potential functions
The result of the present study indicated that differences in habitat type have a significant effect on the composition of theA.japonicusgut microbiota and ambient sediments. Comparative analysis showed that the microbiota and their relative abundance in sediment samples from the two habitats had different characteristics. At the phylum level, the abundance of Firmicutes was significantly higher in seagrass bed sediments than in sediments from the rocky intertidal habitats. Belong to the phylum Firmicutes,Bacillusare probiotic bacteria that are widely distributed in soil and seawater and are known to improve the breeding environment ofA.japonicusand to play a role in the cycling of carbon, nitrogen, phosphorus,and other elements in the ecosystem (Wang et al.,2008). Meanwhile, at the class level, the higher abundance in the seagrass bed sediment samples than in rock intertidal habitat sediment samples was Deltaproteobacteria. The sulfate-reducing bacteria(SRB) belong to Deltaproteobacteria class primarily utilizes organic compounds as donors to reduce sulfate under anaerobic conditions (J?rgensen, 1982).The abundance of the SRB Desulfobulbaceae_uncultured in the sediment from seagrass beds samples was 1.4-fold gather than those from rocky intertidal habitats. This microbe participates in acetate production and nitrogen fi xation in the host gut,additionally can catalyze carbon oxidation in anaerobic marine sediment, making a contribution to promoting the cycling of elements through the environment (Dr?ge et al., 2005). Comparative analysis showed that the microbiota and their relative abundance in the intestinal content ofA.japonicusfrom the two habitats differed significantly.Bacteroidetes and Actinobacteria in the gut content sampled in seagrass bed presented significantly higher abundance than those in rock intertidal habitats.Bacteroidetes were bacteria which closely related to DNA, lipid and protein conversion, so as to promoting absorption of these organic matters (Cottrell and Kirchman, 2000). Due to their potential to produce special and new metabolites, marine Actinobacteria was considered as an important source to develop marine drugs and bioactive substances (Bérdy, 2005;Bull and Stach, 2007). In summary, compared with the rocky intertidal habitats, the seagrass beds revealed higher relative abundance of potential functional microbes in the gut content ofA.japonicusand ambient sediment.
In sea bottom-sowing proliferation areas,A.japonicusfeed only on natural diets without artifi cial feeding. Thus, the composition of gut bacteria of theseA.japonicusis likely to be similar to that of the surrounding habitat (Gao et al., 2014a).Indeed, our fi ndings revealed that the microbiota present in sediment from different habitats significantly inf l uencesA.japonicusgut microbiota composition. Uthicke (2004) showed that the occurrence of eelgrass debris is as high as 60% in the guts ofA.japonicusthat inhabit in seagrass beds naturally. Therefore, in contrast to rocky intertidal habitat ones,A.japonicusof seagrass bed habitats feed on organic debris of decayed eelgrass in order to fulfi ll their nutritional needs, which may significantly increase their growth and excretion rates (Liu et al.,2013). Holothurian are typical deposit-feeders that obtain nutrition from organic matter and microbes in sediment. The seagrass bed sediment provides a highquality food source containing an abundance of beneficial microbes. These beneficial microbes may optimize the gut micro-ecological environment and promoteA.japonicusgrowth and resource recovery(Fan et al., 2015).
4.3 Comparison ofimmune and digestive function in A. japonicus from different habitats
Probiotics exert beneficial effects on the digestive and immune systems of animals (Verschuere et al.,2000). Candidate strains from the host gut microbiota are more likely to successfully colonize in the digestive tract (Wu et al., 2013). Moreover,BacillusandLactobacillushave been successfully used as probiotics in aquaculture successfully (Balcázar et al.,2006; Wang et al., 2008). In this study, OTUs connected with the probiotics of generaBacillus,Carnobacterium,Lactobacillus,Lactococcus,StreptococcusandPseudomonas, were relatively more abundant in the sequencing libraries obtained from seagrass bed habitat samples, suggesting that the seagrass bed ecosystem may represents a better source of candidate probiotics which may be beneficial for the survival ofA.japonicus.
Previous study has revealed that immune enzymes in coelomic fluid reflects the immune status of the animal (Liu et al., 2013). Superoxide dismutase in coelomic fluid, which mediates the removal of free oxygen radicals, is an important class of antioxidant enzymes in holothurian. Indeed, Dynamic changes in superoxide dismutase activity are important indices of antioxidant capacity level and health status of the organism. Lysozymes is another important component of non-Specific immunity in invertebrates, disrupting microbial cells and stimulating phagocytic activity(Johanson et al., 1963). The result of the present study has demonstrated that theA.japonicusfrom the seagrass bed habitats have higher immune activities than those from rocky intertidal habitat. Given that the sediment and gut samples obtained fromA.japonicusderived from the seagrass bed habitat exhibited a greater higher abundance of probiotics useful for aquaculture than the samples derived from rocky intertidal habitat, we hypothesize that these probiotics reinforce immunity and resistance to disease by maintaining the stability of the host gut microbiota and promoting digestive functions(Balcázar et al., 2006). Our hypothesize are in agreement with the research by Wang et al. (2015),the researcher confi rmed that enrichingA.japonicusfeed with a beneficial microbial strain isolated from sea mud, strengthened resistance to viruses and clearly improved the Specific growth rate of enrichedA.japonicusgroups. The ability of probiotics to improve immune function may be attributed to the stimulation ofimmune responses and enzyme activity.Furthermore, probiotics are thought to competitively exclude pathogenic microbes in theA.japonicusgut by producing organic acids that inhibit the growth and reproduction of the latter, thereby reducing the probability of disease and maintaining the balance of the gut microecosystem (Rengpipat et al., 2000).
Changes in the surrounding environment can affect the functional adaptability of animals, thus the organisms can regulate enzyme activity to increase their ability to obtain nutrition from the environment(Hassett et al., 1990). Enriching food with probiotics significantly alters the activity of digestive enzymes,such as protease, amylase, and lipases, in animals,increasing food utilization rates and promoting growth. Yuan et al. (2006) found that feedingBacillusto juvenileA.japonicussignificantly increased their Specific growth rate and led to clearly elevated protease and amylase activity. Meanwhile, Pan and Yang (1997) showed that intestinal protease and amylase activity in an experimental group ofCyprinus carpioenriched with a Bacillus preparation was increased by 20.5% and 61.9%, respectively,compared with the control group. In the present study,A.japonicusobtained from the seagrass bed showed higher digestive enzymes activities than those obtained from rocky intertidal habitat. It speculated that relative abundant probiotics in the seagrass bed could enter theA.japonicusgut by feeding. After colonizing the gut, the probiotic bacteria promote the secretion of digestive enzymes, thereby strengthening the ability of the host to digest and absorb nutrients(S?gaard and Suhr-Jessen, 1990).
5 CONCLUSION
Illumina Miseq high-throughput sequencing was utilized to compare the composition of bacterial communities in the gut content ofA.japonicusand ambient sediments of two different habitats,Z.marinaseagrass bed and rocky intertidal habitat. Additionally,comparison of the activities of digestive and immune system enzymes inA.japonicusfrom the two habitats was performed. The study provided insight into the relationship between the microbial community of theA.japonicusgut microbiota and the surrounding habitat characteristics. The abundant microbiota inZ.marinaseagrass beds was associated with enhanced activity of the enzyme involved inA.japonicusdigestion and immunity. Therefore, compared with the rocky intertidal reef, seagrass bed seems to be more beneficial for the survival ofA.japonicus.
6 DATA AVAILABILITY STATEMENT
The original Miseq sequence data that support the fi ndings of this study have been deposited in GenBank with the accession codes SRP081041.
 Journal of Oceanology and Limnology2018年3期
Journal of Oceanology and Limnology2018年3期
- Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- The post-larval and juvenile fi sh assemblage in the Sukhothai Floodplain, Thailand*
- Effects of probiotic on microf l oral structure of live feed used in larval breeding of turbotScophthalmus maximus*
- Otolith shape analysis for stock discrimination of twoCollichthysgenus croaker (Pieces: Sciaenidae,) from the northern Chinese coast*
- The impact of spatial autocorrelation on CPUE standardization between two different fi sheries*
- Stocking density affects the growth performance and metabolism of Amur sturgeon by regulating expression of genes in the GH/IGF axis*
