Determination of the spatial and temporal variability of phytoplankton community structure in Daya Bay via HPLCCHEMTAX pigment analysis*
WANG Longhua (王龍花) OU Linjian (歐林堅(jiān)) HUANG Kaixuan (黃凱旋)CHAI Chao (柴超) WANG Zhaohui (王朝暉) WANG Xiaomin (王曉敏)JIANG Tao (江濤)
1Key Laboratory of Sustainable Development of Marine Fisheries,Ministry of Agriculture,Yellow Sea Fisheries Research Institute,Chinese Academy of Fishery Sciences,Qingdao 266071,China
2Qingdao Engineering Research Center for Rural Environment,Qingdao Agricultural University,Qingdao 266109,China
3College of Life Science and Technology,Jinan University,Guangzhou 510632,China
AbstractThe spatial and temporal variability of the phytoplankton community structure in Daya Bay,South China Sea, were identified by using HPLC-CHEMTAX analytical techniques. The highest chlorophylla(Chla) concentrations were observed during summer (with an average value of 0.84 μg/L) and lowest ones during winter (with an average value of 0.33 μg/L). CHEMTAX processing revealed the seasonal succession of phytoplankton species in Daya Bay. During winter, diatoms were the dominant phytoplankton species and contributed 41.5% to total Chla. Based on Chlaconcentration, the average ratio of dinof l agellates to total phytoplankton biomass substantially increased with increasing temperature and nitrogen to phosphorus (N/P)ratio, reaching 52.2% in spring. Nutrient limitation shifted from phosphorus to nitrogen during summer.Moreover, this period was associated with the predominance of diatoms, which accounted for 71.1% of Chla. Prasinophytes and cryptophytes were the other two dominant groups and particularly dominated during winter. Cyanobacteria became an important group during summer and autumn. Canonical correspondence analysis suggested that chrysophytes, dinof l agellates, and cryptophytes were strongly associated with high nitrate concentration, ammonium, dissolved inorganic nitrogen (DIN), and N/P ratio, and were negatively associated with temperature and phosphate. Diatoms and cyanobacteria were strongly associated with temperature, phosphate, and salinity, and are negatively inf l uenced by nitrate, ammonium, DIN, and N/P ratio. Microscopic observations and pigment HPLC information were in good agreement for diatoms and dinof l agellates in the bay. This study demonstrated the usefulness of pigment analysis in investigating the distribution of phytoplankton groups in a complex physical environment, such as Daya Bay.
Keyword:phytoplankton; pigments; environmental factor; HPLC-CHEMTAX; Daya Bay
1 INTRODUCTION
Phytoplankton are the primary producers in the marine food chain, thus playing a crucial role in the marine ecosystem. The structure and effciency of food webs, the cycling of nutrients, and the flux of particles to deep waters are inf l uenced by both phytoplankton composition and biomass (Mendes et al., 2011). Moreover, given that phytoplankton is the main source of organic production, information on their biomass, composition, and community structure is necessary in monitoring an aquatic environment(Paerl et al., 2003). Thus, the spatial and temporal distribution of phytoplankton has been widely studied in different aquatic ecosystems (Barlow et al., 2007;Wang et al., 2008; Mendes et al., 2011; He and Peng,2012).
The traditional identifi cation and quantifi cation of phytoplankton via microscopy techniques are timeconsuming and highly dependent on the skills of professionals. In addition, some fragile and small phytoplankton cells are diffcult to identify via microscopy. Although chlorophylla(Chla) has been used to estimate phytoplankton biomass in the marine environment for numerous years, this approach cannot distinguish between different phytoplankton taxonomic groups (Paerl et al., 2003). The characterization of the phytoplankton community structure at the functional group level based on the accurate separation and quantifi cation of various pigments, such as chlorophylls and carotenoids, has been enabled by recent advancements in phytoplankton pigment analysis by using high performance chromatography (HPLC) (Zapata et al., 2000). Certain key pigments are sensitive indicators of phytoplankton community structure. For example, fucoxanthin(Fuco), peridinin (Peri), Chlb, zeaxanthin (Zea), and alloxanthin (Allo) are the diagnostic carotenoids for diatoms, dinof l agellates, chlorophytes, cyanobacteria,and cryptophytes, respectively.
However, pigment data are diffcult to interpret because some pigments are present in several phytoplankton groups. For instance, Fuco is a major pigment in diatoms and is also present in chrysophytes and prymnesiophytes (Wright and Jeffrey, 2006). The problem of nonSpecific pigments has been resolved with the development of statistical tools, such as CHEMTAX (Mackey et al., 1996; Wright et al.,1996). CHEMTAX applies matrix factorization to pigment data to estimate the contribution of phytoplankton groups to total Chla(Mackey et al.,1996). In the past two decades, the HPLC-CHEMTAX approach has been used to effectively determine the abundance of phytoplankton in the marine environment based on the identifi cation of photosynthetic pigments (Mackey et al., 1996, 1998;Schlüter et al., 2000; Carreto et al., 2008).
Daya Bay is one of the largest and most important gulfs along the southern coast of China. The quantities ofindustrial and domestic wastewater that are discharged into Daya Bay have significantly increased over the past several decades with the rapid development ofindustries and harbors and expansion of human populations around the bay (Song et al.,2004). Moreover, Daya Bay has become an important aquaculture area since 1980s. As a result of anthropogenic disturbance, Daya Bay has undergone a shift from oligotrophic to mesotrophic status from the 1980s to recent years (Wang et al., 2008). During this process, the bay’s phytoplankton community has been extensively studied via microscopy methods(Sun et al., 2006; Wang et al., 2006a, b, 2009, 2016).Microscopy results have suggested that diatoms and dinof l agellates are the main phytoplankton species in this area. However, the community structure of phytoplankton in Daya Bay has yet to be determined via the CHEMTAX analysis of HPLC pigments.Therefore, the present study aims to investigate (1)the seasonal and spatial variations of phytoplankton pigments; (2) the community structure of phytoplankton as determined via CHEMTAX analysis of HPLC pigments; and (3) the relationships between environmental factors and phytoplankton distribution.
2 MATERIAL AND METHOD
2.1 Sample collection and analysis
Daya Bay, a semi-enclosed bay situated in the northern part of the South China Sea, covers an area of 600 km2and an average depth of approximately 11 m (Sun et al., 2011). The annual mean air temperature is 22°C. Except for three small rivers that discharge into Dapeng Cove, no major rivers discharge into Daya Bay; most of the water in Daya Bay originates from the South China Sea (Sun et al., 2011).Daya Bay Nuclear Power Plant and Lingao Nuclear Power Plant have operated in the area since 1993 and 2003, respectively (Wang et al., 2008).
Four cruises were conducted at nine stations in Daya Bay during winter (December, 2010), spring(March, 2011), summer (June, 2011), and autumn(November, 2011) (Fig.1). Water temperature and salinity were recorded with YSI 6600 (Yellow Springs Instruments, USA). Five liters of seawater were collected from near surface (0.5 m depth) by a Niskin bottle. Zooplankton was fi rst eliminated by fi ltering the water sample through a 200-μm screen. Then,accurate 1.0 L seawater was fi ltered under gentle vacuum (<0.03 MPa) with Whatman GF/F glass fi ber fi lters (nominal pore size and diameter of 0.7 μm and 47 mm, respectively). The fi ltered samples were immediately deep-frozen in liquid nitrogen for subsequent HPLC pigment analysis. An 80-mL subsample was fi ltered through a pre-ignited GF/F fi lter. The fi ltrate was stored at -20°C for the analysis of dissolved nutrients. Nitrate, nitrite, ammonium,and phosphate were determined with a SKALAR flow analyzer in accordance with standard methods(Hansen et al., 1983). The total of nitrate, nitrite, and ammonium was designated as the concentration of dissolved inorganic nitrogen (DIN).
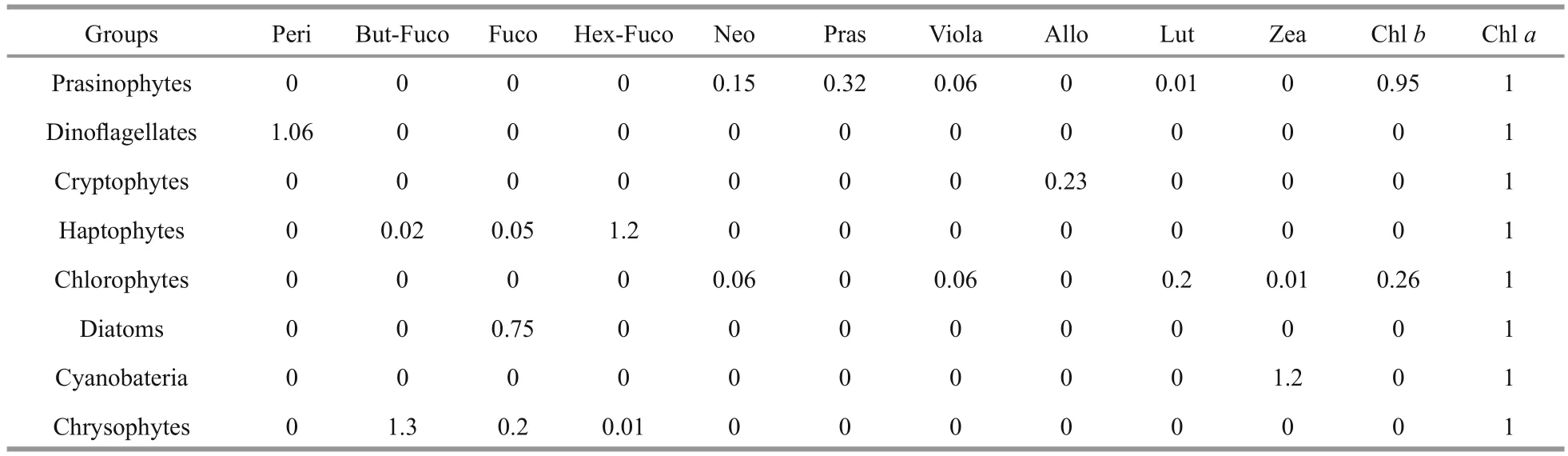
Table 1 Input pigment: chlorophyll a ratios for the Daya Bay
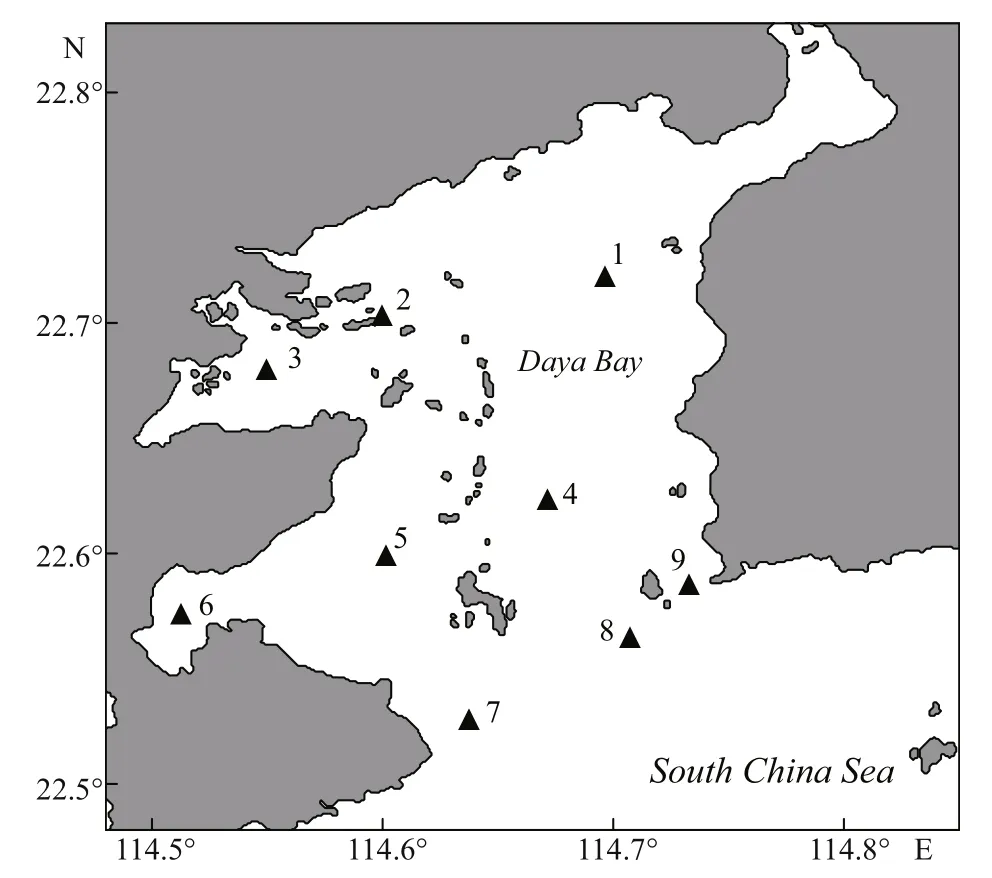
Fig.1 Sampling sites in Daya Bay
2.2 HPLC pigment analysis
The frozen fi lters were placed in screw-cap centrifuge tubes with 3 mL 95% methanol. The tubes were kept in the dark. The fi lters were then disrupted for 5 min in an ice-water bath with a sonicator (Model 100, Fischer scientific). The supernatants were fi ltered through polytetraf l uoroethylene membrane fi lters (0.22-μm pore size) to eliminate fi lter and cell debris from the extract. Then, 100 μL of pigment extract was injected into the HPLC (Agilent 1200)coupled with a reverse-phase Waters C8 column (150 mm×4.6 mm, 3.5-mm particle size). The procedures for HPLC analyses are fully described in the work of Zapata et al. (2000). The HPLC system was previously calibrated with standard pigments from DHI (Institute for Water and Environment, Denmark).
2.3 CHEMTAX analysis and pigment indices
CHEMTAX, a mathematical software, applies matrix factorization to pigment data to estimate the contribution of phytoplankton groups to the total Chla(Mackey et al., 1996; Wright et al., 2009). The basis for calculations and procedures are fully described in Mackey et al. (1996). The initial pigment ratios of major algal classes used in the present study were obtained from the literature (Mackey et al., 1996;Lohrenz et al., 2003; Wang et al., 2015) (Table 1).Based on the detected diagnostic pigments (DP),seven algal groups were loaded to CHEMTAX:diatoms, dinof l agellates, haptophytes, chlorophytes,prasinophytes, chrysophytes, cryptophytes, and cyanobacteria (Table 1). The pigments loaded were Chla, Chlb, Allo, Fuco, Peri, Zea, prasinoxanthin(Pras), lutein (Lut), 19′-butanoyloxyfucoxanthin(But-fuco), 19′-hexanoyloxyfucoxanthin (Hex-fuco),neoxanthin (Neo), and violaxanthin (Viola). The optimized pigment ratio of the matrix generated by CHEMTAX is presented in Table 2. The procedure for CHEMTAX analysis was described in Wright et al. (2009).
Phytoplankton types are conventionally classified based on size as follows: picoplankton (<2 μm),nanoplankton (2-20 μm), and microplankton (20-200 μm) (Nair et al., 2008). DP criteria that were derived from different combinations of marker pigments are presented in Table 3 and roughly correspond to the biomass proportions of various phytoplankton size (Vidussi et al., 2001; Barlow et al., 2007).
2.4 Microscopy
One-liter water samples were collected at the samestations and depths as described for HPLC pigment analysis. The water samples were fi xed in 4%neutralized formalin and then concentrated to 20 mL via sedimentation. Phytoplankton species were identified and counted by microscopic examination under a NIKON (TE-2000U) microscope. The procedures for phytoplankton identifi cation are fully described by Wang et al. (2016).
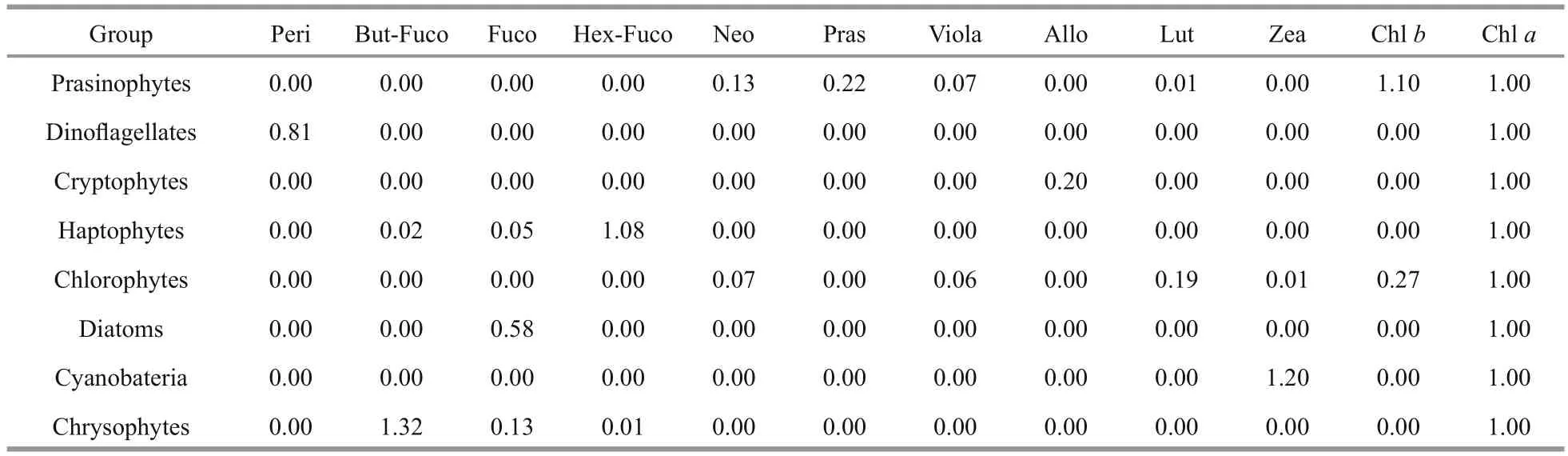
Table 2 Output pigment: chlorophyll a ratios for the Daya Bay
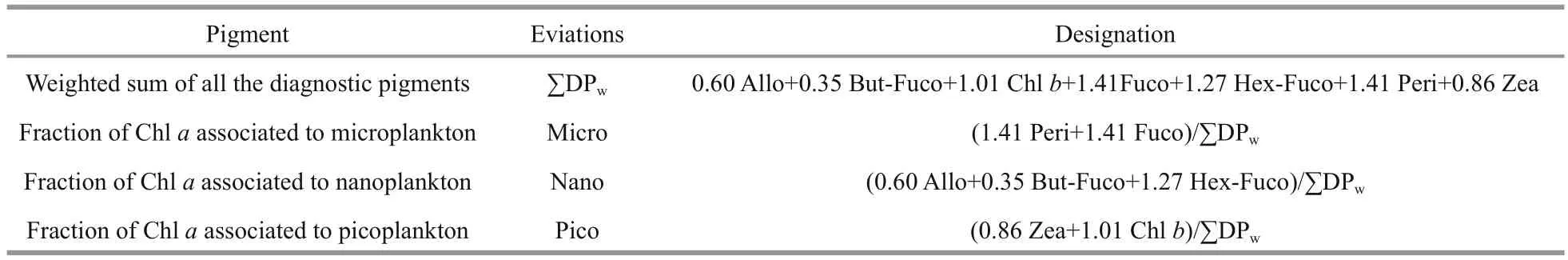
Table 3 Algal pigment indexes and pigment formulae (modified from Uitz et al., 2006; Aiken et al., 2009)
2.5 Statistical analysis
To reveal correlations between phytoplankton communities and environmental factors, Canonical correspondence analysis (CCA) was performed using CANOCO 4.5 (Ter Braak and Smilauer, 2002).Correlation analysis was used to evaluate the agreement between phytoplankton cell counts and CHEMTAX results. Pearson correlation was used for correlation analysis. The statistical signifi cance of environmental variables and pigment concentrations was assessed by standard one-way analysis of variance (ANOVA). ANOVA and correlation analysis were performed using SPSS 16.0.
3 RESULT
3.1 Environmental parameters
Sea surface temperature in Daya Bay varied throughout seasons. The lowest values were recorded during March (18.60°C) and the highest temperatures were recorded during June (28.92°C) (Table 4). Given that no major rivers discharge into the bay, the salinity values, which ranged from 32.81 to 33.92, showed no obvious seasonality (P>0.05). DIN concentration was higher during December and March than during June and November. However, the highest concentration of phosphateoccurred in June (0.71 μmol/L) and was significantly higher than those in other months(P<0.01). The ratio of DIN to(N/P ratio)increased to 87.6 and 194.2 in December and March and decreased to 5.6 in June, which suggested a shift in P limitation to N limitation from the dry to wet season.
3.2 Phytoplankton pigment concentrations
A total of 17 DPs were identified in Daya Bay. The identified pigments included Chla, Chlb, Chlc2, Ch lc3, Peri, Fuco, But-Fuco, Hex-Fuco, Allo, Zea, Pras,Lut, Neo, Viola, Diadino, Diato, and β-Car. Except for Chlbin March, all of these pigments were present during the four cruises in Daya Bay. During the winter and summer cruises, the most abundant pigments were Chlaand Fuco. Peri dominated with Chlaand Fuco during spring and autumn (Table 4). Seasonal variations were also observed for some minor components of DPs. For example, Pras concentration was significantly higher during winter than during other seasons, whereas Zea concentration was highest during summer (P<0.05).
Although Chlaconcentration was highest during summer, Chlaconcentration was not significantlydifferent among different seasons (P>0.05, Table 4).In the inner and middle bay, the seasonally average Chlaconcentration showed an increasing trend from east to west (Fig.2). Average Chlaconcentration at St. 4 was signifi canlty lower than that of St. 3(P<0.05). Otherwise, the former was also sigfi cantly lower than that of St. 9 (P<0.05). However, Average Chlaconcentration showed no significant differences among other stations (P>0.05).
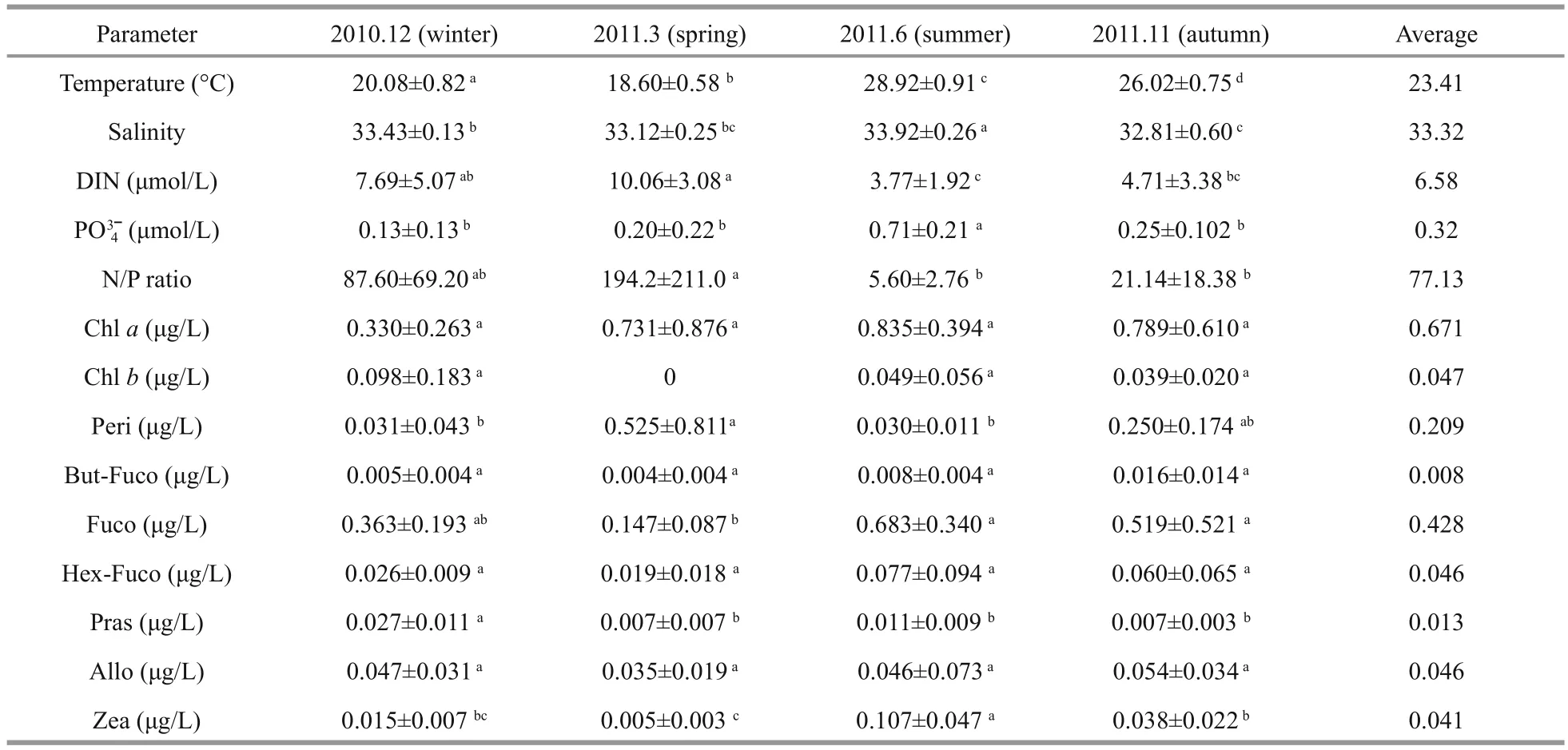
Table 4 Seasonal variations of environmental parameters and pigments in Daya Bay (average±standard deviation)
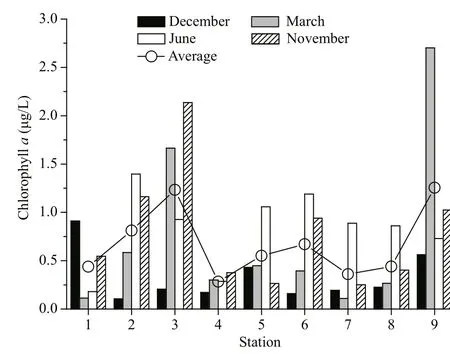
Fig.2 Spatial distribution of chlorophyll a in Daya Bay during different seasons
3.3 Phytoplankton community structure
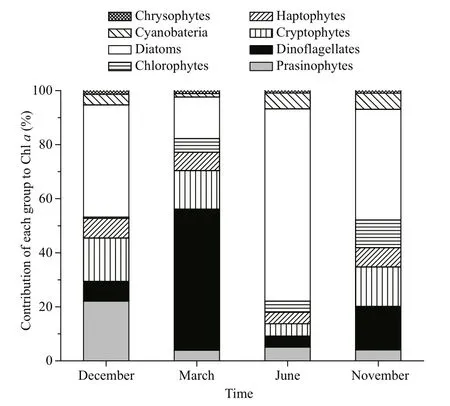
Fig.3 Contribution of various phytoplankton groups to Chlorophyll a concentration in Daya Bay over four sampling cruises
CHEMTAX calculation revealed the seasonal variation in the composition of phytoplankton assemblages in Daya Bay. In December, diatoms were the dominant group of phytoplankton and contributed 41.5% to the total Chla, followed by prasinophytes(22.1%) and cryptophytes (16.1%) (Fig.3). In March,Chlawas mainly contributed by dinof l agellates(52.2%), followed by diatoms (15.3%) and cryptophytes (14.3%). The highest dinof l agellate biomass was observed at St. 9 (Fig.4). In June,diatoms became the most dominant group in this area and accounted for 71.1% of Chla(Fig.3). However,other phytoplankton groups, such as dinof l agellates and cryptophytes, also dominated in the inner and middle bay (such as Sts. 1, 3, and 4) (Fig.4). In November, Chlawas mainly contributed by diatoms(40.9%), followed by cryptophytes (16.1%) and dinof l agellates (14.6%) (Fig.3). Notably,cyanobacteria were an important group during summer and autumn, contributing 5.9% and 6.0% to total Chla, respectively (Figs.3, 4).
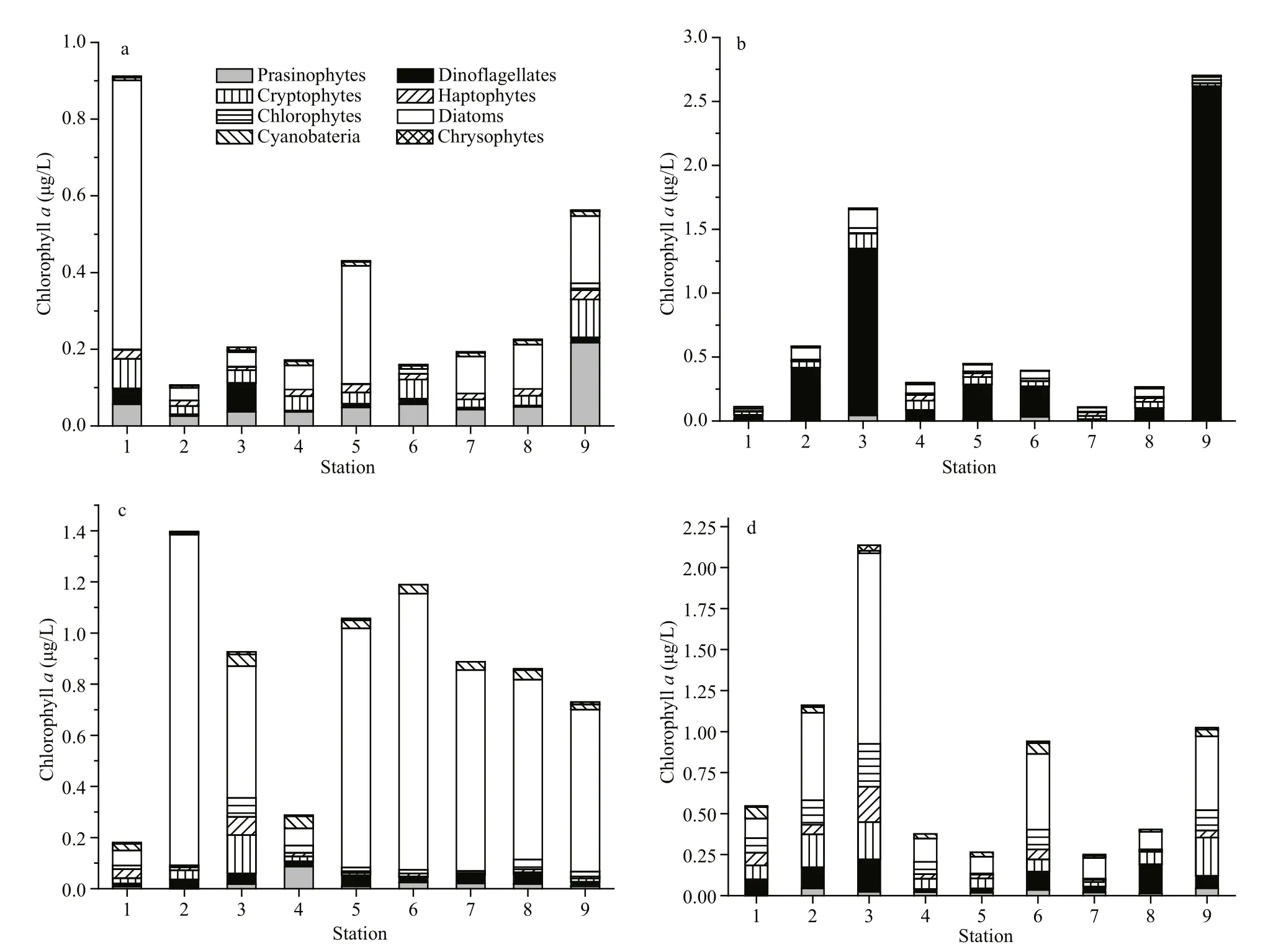
Fig.4 Relative contribution of various functional phytoplankton groups to Chlorophyll a concentration in Daya Bay over four sampling cruisesa. winter; b. spring; c. summer; d. autumn.
3.4 Pigment indices and ratios
Calculating DP concentrations revealed that the contributions of different fractions of phytoplankton did not markedly vary among different seasons(Fig.5). The phytoplankton community was dominated by microplankton, which contributed 74.91% to 88.45% of total Chla. Picoplankton contributed 14.12% of total Chlain June when the water temperature was the highest among the four cruises.By contrast, the proportion of picoplankton was the lowest and contributed only 0.72% to total Chlain March, which presented with the lowest temperature among the four cruises (Fig.5; Table 4).
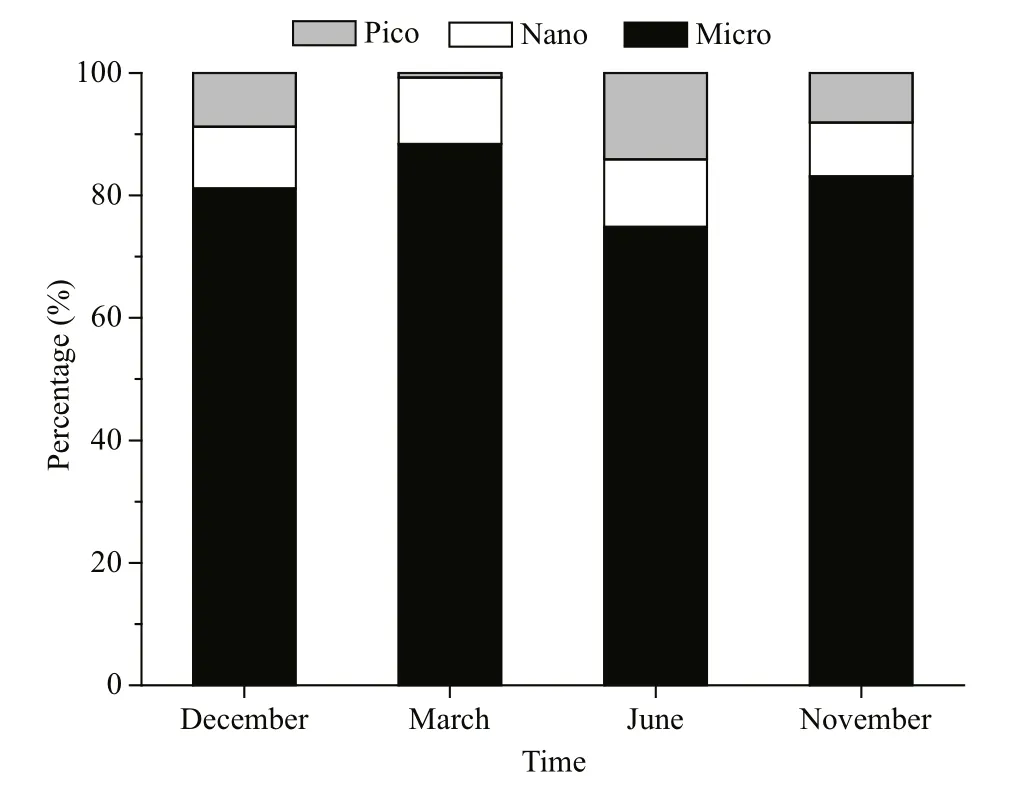
Fig.5 Size distributions of phytoplankton biomass in Daya Bay over four different sampling cruises
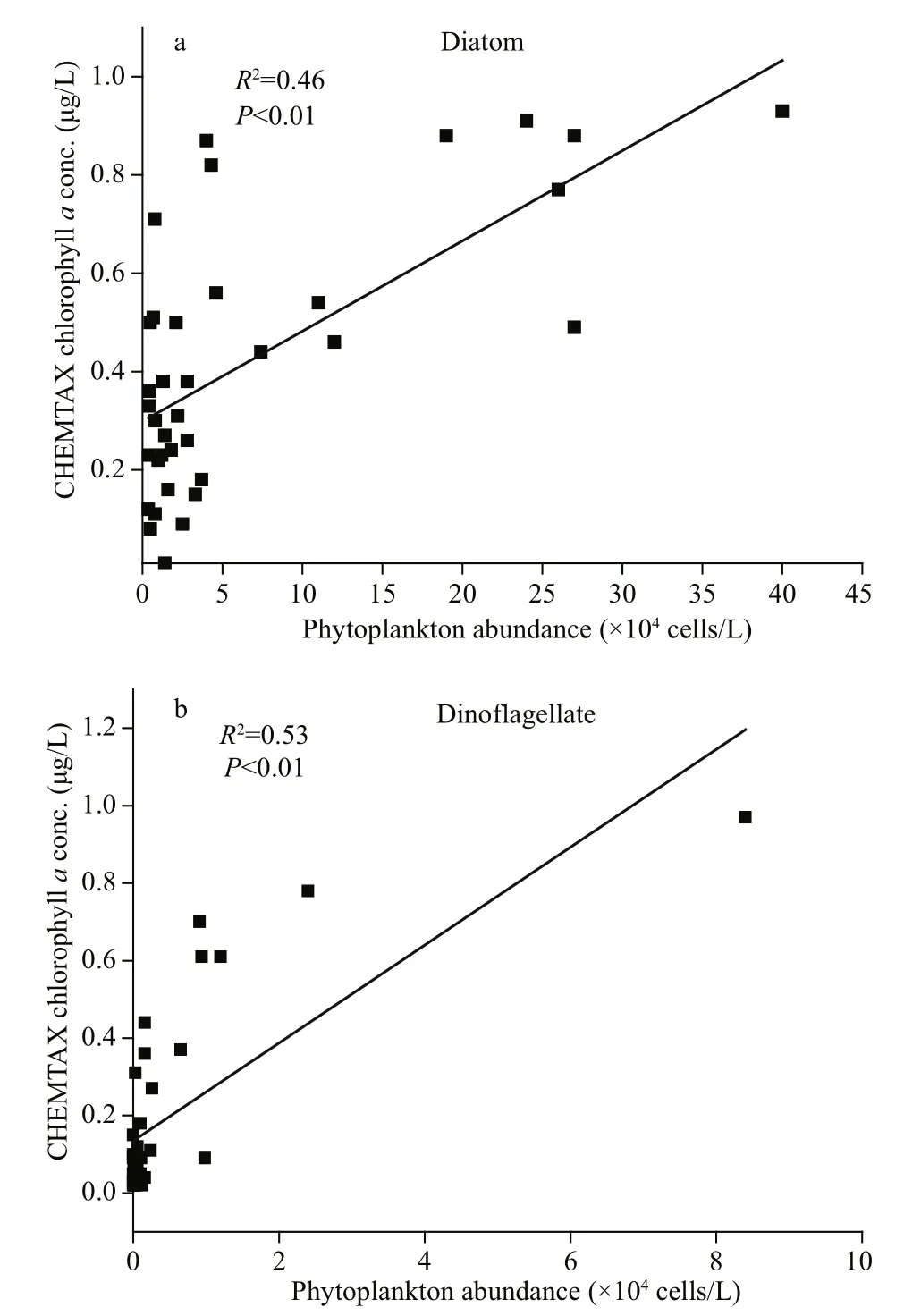
Fig.6 Phytoplankton abundance (cells/L) against chlorophyll a concentrations (μg/L) obtained through CHEMTAX for diatoms and dinof l agellates
3.5 CHEMTAX estimates versus cell counts
Direct comparison revealed that the phytoplankton cell counts obtained via microscopy and the contribution to total Chladetermined via HPLCCHEMTAX were in good agreement (P<0.01; Fig.6).TheR2coeffcients for diatoms and dinof l agellates were 0.46 and 0.53.
3.6 Relationships between environmental factors and phytoplankton distribution
CCA was used to investigate the possible linkages between environmental factors and phytoplankton distribution. The species-environment correlations were 0.68 and 0.82 for the fi rst two axes, respectively,and explained 79.2% of the total spatial distribution of phytoplankton groups (Fig.7). Chrysophytes,dinof l agellates, and cryptophytes were strongly associated with high concentrations of nitrate,ammonium, and DIN, as well as high N/P ratio, and were negatively associated with temperature and phosphate. Diatoms and cyanobacteria were strongly associated with temperature, phosphate, and salinity,and were negatively inf l uenced by nitrate, ammonium,DIN, and N/P ratio. Compared with other phytoplankton assemblages, prasinophytes,haptophytes, and chlorophytes were less controlled by environmental factors (Fig.7).
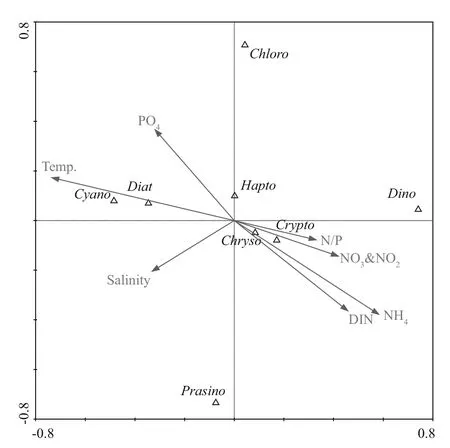
Fig.7 Canonical correspondence analysis ordination plots for environmental parameters and phytoplankton communities in Daya Bay
4 DISCUSSION
The ecosystem of Daya Bay has undergone changes because of anthropogenic perturbations. In the past several decades, DIN content has increased four- to five-fold in Daya Bay. The rapid increase in DIN mainly resulted from nitrogen-based fertilizers, river input, land runoff, waste eラ uents, and atmospheric deposits (Wu et al., 2017). Moreover, marine aquaculture has significantly contributed nitrogen to Daya Bay, especially to the western and northern parts of the water body (Peng et al., 2001; Wu et al.,2017). Phosphate concentration, however, has decreased over time due to the phosphate detergent ban in China. The combined increase in DIN and decrease in phosphate has resulted in the 40-fold increase of the N/P ratio in Daya Bay (Wang et al.,2004, 2009; Wu et al., 2017). Previous studies have suggested that the limiting factors for phytoplankton growth in the bay changed from nitrogen in the 1980s to phosphate in the mid-1990s (Wang et al., 2004,2009; Wu et al., 2017). In this study, the seasonally average N/P ratio reached 77.1, which is substantially higher than the Redfi eld ratio (16:1) (Redfi eld, 1963).These fi ndings further support the potential P limitation to phytoplankton growth in Daya Bay.
Pigment analysis revealed that microphytoplankton,including diatoms and dinof l agellates, dominate in Daya Bay with a distinct seasonal distribution (Figs.3,5). The high N/P ratio was a characteristic feature in the winter and spring (86.7 and 194.2, respectively)(Table 1). In the winter, diatoms were the dominant phytoplankton. This pattern was also observed in previous studies (Wang et al., 2006a; Wu et al., 2017).At low temperature, diatoms have higher NO3ˉ uptake rates than dinof l agellates (Lomas and Glibert, 2000).However, N/P ratio continuously increased with increasing temperature during spring, thus favoring the growth of dinof l agellates. Dinof l agellate species that produce alkaline phosphatase enzymes could grow on organic phosphorus sources (Jauzein et al.,2010). The present study showed that the average ratio of dinof l agellates to total phytoplankton biomass substantially increased and reached 52.2% in spring(Fig.3). The highest ratios of dinof l agellate biomass to total phytoplankton biomass (more than 60.7%)were found at Sts. 2, 3, 5, 6, and 9, where the dinof l agellate speciesAkashiwosanguineaaccumulated (Wang et al., 2016). The highest density ofA.sanguinea, which was 8.2×104cells/L, occurred at St. 9 (Wang et al., 2016). In recent years, dominance has shifted from diatoms to dinof l agellates in Daya Bay (Li et al., 2011). Yu et al. (2007) reported that harmful algal blooms (HABs) that are caused by dinof l agellates, such asScrippsiellatrochoideaandProtoperidiniumquinquecorne, have become increasingly problematic. Before 1993, most HAB species in the area were diatoms. The shift in dominance from diatoms to dinof l agellates has been reported in several other Chinese coastal areas, such as the Changjiang Estuary and Bohai Sea (Wei et al.,2004; Zhou et al., 2008; Jiang et al., 2010). Increased nitrogen input from aquaculture and industrial and domestic sewage and increased N/P ratio are probable reasons for the dominance of for dinof l agellates in Daya Bay (Yu et al., 2007). During summer, however,nutrient limitation shifted from P to N (Table 1). This shift likely occurred because of the increased discharge of P-replete freshwater into Daya Bay.Moreover, the summer is associated with the predominance of diatom species (Fig.3). CCA showed that dinof l agellates are strongly associated with high nitrate and ammonium concentrations, DIN, and N/P ratios, whereas diatoms are correlated withconcentration and temperature (Fig.7). These fi ndings support the importance of nutrient structure (such as DIN and P concentrations, and N/P ratio) and temperature in the succession of both species.
HPLC-CHEMTAX is an essential method for the identifi cation of small-sized cell classes, such as cryptophytes, cyanobacteria, and prasinophytes,which could be neglected by microscopy techniques.The results of our pigment analysis showed that a considerable amount of cryptophytes are present in Daya Bay. Lee (2008) reported that cryptophytes, like dinof l agellates, combine autotrophic with heterotrophic feeding strategies under low nutrient concentrations, thus exhibiting mixotrophy.Cryptophytes are an important group in Jiaozhou Bay(Peng et al., 2010). Cyanobacteria were notably abundant in Daya Bay during summer and autumn.As reported by Jiang et al. (2016), pico-sizedSynechococcusis the dominant cyanobacterial species in Daya Bay. Rajaneesh and Mitbavkar et al. (2013)suggested that the optimum temperature range forSynechococcusin a tropical monsoonal estuary is 27-30°C, which is close to temperatures in the bay during summer and autumn. Chakraborty and Lohrenz(2015) also reported that cyanobacterial species could overcome nitrogen limitation during summer by utilizing alkaline nitrogen. These conclusions were also supported by CCA, which showed that cyanobacterial biomass was highly and positively correlated with temperature and was negatively correlated with DIN concentration and N/P ratio(Fig.7). By contrast, prasinophytes were highly abundant in Daya Bay during winter. Prasinophytes are common in temperate and cold waters (Not et al.,2004). Jiang et al. (2016) reported that the mariculture of oysters may increase the biomass of prasinophytes in Daya Bay during the cold season. Shellfish preferentially fi lter larger cells, thus encouraging the development of the small-celled organisms, such as prasinophytes (Dupuy et al., 2000; Jiang et al., 2016).To summarize, our results confi rmed that small-sized phytoplankton species played an important role in the phytoplankton community of Daya Bay, proving the advantage of HPLC screening methods for determining the composition of the phytoplankton community.
Previous studies that have compared the pigment chemotaxonomy and microscopic analysis of phytoplankton groups suggest that the results of CHEMTAX analysis are consistent with those of microscopy (Wright et al., 1996; Havskum et al.,2004; Brito et al., 2015). Nevertheless, Rodriguez et al. (2002) reported discrepancies between chemotaxonomy and microscopy results for small flagellates and dinof l agellates. Moreover, some pigments are present in several algal groups, making the interpretation of pigment data diffcult. For instance, Fuco, a major pigment in diatoms, is also present in haptophytes (Wright and Jeffrey, 2006). In addition, the microscopic method enumerates dead diatom cells that are not detectable in terms of pigments, thereby resulting in the underestimation of diatoms by CHEMTAX (Brito et al., 2015). Moreover,several dinof l agellates contain pigments other than peridinin as the main carotenoid (Henriksen et al.,2002). For instance,Dinophysisis characterized by pigments that are identical to those in cryptophytes(Meyer-Harms and Pollehne, 1998), resulting in the underestimation of dinof l agellates by CHEMTAX.Despite these limitations, the microscopy observations and pigment HPLC information for diatoms and dinof l agellates in Daya Bay are in good agreement. In this study, HPLC-CHEMTAX provided valuable information on the whole phytoplankton community,especially regarding small-sized groups, whereas microscopy provided good taxonomic reliability for larger cells.
5 CONCLUSION
Pigment analysis revealed that microphytoplankton,including diatoms and dinof l agellates, dominated in Daya Bay with a distinct seasonal distribution. In the winter, diatoms were the dominant phytoplankton and contributed 41.5% to the total Chla. However, N/P ratio continuously increased with increasing temperature during spring, thus favoring the growth of dinof l agellates. This study showed that the average ratio of dinof l agellates to total phytoplankton biomass substantially increased and reached 52.2% in spring.During summer, nutrient limitation shifted from P to N. Consequently, diatoms became the most dominant group in this area and accounted for 71.1% of Chla.Furthermore, this study suggests that HPLCCHEMTAX is an essential method for the identifi cation of small-sized cell classes, such as cryptophytes, cyanobacteria, and prasinophytes,which could be neglected by microscopy techniques.For example, a considerable amount of cryptophytes were present in Daya Bay and cyanobacteria became an important group during summer and autumn. It was suggested that the seasonal variation of composition of phytoplankton assemblages probably associated with environmental parameters, such as temperature and nutrients. Our results evidenced that the microscopy observations and pigment HPLC information for diatoms and dinof l agellates are in good agreement. This study demonstrated the usefulness of pigment analysis in investigating the distribution of phytoplankton groups in Daya Bay.
6 DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
 Journal of Oceanology and Limnology2018年3期
Journal of Oceanology and Limnology2018年3期
- Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- The post-larval and juvenile fi sh assemblage in the Sukhothai Floodplain, Thailand*
- Effects of probiotic on microf l oral structure of live feed used in larval breeding of turbotScophthalmus maximus*
- Comparison ofintestinal microbiota and activities of digestive and immune-related enzymes of sea cucumberApostichopus japonicusin two habitats*
- Otolith shape analysis for stock discrimination of twoCollichthysgenus croaker (Pieces: Sciaenidae,) from the northern Chinese coast*
- The impact of spatial autocorrelation on CPUE standardization between two different fi sheries*
