N-Butane Aromatization Over Hybrid Zeolite Carriers Supported ZnO?
HE Yang,ZHANG Hangfei,HUANG Huizi,MENG Jipeng,LU Jiangyin
(Key Laboratory of Oil and Gas Fine Chemical,Ministry of Education,School of Chemistry and Chemical Engineering,Xinjiang University,Urumqi Xinjiang 830046,China)
0 Introduction
Light aromatics,especially benzene,toluene and xylene(BTX),are important chemical feedstocks in petrochemical industry.However,the petroleum reserves are expected to last only about a few decades.Hence,it is very urgent to f i nd other way to produce aromatics.N-butane is mainly produced as by-products in catalytic cracking and steam pyrolysis,and usually used as fuel,which is not fully utilized their chemical value[1?5].The direct non-oxidative conversion of light alkanes to aromatics is a promising process,which can simultaneously produce high-value aromatic hydrocarbons and relieve the pressure of oil shortage.
Due to the shape selective and adjustable acidic properties of ZSM-5,and the good dehydrogenation performance of Zn and Ga species,many investigations have been conducted to mainly focus on the catalysts based on ZSM-5 zeolite modif i ed most of Ga[6],Zn[7]for light alkanes aromatization.Zn,as a kind of non-noble metal,was in possession of a similar activity to Ga,thus many researchers conveyed their ideas for a potential application of Zn in the industry.However,the strong acidity of Zn/HZSM-5 could result in rapid coke deposition and substantial dry gas(methane and ethane),which are signif i cant obstacles for commercialization[2,7,8].Therefore,stable and highly active catalysts with tunable textural and acid properties are vital to the light alkanes aromatization.
Recently,many studies have reported that zeolite composite could induce remarkable changes in both textural and acid properties,leading to the enhanced catalyst performance.Li and coworkers[9]used HZSM-5/MnAPO-11 for the catalytic conversion of syngas into high-octane hydrocarbons.Compared with HZSM-5,the HZSM-5/MnAPO-11 composite exhibited the highest gasoline yield and iso-paraffin selectivity due to the presence of more mesopores and moderate acid sites.Liu,et al[10]synthesized ZSM-5/SAPO-34 composite as a support by pre-seed method(ZS-PM)and mechanical mixing(ZS-MM)for selective catalytic reduction of NOXand found that Cu/ZS-PM showed a higher deNOXperformance,which should be attributed to that the composites support possessed the advantage of both ZSM-5 and SAPO-34,thus dif f use resistance was reduced and the accessibility of reactants to catalytically active sites was improved.Duan,et al[11]studied on the comparison of ethanol to propylene over HZSM-5/SAPO-34 catalysts prepared by hydrothermal synthesis and physical mixing method.The HZSM-5/SAPO-34 catalyst prepared by physical mixing exhibited the highest propylene yield,which could be attributed to the synergistic ef f ect of HZSM-5 with SAPO-34.
All of research works inspired us to investigate the catalytic performance of Zn/HZ-SA catalyst for n-butane to aromatics.In this study,HZSM-5/SAPO-34 mixtures with dif f erent mass ratio were prepared by mechanical mixing,then ZnO was introduced by an impregnation method.The physicochemical properties of these samples were characterized by scanning electron microscope(SEM),power X-ray dif f raction(XRD)and N2isothermal adsorptiondesorption,temperature-programmed desorption of ammonia(NH3-TPD)and Py-FIIR.Based on the results,compared with HZSM-5,the mechanical mixtures of HZSM-5 and SAPO-34 can ef f ectively alter the textural and acid properties,and the ef f ect of the properties on the catalytic performance of these catalysts were also investigated and discussed in this work.
1 Experiment
1.1 Catalyst preparation
The HZSM-5 zeolite(nSiO2/nAl2O3=50,Nankai University,China)and the SAPO-34 zeolite(Nankai University,China)were put into a mortar at various ratio in mass and grinded carefully till well mixed that the HZSM-5/SAPO-34 mixture zeolite was obtained,marked as HZ-SA.The mixture zeolite was impregnated by zinc nitrate solution in the rotary evaporator for 12 h to get better impregnated sample,then the sample was drained in a vacuum at 80?C,dried at 120?C for 3 h in drying oven,after activated by roasting in muffle for 6 h,the catalysts with dif f erent mass ratio of HZSM-5 and SAPO-34 were obtained.Finally,the powdery catalyst was pressed,crushed and sieved to particles in the range of 40-60 mesh for evaluation tests.In this paper catalysts were named asxZn/HZ-SA-mnxexpressed Zn contents of the catalyst and mn was HZSM-5/SAPO-34 mass ratio in mixture.Generally,x=6 was represented that the Zn contents of catalyst was 6 wt%,if not mentioned in this paper.
1.2 Catalyst characterization
The X-ray dif f raction(XRD)date was acquired using a M18XHF22-SRA dif f ractometer operating at 50 kV and 100 mA with Cu-Kα irradiation in the scan range of 2θ between 5?and 60?.NH3temperature-programmed desorption(NH3-TPD)experiments were performed in an Autosorb-TP-5080 apparatus(Xianquan,China).In the NH3-TPD experiment,0.1 g catalyst was placed in the quartz tube.First,the samples were pretreated at 400?C in helium f l ow for 30 min,then cooled down to 100?C.Switched helium to NH3and adsorbed NH3for 30 min.Finally,heat the samples from 100?C to 900?C at the rate of 10?C min?1
Scanning electron microscope(SEM)analysis was performed using a LEO1430VP detector(LEO Corporation,Germany)todeterminethemorphologyofthesamples.Thenitrogenadsorption-desorptionmeasurementswerecarried out in a JW-BK Brunauer-Emmett-Teller(BET)equipment(the samples were dried at 80?C for 10 h)using nitrogen adsorption/desorption isotherm at-196?C.Zinc content was detected using an atomic absorption spectrophotometer(AAS)using a Z-5000 instrument.The nature of the acid sites of the catalysts was determined by pyridine-FTIR on a MAGNAIR 560 FTIR instrument(Nicolet Co,US)with a resolution of 1 cm?1.The samples were dehydrated at 500?C for 5 h under a vacuum of 1.33 × 10?3Pa,followed by adsorption of purif i ed pyridine vapor at room temperature for 20 min.The system was then degassed and evacuated at 200?C and 350?C,and the IR spectra were recorded.The absolute amount of pyridine adsorbed on Br?nsted acid sites and Lewis acid sites can be calculated using IMEC(B)=1.88 cm mmol?1,IMEC(L)=142 cm mmol?1described by Emeis[12].
1.3 Catalytic performance tests
The catalyst(0.3g)performance tests were evaluated in a f i xed bed with a 6-mm-diameter quartz tube micro reactor operating just at normal pressure,n-butane(99%)was used as the feed gas and high purity nitrogen(99.99%)as the purge gas.The major products of n-butane aromatization including methane,ethylene,ethane,propylene,propane,benzene,toluene,xylene and C9+(the content of these components are extremely low,so they can be ignored).The gaseous products were analyzed on-line using two gas chromatographs(GC-2014C),and each equipped with a sixport automatic sampling valve,quantitative loops(200μL)were connected to the six-port valve.BTX and other aromatics were separated by Chemipak PH packed column(column temperature was 215?C)and detected by FID detector(250?C).Methane,ethylene,ethane,propylene,propane,butane and other light alkenes were separated by Porapak-N packed column(column temperature was 100?C)and detected by FID detector(140?C).
2 Result and discussion
2.1 Catalysts characterization
2.1.1 XRD analysis
Fig 1 showed XRD patterns of Zn/HZSM-5,HZSA,Zn/HZ-SA,SAPO-34 and HZSM-5 as references.The peaks at 2θ=8.0?,8.9?,22.9?,23.9?,24.2?are the characteristics dif f raction peaks of ZSM-5 zeolite,and the peaks at 2θ=9.5?,16.0?,20.5?,26.0?,31.0?are the SAPO-34 characteristics dif f raction peaks,in consistence with the Shonrer Fatern[13].No dif f raction peaks other than those of HZSM-5 and SAPO-34 zeolites were found from HZ-SA samples.Hence,it indicated that the HZSM-5 and SAPO-34 are evenly mixed.In addition,compared to the pristine HZSM-5 and SAPO-34,the dif f raction peaks intensity of mixture are weaker,indicating the reduction crystallinity or grain fragmentation of HZSM-5 and SAPO-34 in physic mixing period by mechanical disruption.

Fig 1 XRD patterns of these samples
After the Zn loading,no dif f raction peaks for Zn oxide crystallites were observed,indicating either good Zn dispersion,or the formation of amorphous ZnO on the zeolite surface[14].The similarity in the spectra before and after Zn loading suggested that the crystal framework of carrier zeolite is preserved.In addition,the intensity of the dif f raction peaks was obviously decreased with the Zn loading,which could be due to a strong interaction between Zn and the Al framework on the catalyst surface,leading to lower crystallinity.
2.1.2 SEM characterization
In order to observe the morphology and get further understanding of the surface structure of dif f erent samples,Figure 2 showed SEM images of HZSM-5,SAPO-34,HZ-SA and some Zn contained samples.HZSM-5 showed block-shaped particles without any agglomeration with the size of ca.3μm.A clear cubic particle with size of 1~2μm was observed in SEM image of SAPO-34 sample.We can see from Figure 2c,after the physically mixing,some HZSM-5 and SAPO-34 particles has been destroyed,and this could result in a reduction of binary structure crystallinity of HZ-SA carrier,in good agreement with the result of XRD.Figure 2d-f exhibited that the introduction of Zn with molecular sieve had not af f ected on carrier morphology.In addition,the HZ-SA material possessed the structure and morphology of both the parent HZSM-5 and SAPO-34 zeolites to some extent,which suggested that the hybird structure HZ-SA carrier could be prepared by physically mixing,similar with the Duan Chao et al[14].

Fig 2 SEM images of these samples
2.1.3 N2isothermal adsorption-desorption characterization

Table 1 Composition,specific surface area and pore volume of these samples
Data were derived from a)AAS technique;b)BET method;c)the t-plot method.
The BET,microporous and external surface area,microporous volume of the studied samples was measured by the nitrogen adsorption–desorption isotherms at 77 K and the results are listed in Table 1.SAPO-34 exhibited a large BET surface of 406 m2·g?1and microporous volume of 0.18 cm3·g?1.For mixture supports,both specif i c surface area and pore volume of support increased with the increasing of the mass fraction of SAPO-34 in HZ-SA mixtures,which will be benef i ted for the dispersibility of the active components and improved the reaction performance.In addition,HZSM-5 and SAPO-34 are in possession of dif f erent pore size,pore type,and shape-selective catalysis function,which may af f ect the dif f usion of reactants and products,and have a great inf l uence on the catalytic activity and product distribution.The surface area and the microporous volume show an evidenced decrease upon the Zn loading,which could be due to that Zn deposited inside the microporous channels[15],or some pore structures were blocked owing to the strong interaction between Zn and external Brnsted acid sites on the catalyst external surface[16].
Figure 3 showed the nitrogen adsorption-desorption isotherms for various samples.All samples exhibited typical type-I isotherms characteristic of microporous materials[10].Zn/HZ-SA catalysts isotherms showed a small hysteresis loop in the range ofP/P0=0.42-1.0,meaning some mesoporosity in catalysts,which may be due to the fragmentation of HZSM-5 and SAPO-34 in physically mixing process.The presence of a certain amount of mesopores may be in favor of the dif f usion of reactants and products,which can improve the catalytic performance.

Fig 3 N2adsorption-desorption isotherms of these samples
2.1.4 NH3-TPD and Py-FTIR characterization
The total number of acid sites on the catalysts was measured by the NH3-TPD technique.This characterization can provide information about acid site strength and distribution on the catalyst surface.Figure 4a showed the NH3-TPD prof i les of HZSM-5,SAPO-34 and mixture carrier with the dif f erent HZ/SA ratio.All the samples exhibited two evident ammonia desorption peaks.The ammonia desorption peaks at low temperature stands for the weak acid sites and the high temperature desorption peaks corresponds to strong acid sites[17,18].The strong acid sites contain not only the strong Br?nsted acid sites but also the strong Lewis acid sites[18,19].Obviously,compared with HZSM-5,both the strength and the number of acid sites of SAPO-34 were lower.It is interesting that the SAPO-34 has a similar amount of strong and weak acid sites,and it is in good agreement with the literature[20].The density and distribution of acid sites in the mixture carrier showed a certain regular change with the increase of the proportion of SAPO-34.The number and strength of acid sites showed a decline trend,which means that the acidity of the mixture can be ef f ectively changed by mixing SAPO-34,and this result agree well with literature[11].
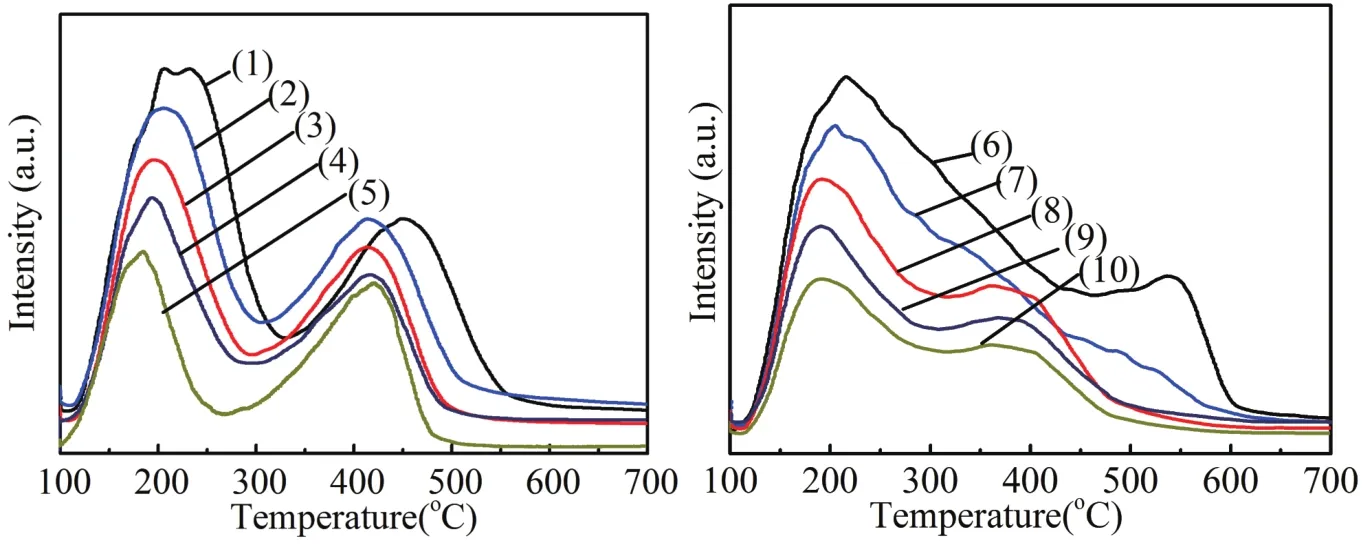
Fig 4 NH3-TPD prof i les of these samples
After Zn loading,the strong acid sites of all samples decreased signif i cantly,while a large number of mediumstrong acid sites appeared,which could be due to the strong interaction of Zn species and molecular sieve strong acid[2,14].With an increase of the proportion of SAPO-34,the density and strength of acidity of Zn/HZ-SA catalysts weakened gradually,which is similar with the trend of the parent mixture support.
The acidities of some catalysts have been further studied by FI-IR-pyridine after the degassing of pyridine at 200?C(Figure 5a)and 350?C(Figure 5b).The peak at 1 445 cm?1,1 545 cm?1represents the vibration of pyridine adsorbed on the Lewis and Br?nsted acid sites,respectively[21].An additional band around 1 490 cm?1is attributed to both pyridine-bound Lewis and Br?nsted acid sites[22,23].Obviously,after Zn loading,there is a signif i cant peak near 1 606 cm?1,which is considered to be a new active centre by the interaction of Zn with Br?nsted acid sites,and it has a dehydrogenation function of Lewis acid sites[19].The concentrations of the dif f erent acid sites were calculated and the results were displayed in Table 2.It can be seen from the table that the B/L ratio of HZSM-5 was greater than 1 and the B/L ratio of SAPO-34 was not.After physically mixing of HZSM-5 and SAPO-34,a mixture support with a moderate proportion of B/L can be obtained.After Zn loading,the number and type of catalyst acid sites changed signif i cantly,the Br?nsted acid sites decreased,while Lewis acid sites increased distinctly,which could be caused by that a lot of Br?nsted acid sites consumed and a large number of Lewis acid sites were generated during the interaction of Zn species with Br?nsted acid sites,in consistent with the literature[2,15].Samples with dif f erent HZ/SA ratios have dif f erent changes in the density and type of acid sites,which suggested that the mix of SAPO-34 could ef f ectively inf l uence the acidity of the catalyst,and it agreed well with the results of NH3-TPD characterization.
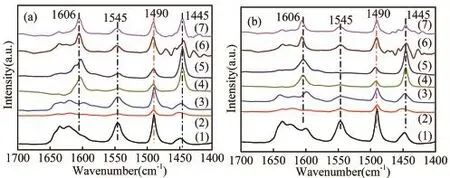
Fig 5 FTIR spectra of pyridine adsorbed on these samples
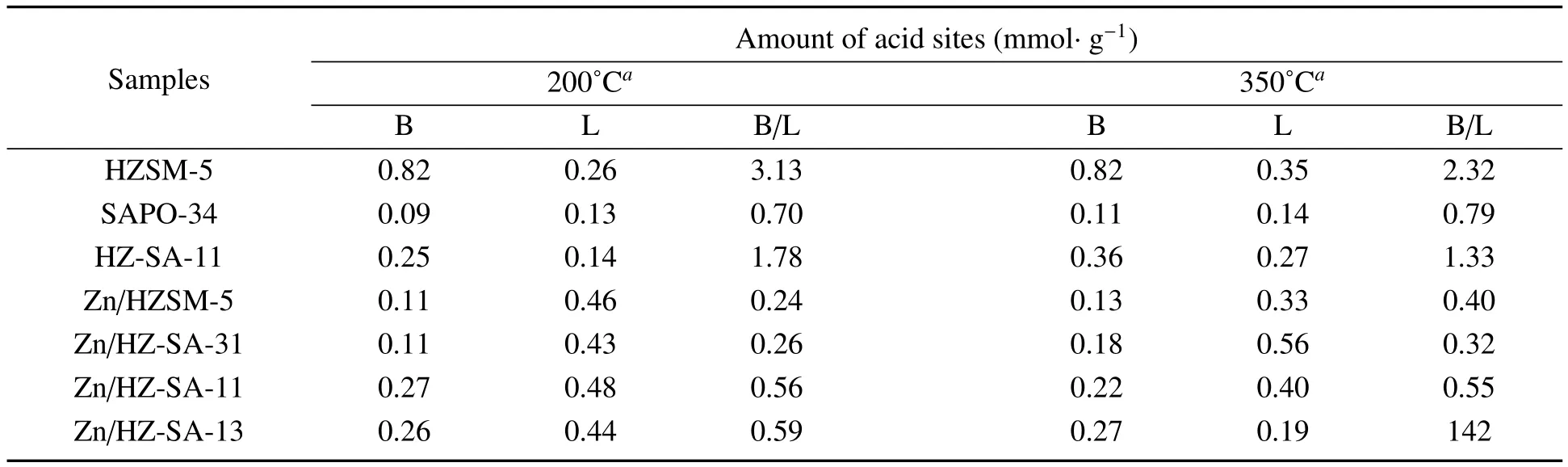
Table 2 Acidic properties of some samples
2.2 Catalytic performance tests
It is generally agreed that metal zeolite catalyst is a bifunctional catalyst.The reaction mechanism of light alkanes aromatization in metal zeolite catalysts is thought to consist of a complex scheme involving dehydrogenation,oligomerization and ring-closure steps[24,25].Generally,light alkanes will undergo the dehydrogenation on the dehydrogen species over the Zn/MFI acid zeolite catalysts,or some activity intermediate products,such as olef i ns&Zn-alkyl species,were generated through proton cracking at the protonic acid sites and pyrolysis reaction[26],etc.,and then the intermediate products undergo various secondary reactions(including oligomerization,cyclization,hydrogen transfer,aromatization et al.)on the acid sites,leading to thermodynamically stable products,i.e.aromatic hydrocarbons.In addition,this series of processes also involves the reactants,intermediates and product dif f usion and migration.Therefore,the performance of light alkanes aromatization are closely associated with the catalyst property of texture and acidity,and the state of the metal species[22,24].
Figure 6 showed time courses of n-butane conversion over various catalysts located in the isothermal f i xed-bed reactor.Although Zn/SAPO-34 has the largest specif i c surface area,the highest n-butane conversion was only 19.6%,a signif i cantlylowvaluecompared to Zn/HZSM-5(49.8%)and Zn/HZ-SA(more than 55.7%).The resultcouldbedueto the weakest acid strength and lowest acid density of the Zn/SAPO-34 catalyst.During the reaction time of investigated,all Zn/HZ-SA exhibited higher conversion compared with the Zn/HZSM-5 catalyst,which could be attributed to the increased surface area of the catalyst by mixing SAPO-34 and the enhanced number of active sites available.However,for Zn/HZ-SA,the n-butane conversion decreased with an increase in SAPO-34 mass ratio in the mixture support.The acidity of the catalyst had a pronounced ef f ect on the n-butane activation.Depended on the results of NH3-TPD characterization,the acidity strength and density reduced gradually with a raise in SAPO-34 mass ratio,which is disadvantageous to the n-butane conversion.In addition,with the reaction time increases,the n-butane conversion of Zn/SAPO-34decreasedfrom19.6%to12%(decreasingamplitudereached38.8%),Zn/HZSM-5decreasedfrom49.9%to 35.2%(29.5%),Zn/HZ-SA-31 decreased from 73.1%to 63.4%(13.3%),Zn/HZ-SA-11 decreased from 65.5%to 55.2%(15.7%),Zn/HZ-SA-13 decreased from 56%to 47.8%(14.6%).This results suggested that Zn/HZ-SA catalysts possessed more stable activity than Zn/SAPO-34 and Zn/HZSM-5.
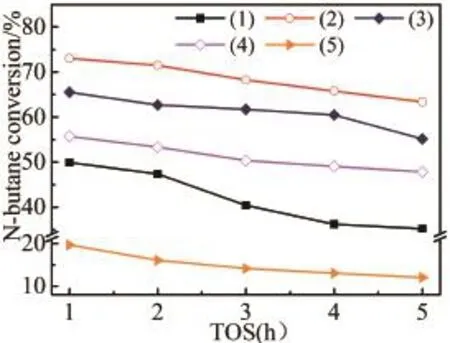
Fig 6 Catalytic conversion of n-butane for different samples
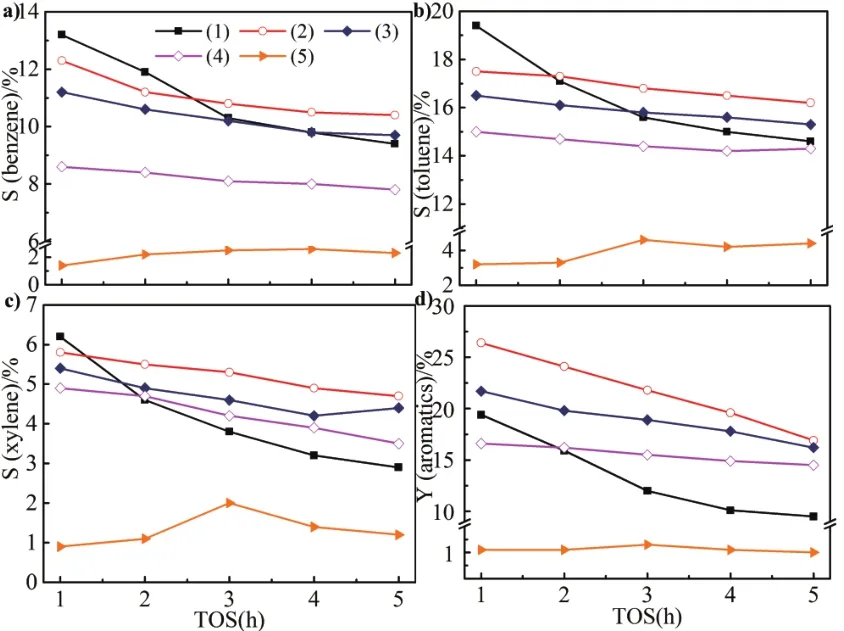
Fig 7 Catalytic selectivity and yield of aromatics for dif f erent samples

Fig 8 Catalytic selectivity of olef i ns and alkanes for dif f erent samples
Selectivity of a)benzene,b)toluene,c)xylene.d)yield of aromatics(1)Zn/HZSM-5;(2)Zn/HZ-SA-31;(3)Zn/HZ-SA-11;(4)Zn/HZ-SA-13;(5)Zn/SAPO-34.Reaction conditions:0.1 Mpa,0.3 g catalyst,temperature=580?C,space velocity=1500 mL·g?1·h?1.
Selectivity of a)methane and ethane,b)ethylene,c)propylene and propane.(1)Zn/HZSM-5;(2)Zn/HZ-SA-31;(3)Zn/HZ-SA-11;(4)Zn/HZ-SA-13;(5)Zn/SAPO-34.Reaction conditions:0.1 Mpa,0.3 g catalyst,temperature=580?C,space velocity=1500 mL·g?1·h?1
The distribution and total yield of aromatics over dif f erent samples with time on stream has been given in Figure 7.Zn/SAPO-34 exhibited the lowest aromatics selectivity(less than 10%)and yield(less than 1.5%).In the initial period,Zn/HZSM-5 showed the highest aromatics selectivity(38.8%),however,after f i ve hours of reaction,the decline was signif i cant.Compared with Zn/HZSM-5,Zn/HZ-SA catalysts exhibited more stable aromatics selectivity and yield,after f i ve hours of reaction,the selectivity of aromatics for Zn/HZ-SA-31 and Zn/HZ-SA-11 can achieve 31.3%and 29.4%respectively,slightly higher than Zn/HZSM-5(26.7%).The total yield of aromatics for Zn/HZ-SA-31 and Zn/HZ-SA-11 can achieve 19.8%and 16.3%respectively,observably higher than Zn/HZSM-5(9.4%).
Figure 8 displayed the other products distribution over dif f erent catalysts with time on stream.In the initial period,Zn/SAPO-34 showed the highest ethylene selectivity at 27.1%and then decreased to 22.5%,the total selectivity of methane and ethane increased from 25.8%to 29%,and the total selectivity of propane and propylene changed slightly.Zn/HZSM-5 possessed lowest total selectivity of propane and propylene at initially,and then increased from 8.7%to 15.9%,meanwhile,the ethylene selectivity increased from 18%to 23.8%.For Zn/HZ-SA the total selectivity of methane and ethane decreased with the increase of the proportion of SAPO-34,however,the selectivity of ethylene,propane and propylene presented reverse trend.
All above results indicated that n-butane conversion over Zn/SAPO-34 mainly undergoes a thermal cracking reaction.it has a lot of products of ethylene,propane and propylene.Zn/SAPO-34 showed lowest total selectivity and yield,owing to the absence of a shape-selective environment and a certain number of Br?nsted acid sites on SAPO-34.Although,in the initial period,Zn/HZSM-5 showed the highest aromatic selectivity,however,with the increased of reaction time,the aromatics selectivity decreased while the olefin increased.This results could indicated that the coke deposited has been formed gradually,which could cover the actives sites and block the pores,resulting in the inhibition for the shape-selective conversion of olef i ns to aromatics[27].This suggested that single HZSM-5 or SAPO-34 probably not a suitable supporter for n-butane aromatization reaction under studied condition.Evidently,Zn/HZ-SA showed better catalytic performance than Zn/HZSM-5 in both of stability and yield of aromatics,which means that SAPO-34 enhanced the catalytic capability of the Zn/HZ-SA.And this can be explained by the following reasons:1)Zn/HZ-SA possessed a greater specif i c surface area and a substantial number of available active sites,which could enhance the conversion of n-butane;2)Zn/HZ-SA had medium strength and quantity of acid sites,which could retard the rate of catalysis deactivation;3)Zn/HZ-SA owned diversif i ed pore structure,which may be benef i cial for the dif f usion and migration of the reaction substance.In the case of Zn/HZ-SA catalyst,it was noteworthy that Zn/HZ-SA-31 had the highest yield of both aromatics and olef i ns,which could be attributed to maximum strong Lewis acid density in Zn/HZSA-31 sample.Many investigations have demonstrated that,this strong Lewis acid sites was generation by the strong interaction of Zn species and Br?nsted acid sites,and it has a excellent dehydrogenation function,which can inhibit the conversion of olefins into alkanes during the hydrogen transfer process thus in favor of the formation of aromatics[19].
3 Conclusions
In current work,a series of HZ-SA mixed carriers with dif f erent HZ/SA mass ratios were prepared by mechanical mixing,and then Zn was introduced by impregnating.The catalytic performance of Zn/HZ-SA catalysts was investigated for the f i rst time in aromatization of n-butane.Based on the performance results,the Zn/HZ-SA catalysts exhibited superior catalytic behaviours,including n-butane conversion,the total yield of aromatics and catalyst stability,as compared to Zn/HZSM-5 catalyst and Zn/SAPO-34 catalyst.The results from XRD and SEM showed that the mixture supports basically maintained the original morphology of the parent molecular sieve,but the grain structure was suf f ered certain degree of damage.The results from N2-physisorption revealed that the addition of SAPO-34 appreciably increased the specif i c surface area of the catalyst and the number of available active sites.We can learned from the results of nitrogen adsorption-desorption isotherms that Zn/HZ-SA owned an amount of mesopores,which may be due to the stacking of dif f erent grain size of molecular sieve or fragments and/or f i nes.The presence of mesopores reduces the mass transfer resistance,which is benef i cial to the activity and stability of the catalyst.The NH3-TPD and Py-FIIR results indicated that both the strength,density and type of acid sites of Zn/HZ-SA changed signif i cantly,which may be responsible for high aromatics yields and better catalytic stability.A reasonable explanation of super catalytic behaviours of Zn/HZ-SA was that the properties of texture and acid via doping SAPO-34 were ameliorated,or that synergistic ef f ect of HZSM-5 with SAPO-34.
References:
[1]Lim D,Jang J,Kim T,et al.Selective hydrodealkylation ofaromatics to benzene,toluene,and xylenes(BTX)over a Pt/H-ZSM-5 catalyst[J].Journal of Molecular Catalysis A Chemical,2015,407:147-151.
[2]Sha N L H A,Liu J,Ning H E,et al.Catalytic conversion of n-butane over Au-Zn-modif i ed nano-sized HZSM-5[J].Chinese Journal of Catalysis,2013,34(6):1262-1266.
[3]Maia A J,Oliveira B G,Esteves P M,et al.Isobutane and n-butane cracking on Ni-ZSM-5 catalyst:Ef f ect on light olef i n formation[J].Applied Catalysis A General,2011,403(1):58-64.
[4]Xu Yuebing,Lu Jiangyin,Zhong Mei,et al.Dehydrogenation of n-butane over vanadia catalysts supported on silica gel[J].Journal of Natural Gas Chemistry,2009,18(1):88-93.
[5]Jiang G,Li Z,Zhao Z,et al.Highly ef f ective P-modif i ed HZSM-5 catalyst for the cracking of C 4,alkanes to produce light olef i ns[J].Applied Catalysis A General,2008,340(2):176-182.
[6]Rodrigues V D O,J′unior A C F.On catalyst activation and reaction mechanisms in propane aromatization on Ga/HZSM5 catalysts[J].Applied Catalysis A General,2012,435(17):68-77.
[7]Krishnamurthy G,Bhan A,Delgass W N.Identity and chemical function of gallium species inferred from microkinetic modeling studies of propane aromatization over Ga/HZSM-5 catalysts[J].Journal of Catalysis,2010,271(2):370-385.
[8]Liu J,Hong A S N L,He N,et al.The crucial role of reaction pressure in the reaction paths for i-butane conversion over Zn/HZSM-5[J].Chemical Engineering Journal,2013,218(4):1-8.
[9]Li J,Tan Y,Zhang Q,et al.Characterization of an HZSM-5/MnAPO-11 composite and its catalytic properties in the synthesis of high-octane hydrocarbons from syngas[J].Fuel,2010,89(11):3510-3516.
[10]Liu J,Song W,Xu C,et al.The selective catalytic reduction of NOx over a Cu/ZSM-5/SAPO-34 composite catalyst[J].Rsc Advances,2015,5(127):104923-104931.
[11]Duan C,Zhang X,Zhou R,et al.Comparative studies of ethanol to propylene over HZSM-5/SAPO-34 catalysts prepared by hydrothermal synthesis and physical mixture[J].Fuel Processing Technology,2013,108:31-40.
[12]C A Emeis.Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts[J].Journal of Catalysis,1993,24(38):347354
[13]Razavian M,Fatemi S.Synthesis and application of ZSM-5/SAPO-34 and SAPO-34/ZSM-5 composite systems for propylene yield enhancement in propane dehydrogenation process[J].Microporous&Mesoporous Materials,2015,201:176-189.
[14]Chao D,Xin Z,Rui Z,et al.Hydrothermally synthesized HZSM-5/SAPO-34 composite zeolite catalyst for ethanol conversion to propylene[J].Catalysis Letters,2011,141(12):1821-1827.
[15]Abdelsayed V,Smith M W,Shekhawat D.Investigation of the stability of Zn-Based HZSM-5 catalysts for methane dehydroaromatization[J].Applied Catalysis A General,2015,505:365-374.
[16]Abdelsayed V,Shekhawat D,Smith M W.Ef f ect of Fe and Zn promoters on Mo/HZSM-5 catalyst for methane dehydroaromatization[J].Fuel,2015,139:401-410.
[17]Kong L,Shen B,Zhao J,et al.Comparative study on the chloromethane to olef i ns reaction over SAPO-34 and HZSM-22[J].Industrial&Engineering Chemistry Research,2014,53(42):16324-16331.
[18]Cui Y,Xu Y,Lu J.The ef f ect of zeolite particle size on the activity of Mo/HZSM-5 in non-oxidative methane dehydroaromatization[J].Applied Catalysis A General,2011,393(1):348-358.
[19]Song C,Li X,Zhu X,et al.Inf l uence of the state of Zn species over Zn-ZSM-5/ZSM-11 on the coupling ef f ects of cofeeding n-butane with methanol[J].Applied Catalysis A General,2016,519:48-55.
[20]Kim J,Choi M,Ryoo R.Ef f ect of mesoporosity against the deactivation of MFI zeolite catalyst during the methanol-to-hydrocarbon conversion process[J].Journal of Catalysis,2010,269(1):219-228.
[21]Chen X,Dong M,Niu X,et al.Inf l uence of Zn species in HZSM-5 on ethylene aromatization[J].Chinese Journal of Catalysis,2015,36(6):880-888.
[22]Kub˙u M,ˇZilkov′a N,ˇCjka J.Post-synthesis modif i cation of TUN zeolite:Textural,acidic and catalytic properties[J].Catalysis Today,2011,168(1):63-70.
[23]Singh A K,Yadav R,Sakthivel A.Synthesis,characterization,and catalytic application of mesoporous SAPO-34(MESO-SAPO-34)molecular sieves[J].Microporous&Mesoporous Materials,2013,181(11):166-174.
[24]Aditya Bhan W,Nicholas Delgass.Propane aromatization over HZSM and Ga/HZSM catalysts[J].Catalysis Reviews Science&Engineering,2008,50(1):19-151.
[25]Caeiro G,Carvalho R H,Wang X,et al.Activation of C2-C4alkanes over acid and bifunctional zeolite catalysts[J].Journal of Molecular Catalysis A Chemical,2006,255(1):131-158
[26]Guisnet M,Gnep N S.Mechanism of short-chain alkane transformation over protonic zeolites alkylation,disproportionation and aromatization[J].Applied Catalysis A General,1996,146(1):33-64.
[27]Tempelman C H L,Hensen E J M.On the deactivation of Mo/HZSM-5 in the methane dehydroaromatization reaction[J].Applied Catalysis B Environmental,2015,176-177:731-739.

