Sex determination and differentiation in Aurelia sp.1: the absence of temperature dependence*
LIU Chunsheng (劉春勝) , GU Zhifeng (顧志峰) , XING Mengxin (邢孟欣) ,SUN Yun (孫云) , , CHEN Siqing (陳四清) , , CHEN Zhaoting (陳昭廷)
1 State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan University, Haikou 570228, China
2 Key Laboratory of Tropical Hydrobiogical Technology, Ocean College, Hainan University, Haikou 570228, China
3 Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
1 INTRODUCTION
Evolutionary mechanisms relating to sex determination and sex ratio have long been of interest to population biologists (Conover and Kynard, 1981).In most metazoans, sex is genetically determined and irreversibly defined at fertilization (Carré and Carré,2000). Nevertheless, a number of environmental factors have been found to have an influence on sex determination and sexual plasticity, particularly in certain reptiles, fish, and cnidarians (Janzen, 1994;Littlefield, 1994; Chen et al., 1995, 2008; Devlin and Nagahama, 2002; Morjan, 2003). Among the environmental factors studied so far, the thermal condition during the period of primary sex differentiation appears to be the main environmental determinant of sex (Blázquez et al., 1999). Taking sexual phenotype in gonochoristic species of hydra as an example, males could switch to females at high temperature because of losing the sperm lineage(Littlefield, 1994). It is inferred that these phenomena,observed in poikilothermic animals, are associated with full exposure to the external physical environment at certain differentiation stages (Devlin and Nagahama, 2002).
Cnidarians are generally regarded as one of the simplest radially symmetrical living animals. They characteristically have only two germ layers and have been placed as the major sister clade to the Bilateria(protostomes + deuterostomes), which encompasses 99% of living animal species (Glenner et al., 2004;Dunn et al., 2008; Philippe et al., 2009; Wheeler et al.,2009; Pick et al., 2010; Erwin et al., 2011). Because of their unique position in terms of metazoan evolution, there has been recent interest in cnidarians(Technau and Steele, 2011).
There are four distinct classes of Cnidaria: the Anthozoa, Hydrozoa, Scyphozoa, and Cubozoa(Houliston et al., 2010). Sex differentiation and sex determination in cnidarians have been studied for the past 30 years. Based on evidence from morphological,cellular, and molecular studies, it appears that environmental factors play an important role in the development of germ cells, sex determination, and sex inversion, particularly in the Hydrozoa and Anthozoa (Littlefield et al., 1991; Littlefield, 1994;Carré and Carré, 2000; Schlesinger et al., 2010;Nishimiya-Fujisawa and Kobayashi, 2012). Unlike other well-known cnidarian taxa, such as hydra(Hydrozoa) and sea anemones (Anthozoa), the life history of Scyphozoa alternates between a freeswimming medusa phase and a benthic polyp phase.Traditionally, the latter phase is usually called asexual stage because of their reproduction (including budding, podocyst and strobilation). However,whether polyps are really non-sex or asexual? To our knowledge, none has actually raised this question in scyphozoan jellyfish.
Moreover, when studying the sex determination mechanism of these simple animals, it is important to note the following factors: that some variation, in terms of sex differentiation, has been noted among different cnidarian species; that a high degree of sex plasticity has been observed among different cnidarian species; and that the mechanisms controlling sex determination in these organisms are still poorly understood (Bosch and David, 1986, 1987; Ayre,1988; Carré and Carré, 2000; Fautin, 2002; Gold and Jacobs, 2013). These observations suggest that more than one mechanism for sex determination may exist among cnidarians.
Aureliaaurita, a cosmopolitan scyphozoan species,has often been regarded as representative of scyphozoan species (M?ller, 1980; Miyake et al.,1997; Lucas and Lawes, 1998; Lucas, 2001). As a result, the aims of this study were to determine the life cycle stage at which the sex ofA.auritadifferentiates and to examine the relationships between temperature variation and sexual plasticity ofA.aurita.
2 MATERIAL AND METHOD
2.1 Strains and preparation
Aureliasp.1 (Wang et al., 2015) polyps were bred from mature medusae (10–15 cm in diameter)collected from Jiaozhou Bay, Qingdao, China, where the temperature was about 25°C. The polyps were transferred to a laboratory, where they were maintained for about 3 years.
When required for experiments, polyps were randomly detached from the corrugated plate surface using a sharp scalpel, placed individually in 500-mL breakers, and cultured in fresh seawater. Detached polyps were induced to re-attach in a fully darkened incubator at 20°C and did not receive food for 5 days.Reattached polyps were fed with freshly-hatchedArtemianauplii twice a week prior to preparation for experimentation.
2.2 General procedures of Aurelia sp.1 rearing
The general procedures of rearing from polyps to the sexual maturation of medusae were as follows.Polyps were rearing at strobilation temperature (10–15°C) and dark condition for 20 days, and then new born ephyrae were carefully collected and reared in 2-L beakers for 10 days. At last, 1-cm medusae were cultured in 300-L special tanks for about 60 days. All life stages ofAureliasp.1 were fed with enough freshly-hatchedArtemianauplii twice a day, and about 50% of water in container was replaced with fresh seawater every day.
2.3 Sex identification of medusae and polyps
The sex of medusae was determined by observation of gamete type (oocyte or spermatid). Sampled individual medusae were anesthetized in 0.5% MgCl2and placed in glass Petri dishes with the mouth facing upwards. A small incision was cut in the horseshoeshaped evaginations using surgical scissors, which allowed for the removal by microdissection of sections of the gonad. To observe gamete type, a minimum of three 2 mm×2 mm sections (sampled from three horseshoe-shaped gonads) from each medusa were prepared, squashed on microscope slides, and examined under a Nikon 80i microscope(Nikon, Japan). Gonad-cut medusae recuperated in fresh seawater and were re-reared, if necessary.
To redetect the sex and developmental stage of meduase, the gonad of a mature medusa along with a patch of mesoglea was carefully cut with a sharp scalpel, washed three times, and fixed with 4%formaldehyde. After 24 h the fixative was removed and the specimen rinsed in filtered water. After excising any excess mesoglea, the gonad was placed in 70% ethanol, after which the fixed specimen was embedded in paraffin and an ultramicrotome was used to cut thin sections (of 5 μm thickness), which were mounted on glass slides. Sections were stained using hematoxylin-eosin. Histological sections were examined under a light microscope to determine the presence and type of gonad.
The sex of polyps was determined by detecting the sex of all released medusae. 18 polyps were randomly picked, and strobilation was induced by incubation at(13± 0.5)°C under dark-condition exposure. Following their release from polyps, the ephyrae were maintained in 18 2-L beakers at room temperature (23–25°C) and fed twice daily with newly-hatchedArtemianauplii.The seawater in each beaker was changed twice daily an hour after feeding. Medusae, which were released from polyps after reaching a bell diameter of 1 cm,were cultured in 18 300-L special tanks. Sex identification of medusae was determined using the method mentioned above.
In other case, the gonad development of both male and femaleAureliasp.1 medusae was observed every 5 days at 20°C in laboratory conditions. The width and sex of medusae gonad was measured under dissecting microscope. In detail, medusae were anesthetized in 0.5% MgCl2and placed in glass Petri dishes with the mouth facing upwards. A small incision was cut in the horseshoe-shaped evaginations using surgical scissors, and then the sex and width of exposed gonad (vertical distance from junction with gut to the other gonad edge) was measured.
2.4 effect of temperature on sex determination
Raised temperature on the sex of polyp clones: To examine the effect of temperature on the sex of polyp budding clones from a parent individual of known sex, sixteen polyps (eight males; eight females) were divided into four groups, each of two males and two females. Polyps of each group budded asexual clones at four different temperatures (15°C, 20°C, 25°C, and 30°C) for 60 days. DailyArtemianauplii consumption of each group was 4 ind./(polyp?d). After 2 months,clones were stimulated to strobilation at (13± 0.5)°C for 20 days. Ephyrae released from each breaker were collected and reared in sixteen 20-L tanks. Following the development of ephyrae into 1 cmmedusae, the experimental animals were transported to medusae culture apparatus. In order to keep medusae in a healthy state, two rearing tanks were used for each group with medusae number being of more than 200.The gender of each medusa was detected using the method mentioned above.
Strobilation temperature on the sex of medusae:According to ecological characteristics of polyps used in this experiment, strobilation temperatures were divided into three levels, 10°C, 12°C, and 14°C.45 male and 45 female polyps were respectively divided into 3 groups named M1, M2, M3 for males,and F1, F2, F3 for females. All polyps were maintained at 15°C for two days. Then they were stimulated to strobilation at different temperature (M1 and F1 at 10°C; M2 and F2 at 12°C; M3 and F3 at 14°C) for 20 days. All ephyrae released from each group were collected and reared in the same tanks. Medusae rearing andsex identification methods were performed as described above.
Raised temperature on the sex of medusae: To examine the sexual plasticity of medusae, ephyrae that had been released from same-gender polyps were reared at four different temperatures: 15°C, 20°C,25°C, and 30°C. In detail, three male polyps and three female polyps were stimulated to bud asexual clones respectively for 20 days under the following conditions: 25°C, in the dark, and at a daily consumption ofArtemianauplii (for each group) of about 4 ind./(polyp?day). Then male and female polyp groups were all stimulated to strobilation at(13± 0.5)°C. The new ephyrae released from male or female polyps were divided into four groups and reared in 15°C, 20°C, 25°C, and 30°C seawater until the sex could be distinguished.
3 RESULT
3.1 Determination of sex in medusae
Sex identification in both polyps and medusae was examined according to observations of the medusa gonad. In laboratory culture, the growth rates of both the female and the male medusae reared under the same conditions were the same (Fig.1a). Planulae were observed in the oral arms of females at a size of about 130 mm, indicating synchronous maturity of ovary and testis when male and female medusae were reared together. Examination under the light microscope indicated that female gonad development occurred at an earlier stage than was the case for males.
Sex identification in male and female medusaebecame possible at about 40 mm and 69 mm,respectively (Fig.1a and b). Microscopic analysis of female and male gonads revealed that the ovaries were filled with oocytes of different sizes, whereas the testes of the male medusa were filled with irregular-shaped sperm follicles (Fig.2). Histological examination confirmed the existence of these traits, as indicated by the presence of both small immature oocytes as well as large well-developed oocytes in the female gonads. Observations of the mature male gonads indicated a large number of sperm follicles of almost uniform size as well as the occurrence of spermiation (Fig.3b: indicated by arrows).
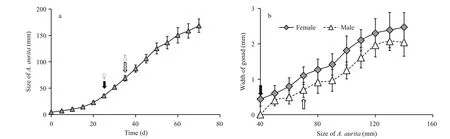
Fig.1 Gonad development of Aurelia sp.1 medusa
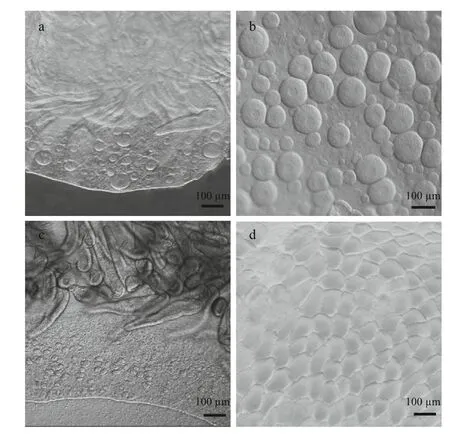
a. female gonad of a 40 mm medusa; b. mature female gonad; c. male gonad of a 70 mm medusa; d. mature male gonad.
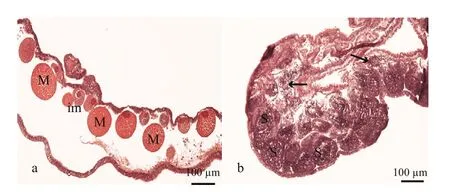
Fig.3 Histology of mature Aurelia sp.1 meudsae gonads
3.2 Determination of sex in medusae originated from a single polyp
Sex determination of 18 polyps (marked from No.1 to No.18) sampled from laboratory-reared specimens showed that medusae originating from a given polyp(in which the release rate was estimated at about 6–14 medusae per polyp) shared the same sex (Table 1). As a result, 10 expected-male and 8 expected-female polyps were identified among the sampled specimens.Moreover, the strobilation rate (6–14 ephyrae per polyp), as well as the survival rate (more than 95%)from newly released ephyrae to mature medusae, of both expected-male and expected-female polyps were almost the same, and no significant differences were found.
3.3 Determination of sex in medusae originated from a polyp clone
In laboratory cultures, the excepted sex of polyps budding from one individual of known sex was stable that was the same as its parents throughout the experiment at four different rearing temperatures(15°C, 20°C, 25°C and 30°C) (Table 2). Moreover,higher strobilation rates of both male and female polyps in lower rearing temperature were found(60.2% and 69.2% at 15°C groups of female and male respectively), comparing with higher rearing temperature (53.3% and 54.3% at 30°C groups of female and male respectively) (date not showed in paper). The number of ephyrae per strobila varied from 9.9 to 12.3 (Table 2).
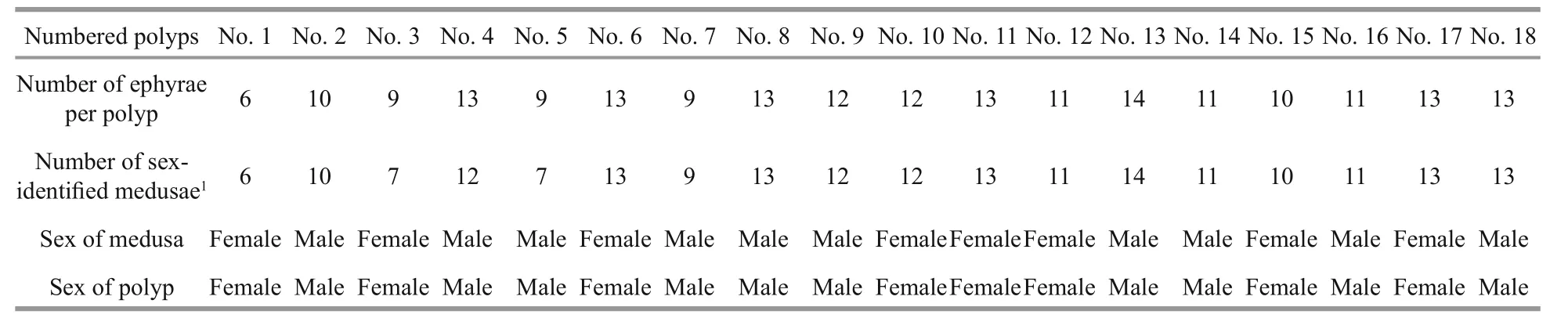
Table 1 Sex identification of 18 polyps
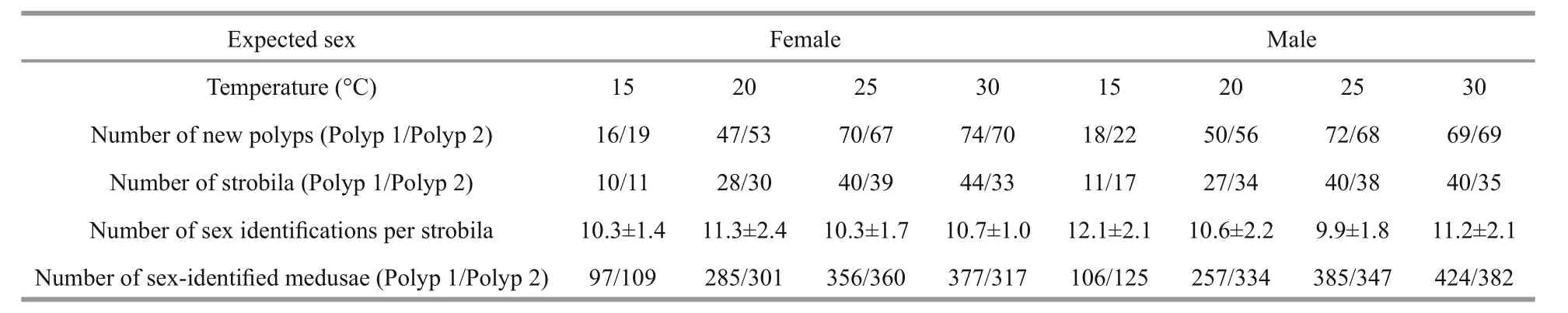
Table 2 Sex identification of polyp clones budding from expected-sex individuals at four rearing temperatures
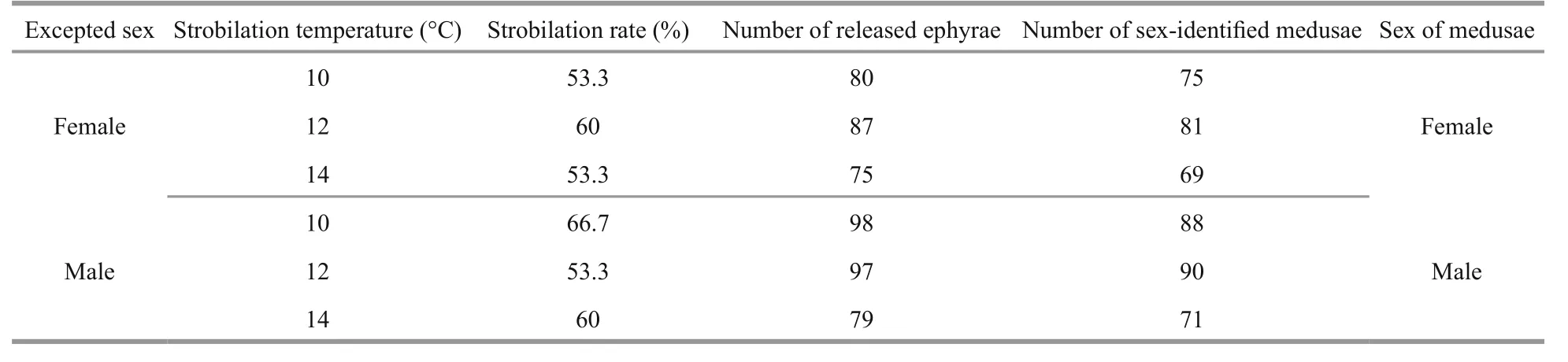
Table 3 Sex identification of medusae released from known-sex polyps at three strobilation temperatures
3.4 effect of strobilation and rearing temperature on the sex of medusae
In order to examine the effect of temperature on the sex of meduasae, (1) polyps were induced to strobilation at three temperature levels, 10°C, 12°C, and 14°C. The results showed that the sex of medusae released from polyps of known sex was the same and strongly dependent on the sex of the original polyps from which they grew (Table 3). (2) Ephyrae released from samegender polyps (female or male) were reared at four different temperatures levels, 15°C, 20°C, 25°C, and 30°C. The results showed that sex of medusae was all the same as their known-sex polyps, and had no relationship to rearing temperature (Table 4).
4 DISCUSSION
To the best of our knowledge, there have been no studies of the mechanism of sex determination in either benthic polyps or pelagic medusae ofAurelia aurita. In this study we initially explored the sexual characteristics and the sex determination mechanism ofAureliaspecies (Aureliasp.1) from Jiaozhou Bay,Qingdao, China. The results clearly indicated thatAureliasp.1 was gonochoric and that the sexual characteristics, which were independent of rearing temperature for both genders, remained stable throughout the polyp and medusa stages.
4.1 Sex differentiation of A. aurita
Sex differentiation refers to the physical realization of testicular or ovarian development, which is identified by morphological, cellular, and molecularmethods (Devlin and Nagahama, 2002). Certain phenotypic characteristics (such as the color and gross morphology of gonads) can be used for sex determination in most cnidarians. For example, the gonad characteristics of scyphozoans have been reported for the medusae ofCyaneanozakii,Nemopilemanomurai, andA.aurita(Eckelbarger and Larson, 1988; Ohtsu et al., 2007; Liu et al., 2015). InA.auritathere were no suitable markers for female or male germ cell analysis, and we deduced that sex differentiation of medusa could be determined by observing the gonads. Our results indicated that the sexual characteristics of polyps could be identified from the sex of the ephyrae released from such polyps.Moreover, sex differentiation was discovered at three life stages ofA.aurita, including polyp, ephyra and medusa, which meant that the sex determination took place at polyp stage, or maybe earlier. However, no special molecular markers indicating female or male individuals have been identified inA.aurita. So it was impossible, at least in our paper, to identify the sex of embryo and planula.
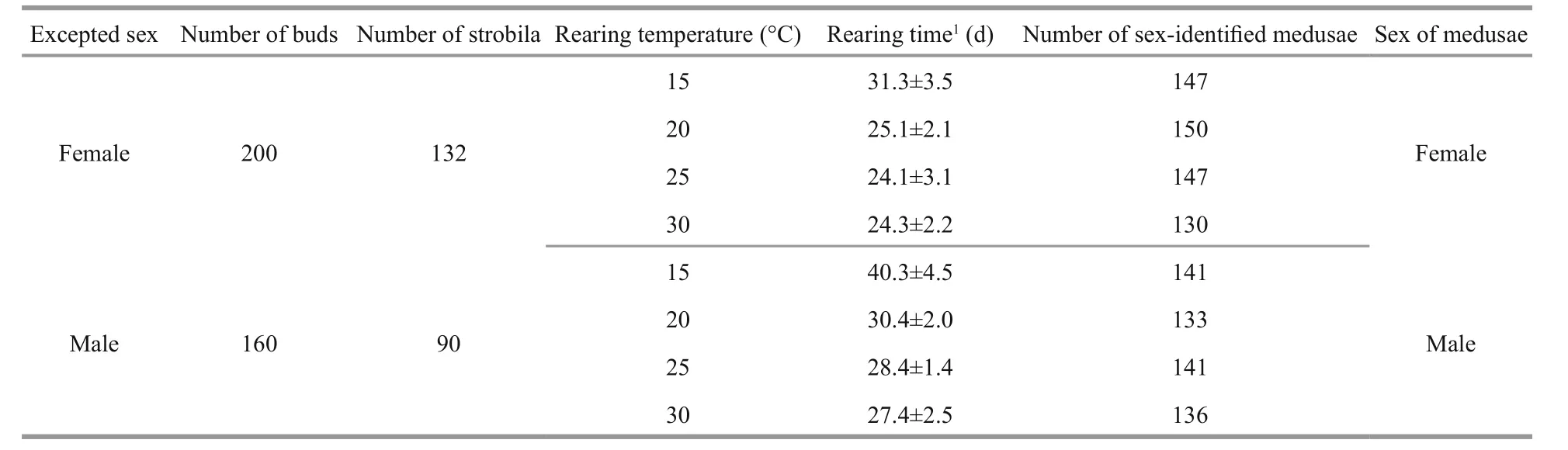
Table 4 Sex identification of medusae released from excepted-sex polyps at four rearing temperatures
In this context it should be noted that the sex identifications for the medusa stage ofA.auritahave been previously reported. It was showed that both male and female medusae were detected in artificial seawater at 21–24°C, while only male medusae were observerd at lower temperature (17°C for one month,an then 22°C for three months) (Spangenberg, 1965;Vagelli, 2007). Therefore, the phenotypic sex ofA.auritaat medusa stage controlled by temperature was concluded (Vagelli, 2007). However, our experimental results indicated relatively stable sex characteristics inA.aurita, which inferred that differentAureliaspecies were varies in sex stability.
Though the period required to develop gonads ofA.auritawas influenced by temperature, food, and photoperiod, differences of gonad development in female and male medusae were observed (Lucas,1996, 2001). In our rearing condition, the periods from new born ephyrae to sex-identifiable female and male medusae were 25 days and 35 days, respectively,while the mature periods for females and males were more or less the same. These results clearly indicated a longer growth period for the development of the ovary compared to that of the testis. The same results were also concluded in Spangenberg’s report(Spangenberg, 1965).
4.2 Sexual plasticity and sex determination of A. aurita
Sexual plasticity and the sex determination of polyps have been studied extensively, particularly in sister species within the Scyphozoa, Anthozoa, and Hydrozoa. Spontaneous sex reversal phenomena were frequently observed in certain Hydrozoa, such asHydraattenuata,Hydracarnea, andHydra magnipapillata(Tardent, 1968; Littlefield, 1984;Bosch and David, 1986, 1987). In comparison to the high sex plasticity of these species, the sex reversal phenomenon inA.auritawas more restricted, or was not even observed, at a variety of temperature regimes during the entire period of our experiments. Thus, two different adaptation mechanisms may exist in benthiconly and polyp-medusae metagenesis cnidarians. For benthic-only species, asexual reproduction results in low genetic and phenotypic diversity in a clonally derived population in which all individuals have the same sexual characteristics (Fautin, 1990; Frank and Mokadi, 2002). One way in which to overcome this problem would be to produce sexually plastic individuals that could result in successful fertilization within the same clonal population (Carlon, 1999;Vollmer and Palumbi, 2002; Schlesinger et al., 2010).Nevertheless, inA.aurita, which has a complex metagenesis life cycle, the gonad developed and matured at the free-swimming medusa stage. This implies that gametes originating from different polyp clones have the capacity for fertilization and the formation of a new population with high genetic diversity. This leads to the conclusion that sexual plasticity of polyps inA.auritais not necessarily a genotypic characteristic.
As the most important environmental sex determination factor, temperature-dependent sex determination has been studied extensively in reptiles,where exposure to elevated temperature results in female development in some species (Bull and Vogt,1979; Vogt and Bull, 1982). These observations suggest that temperature dramatically influences the structure and function of proteins and other macromolecules that control biochemical pathways related to sex determination (Devlin and Nagahama,2002). The mechanisms controlling sex determination in cnidarians are still poorly understood (Fautin,2002). In some cnidarian species, environmental parameters such as water temperature or food availability play a role in this process (Littlefield,1994; Chen et al., 1995, 2008). For example, sex determination inHydrahas a temperature-dependent component (Littlefield, 1994). Carré and Carré (2000)also demonstrated temperature-dependent sex inversion inClytiahemisphaericamedusae at release time (with 80% female individuals being released at 24°C and 85% male individuals being released at 15°C). In our experiments onAureliasp.1, no sex reversal phenomena were observed in either the polyp or medusa stages at four different rearing temperatures(15°C, 20°C, 25°C, and 30°C), indicating that in this species genetic determination, but not temperature,plays a significant role in gonad generation. Ayre(1988) also concluded that determination of sex inActiniatenebrosawas genetically controlled.
5 CONCLUSION
This study clearly indicated that sex differentiation ofA.auritahas been observed at the polyp stage and that all medusae originating from a given polyp are,phenotypically, of the same sex. Moreover, the sexual characteristics, which were independent of rearing temperature for both genders, remained stable throughout the polyp and medusa stages.A comparison of variability in terms of sexual plasticity ofA.auritawith that of Hydrozoa and Anthozoa suggests that species characterized by a free-swimming medusa life stage have a high dispersal potential, which probably results in a lower rate of sex reversal.
Ayre D J. 1988. Evidence for genetic determination of sex inActiniatenebrosa.J.Exp.Mar.Biol.Ecol.,116(1): 23-34.
Blázquez M, Carrillo M, Zanuy S, Piferrer F. 1999. Sex ratios in off spring of sex-reversed sea bass and the relationship between growth and phenotypic sex differentiation.J.FishBiol.,55(5): 916-930.
Bosch T C G, David C N. 1986. Male and female stem cells and sex reversal inHydrapolyps.Proc.Natl.Acad.Sci.U.S.A.,83(24): 9 478-9 482.
Bosch T C G, David C N. 1987. Stem cells ofHydra magnipapillatacan differentiate into somatic cells and germ line cells.Dev.Biol.,121(1): 182-191.
Bull J J, Vogt R C. 1979. Temperature-dependent sex determination in turtles.Science,206(4423): 1 186-1 188.
Carlon D B. 1999. The evolution of mating systems in tropical reef corals.TrendsEcol.Evol.,14(12): 491-495.
Carré D, Carré C. 2000. Origin of germ cells, sex determination,and sex inversion in medusae of the genusClytia(Hydrozoa, Leptomedusae): the influence of temperature.J.Exp.Zool.A,287(3): 233-242.
Chen C L A, Chen C P, Chen I M. 1995. Spatial variability of size and sex in the tropical corallimorpharianRhodactis(=Discosoma)indosinensis(Cnidaria: Corallimorpharia)in Taiwan.Zool.Stud.,34(2): 82-87.
Chen C, Soong K, Chen C A. 2008. The smallest oocytes among broadcast-spawning actiniarians and a unique lunar reproductive cycle in a unisexual population of the sea Anemone,Aiptasiapulchella(Anthozoa: Actiniaria).Zool.Stud.,47(1): 37-45.
Conover D O, Kynard B E. 1981. Environmental sex determination: interaction of temperature and genotype in a fish.Science,213(4507): 577-579.
Dawson M N, Jacobs D K. 2001. Molecular evidence for cryptic species ofAureliaaurita(Cnidaria, Scyphozoa).Biol.Bull.,200(1): 92-96.
Devlin R H, Nagahama Y. 2002. Sex determination and sex differentiation in fish: an overview of genetic,physiological, and environmental influences.Aquaculture,208(3-4): 191-364.
Dunn C W, Hejnol A, Matus D Q, Pang K, Browne W E, Smith S A, Seaver E, Rouse G W, Obst M, Edgecombe G D,S?rensen M V, Haddock S H D, Schmidt-Rhaesa A,Okusu A, Kristensen R M, Wheeler W C, Martindale M Q, Giribet G. 2008. Broad phylogenomic sampling improves resolution of the animal tree of life.Nature,452(7188): 745-749.
Eckelbarger K J, Larson R L. 1988. Ovarian morphology and oogenesis inAureliaaurita(Scyphozoa: Semaeostomae):ultrastructural evidence of heterosynthetic yolk formation in a primitive metazoan.Mar.Biol.,100(1): 103-115.
Erwin D H, Laflamme M, Tweedt S M, Sperling E A, Pisani D,Peterson K J. 2011. The Cambrian conundrum: early divergence and later ecological success in the early history of animals.Science,334(6059): 1 091-1 097.
Fautin D G. 1990. Cnidaria.In: Adiyodi K G, Adiyodi R G eds.Reproductive Biology of Invertebrates. Oxford and I. B.H, New Delhi. p.31-55.
Fautin D G. 2002. Reproduction of Cnidaria.Can.J.Zool.,80(10): 1 735-1 754.
Frank U, Mokadi O. 2002. Coral biodiversity and evolution:recent molecular contributions.Can.J.Zool.,80(10):1 723-1 734.
Fukuda Y, Naganuma T. 2001. Potential dietary effects on the fatty acid composition of the common jellyfishAurelia aurita.Mar.Biol.,138(5): 1 029-1 035.
Glenner H, Hansen A J, S?rensen M V, Ronquist F, Huelsenbeck J P, Willerslev E. 2004. Bayesian inference of the metazoan phylogeny: a combined molecular and morphological approach.Curr.Biol.,14(18): 1 644-1 649.
Gold D A, Jacobs D K. 2013. Stem cell dynamics in Cnidaria:are there unifying principles?Dev.GenesEvol.,223(1-2):53-66.
Houliston E, Momose T, Manuel M. 2010.Clytia hemisphaerica: a jellyfish cousin joins the laboratory.TrendsGenet.,26(4): 159-167.
Janzen F J. 1994. Climate change and temperature-dependent sex determination in reptiles.Proc.Natl.Acad.Sci.U.S.A.,91(16): 7 487-7 490.
Littlefield C L, Finkemeier C, Bode H R. 1991. Spermatogenesis inHydraoligactis: II. How temperature controls the reciprocity of sexual and asexual reproduction.Dev.Boil.,146(2): 292-300.
Littlefield C L. 1984. The interstitial cells control the sexual phenotype of heterosexual chimeras of hydra.Dev.Biol.,102(2): 426-432.
Littlefield C L. 1994. Cell-cell interactions and the control of sex determination in hydra.Semin.Dev.Biol.,5(1): 13-20.
Liu C S, Zhuang Z M, Chen S Q, Yan J P, Liu C L, Sun J M.2015. A large-scale breeding experiment and observations on development ofCyaneanozakii(Scyphozoa,Cyaneidae) from fertilized eggs to larval medusae.Hydrobiologia,754(1): 113-123.
Lucas C H, Lawes S. 1998. Sexual reproduction of the scyphomedusaAureliaauritain relation to temperature and variable food supply.Mar.Biol.,131(4): 629-638.
Lucas C H. 1996. Population dynamics ofAureliaaurita(Scyphozoa) from an isolated brackish lake, with particular reference to sexual reproduction.J.Plankton Res.,18(6): 987-1 007.
Lucas C H. 2001. Reproduction and life history strategies of the common jellyfish,Aureliaaurita, in relation to its ambient environment.Hydrobiologia,451(1-3): 229-246.
Miyake H, Iwao K, Kakinuma Y. 1997. Life history and environment ofAureliaaurita.SouthPac.Study,17(2):273-285.
M?ller H. 1980. Population dynamics ofAureliaauritamedusae in Kiel Bight, Germany (FRG).Mar.Biol.,60(2-3): 123-128.
Morjan C L. 2003. Variation in nesting patterns affecting nest temperatures in two populations of painted turtles(Chrysemyspicta) with temperature-dependent sex determination.Behav.Ecol.Sociobiol.,53(4): 254-261.
Nishimiya-Fujisawa C, Kobayashi S. 2012. Germline stem cells and sex determination inHydra.Int.J.Dev.Biol.,56(6-8): 499-508.
Ohtsu K, Kawahara M, Ikeda H, Uye S I. 2007. Experimental induction of gonadal maturation and spawning in the giant jellyfishNemopilemanomurai(Scyphozoa:Rhizostomeae).Mar.Biol.,152(3): 667-676.
Orton J H. 1922. The mode of feeding of the jelly-fish,Aurelia aurita, on the smaller organisms in the Plankton.Nature,110(2753): 178-179.
Philippe H, Derelle R, Lopez P, Pick K, Borchiellini C, Boury-Esnault N, Vacelet J, Renard E, Houliston E, Quéinnec E,Da Silva C, Wincker P, Le Guyader H, Leys S, Jackson D J, Schreiber F, Erpenbeck D, Morgenstern B, W?rheide G,Manuel M. 2009. Phylogenomics revives traditional views on deep animal relationships.Curr.Biol.,19(8):706-712.
Pick K S, Philippe H, Schreiber F, Erpenbeck D, Jackson D J,Wrede P, Wiens M, Alié A, Morgenstern B, Manuel M,W?rheide G. 2010. Improved phylogenomic taxon sampling noticeably aff ects nonbilaterian relationships.Mol.Biol.Evol.,27(9): 1 983-1 987.
Schlesinger A, Kramarsky-Winter E, Rosenfeld H, Armoza-Zvoloni R, Loya Y. 2010. Sexual plasticity and selffertilization in the sea anemoneAiptasiadiaphana.PLoS One,5(7): e11874.
Schroth W, Jarms G, Streit B, Schierwater B. 2002. Speciation and phylogeography in the cosmopolitan marine moon jelly,Aureliasp.BMCEvol.Biol.,2(1): 1.
Shimauchi H, Uye S I. 2007. Excretion and respiration rates of the scyphomedusaAureliaauritafrom the Inland Sea of Japan.J.Oceanogr.,63(1): 27-34.
Spangenberg D B. 1965. Cultivation of the life stages ofAureliaauritaunder controlled conditions.J.Exp.Zool.,159(3): 303-318.
Tardent P. 1968. Experiments about sex determination inHydraattenuataPall.Dev.Boil.,17(5): 483-511.
Technau U, Steele R E. 2011. Evolutionary crossroads in developmental biology: Cnidaria.Development,138(8):1 447-1 458.
Vagelli A A. 2007. New observations on the asexual reproduction ofAureliaaurita(Cnidaria, Scyphozoa)with comments on its life cycle and adaptive significance.Invert.Zool.,4(2): 111-127.
Vogt R C, Bull J J. 1982. Genetic sex determination in the spiny softshellTrionyxspiniferus(Testudines:Trionychidae)?Copeia,1982(3): 699-700.
Vollmer S V, Palumbi S R. 2002. Hybridization and the evolution of reef coral diversity.Science,296(5575):2 023-2 025.
Wang Y T, Zheng S, Sun S, Zhang F. 2015. effect of temperature and food type on asexual reproduction inAureliasp.1 polyps.Hydrobiologia,754(1): 169-178.
Wheeler B M, Heimberg A M, Moy V N, Sperling E A, Holstein T W, Heber S, Peterson K J. 2009. The deep evolution of metazoan microRNAs.Evol.Dev.,11(1): 50-68.
 Journal of Oceanology and Limnology2018年2期
Journal of Oceanology and Limnology2018年2期
- Journal of Oceanology and Limnology的其它文章
- Editorial Statement
- Hydroacoustic estimates of fish biomass and spatial distributions in shallow lakes*
- A comparison between benthic gillnet and bottom trawl for assessing fish assemblages in a shallow eutrophic lake near the Changjiang River estuary*
- Morphological beak differences of loliginid squid, Uroteuthis chinensis and Uroteuthis edulis, in the northern South China Sea*
- Muelleria pseudogibbula, a new species from a newly recorded genus (Bacillariophyceae) in China*
- Planaxidae (Mollusca, Gastropoda) from the South China Sea*
