Notch pathway inhibitor DAPT enhances Atoh1 activity to generate new hair cells in situ in rat cochleae
Wen-wei Luo, Zhao Han, Dong-dong Ren Xin-wei Wang Fang-lu Chi, Juan-mei Yang
1 Department of Otolaryngology, Eye & ENT Hospital of Fudan University, Shanghai, China
2 Research Institute of Otolaryngology, Fudan University, Shanghai, China
Notch pathway inhibitor DAPT enhances Atoh1 activity to generate new hair cells in situ in rat cochleae
Wen-wei Luo1,2,#, Zhao Han1,2,#, Dong-dong Ren1,2, Xin-wei Wang1,2, Fang-lu Chi1,2,*, Juan-mei Yang1,2,*
1 Department of Otolaryngology, Eye & ENT Hospital of Fudan University, Shanghai, China
2 Research Institute of Otolaryngology, Fudan University, Shanghai, China
Atoh1 overexpression in cochlear epithelium induces new hair cell formation. Use of adenovirus-mediated Atoh1 overexpression has mainly focused on the rat lesser epithelial ridge and induces ectopic hair cell regeneration. The sensory region of rat cochlea is difficult to transfect, thus new hair cells are rarely producedin situin rat cochlear explants. After culturing rat cochleae in medium containing 10%fetal bovine serum, adenovirus successfully infected the sensory region as the width of the supporting cell area was signi ficantly increased.Adenovirus encoding Atoh1 infected the sensory region and induced hair cell formationin situ. Combined application of the Notch inhibitor DAPT and Atoh1 increased the Atoh1 expression level and decreased hes1 and hes5 levels, further promoting hair cell generation.Our results demonstrate that DAPT enhances Atoh1 activity to promote hair cell regeneration in rat cochlear sensory epitheliumin vitro.
nerve regeneration; Atoh1; DAPT; transdifferentiation; gamma secretase inhibitor; cochlea; sensory epithelium; fetal bovine serum;hair cell; supporting cell; hair cell regeneration; neural regeneration
Introduction
Sensory hair cells in the mammalian cochlea convert mechanical stimuli into electrical impulses that allow hearing(Fuchs et al., 2003; Grant et al., 2011; Fujikawa et al., 2014).Loss of hair cells in lower vertebrates causes the surrounding support cells to re-enter the cell cycle and regenerate new hair cells (Corwin and Cotanche, 1988; Ryals and Rubel,1988); however, lost sensory hair cells in the mammalian organ of Corti do not regenerate spontaneously (Izumikawa et al., 2005; Yang et al., 2012).
The basic helix-loop-helix transcription factor, atonal homolog 1 (Atoh1), plays a critical role in hair cell development and regeneration (Bermingham et al., 1999; Zheng and Gao, 2000; Chen et al., 2002; Kawamoto et al., 2003; Woods et al., 2004). Overexpression ofAtoh1induces hair cell formation in the inner ear of mammals (Izumikawa et al., 2005;Staecker et al., 2007; Gubbels et al., 2008; Huang et al., 2009;Han et al., 2010; Kelly et al., 2012; Pan et al., 2012; Chonko et al., 2013; Atkinson et al., 2014; Luo et al., 2014; Gao et al.,2016). Newly generated hair cells were mainly located in the lesser epithelial ridge and the greater epithelial ridge in rat cochleae (Zheng and Gao, 2000; Yang et al., 2012, 2013; Luo et al., 2014, 2017). In addition, spiral ganglion neurons in sensory regions extended dendrites toward the ectopic haircell-like cells (Yang et al., 2012) and formed afferent synapses with ectopic hair-cell-like cells (Luo et al., 2017), which is important for restoration of hearing.
The Notch signaling pathway is vital in determining the fate of hair cells and supporting cells, by lateral inhibition,during inner ear development (Lanford et al., 1999; Zine et al., 2000; Daudet and Lewis, 2005; Yamamoto et al., 2006;Kopan and Ilagan, 2009; Mizutari et al., 2013). Pharmacological inhibition of Notch signaling by γ-secretase inhibitors leads to the generation of supernumerary hair cellsin situin cultured embryonic or neonatal cochleae (Mizutari et al.,2013; Li et al., 2015; Ni et al., 2016; Ren et al., 2016), which mainly involves direct transdifferentiation of supporting cells (Yamamoto et al., 2006; Doetzlhofer et al., 2009; Zhao et al., 2011; Mizutari et al., 2013). A combination of different signals has been shown to promotein situcochlear hair cell regeneration after neomycin insult in mice (Doetzlhofer et al., 2009; Zhao et al., 2011; Masuda et al., 2012; Chen et al., 2013; Ikeda et al., 2015; Ni et al., 2016; Ren et al., 2016;Walters et al., 2017). However, generation of new hair cells in rat cochleain situis difficult due to the low infection efficiency of adenovirus in sensory epithelium. The induction of sufficient numbers of new hair cells in rat cochleain situto replace the lost hair cells could facilitate the formation of synapses between new hair cells and the remaining spiral ganglion cells (Zhao et al., 2011; Chen et al., 2013; Ikeda et al., 2015). Stimulating the formation of a sufficient number of hair cellsin situwould be a useful way to promote hearing recovery in the future.
To increase the infection efficiency of adenovirus in the sensory epithelium, we applied a high concentration of fetal bovine serum and prolonged tissue culture prior to viral infection to increase the width of the sensory epithelium. We successfully generated new hair cells after viral gene transfer of Atoh1in situ, but the number was insufficient. Concurrent application of the γ-secretase inhibitor N-[N-(3,5-Di fluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT)with Atoh1 overexpression increased the rate of supporting cell conversion and produced a greater number of new hair cellsin situ.
Materials and Methods
Animals
A total of fifty-five one-day-old (P1) Sprague-Dawley rat pups, weighing 5 g and of both sexes, were provided by the Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai,China). The protocol for animal use was reviewed and approved by the Institutional Animal Care and Use Committee of Eye & ENT Hospital of Fudan University of China. The experimental procedures followed theUnited States National Institutes of Health Guide for the Care and Use of Laboratory Animals(NIH Publication No. 85-23, revised 1985).
For measuring the width of sensory epithelium in organs of Corti, rat pups were randomized into four groups: neonatal day 1 (n= 5); 5% fetal bovine serum (FBS) for 24 hours (n= 5); 5% FBS for 48 hours (n= 5); 10% FBS for 48 hours (n=5). The rat pups were randomized into five groups for measuring hair cell numbers or relative mRNA expression levels in sensory epithelium: neomycin-green fluorescent protein(neo-GFP) (n= 5), neo-dimethyl sulfoxide (neo-DMSO) (n= 5), neo-DAPT (n= 5), neo-Atoh1 (n= 5), and neo-Atoh1-DAPT (n= 5).
Cochlear cultures and Atoh1 infection
Rats were sacri ficed by CO2asphyxiation and cochleae dissected. Only the middle-basal turns were explanted onto poly-L-lysine (Sigma, St. Louis, MO, USA) coated glass coverslips in Dulbecco’s modi fied Eagle medium/nutrient mixture F12 (DMEM/F12, Life Technologies, Carlsbad, CA, USA)containing 5% FBS (Life Technologies).
On the second dayin vitro, 1.5 mM neomycin was applied for 24 hours. On the third dayin vitro, the medium was replaced with one of the following: DMEM/F-12 containing 5% FBS for 24 hours (the 5% FBS for 24 hours group),DMEM/F-12 containing 5% FBS for 48 hours (the 5% FBS for 48 hours group), or DMEM/F-12 containing 10% FBS for 48 hours (the 10% FBS for 48 hours group). On the fifth dayin vitro, all of the media were replaced with serum-free DMEM/F-12 supplemented with B27 (Life Technologies).This culture medium was replaced every other day.Ad5-GFP-Atoh1orAd5-Atoh1(Huang et al., 2009; Han et al.,2010; Yang et al., 2012, 2013) was co-incubated with the cochlear explants on the fifth dayin vitrofor 20 hours, to overexpress Atoh1 in the cochlear segments, and the control group was infected with Ad5-GFP. For the DAPT experiments, DAPT was added to the medium 24 hours after adding Ad5-vector and the culture medium containing DAPT was replaced every other day until the explants were fixed.Culture medium containing 0.1% DMSO was used as a control. The culture dishes were placed in a 37°C incubator containing 5% CO2in a humid environment.
The final concentration of the Ad5 vector was 1.6 × 108PFU/mL in serum-free DMEM/F-12 medium. The viruses used in the present experiment have been described in previous reports (Huang et al., 2009; Han et al., 2010; Yang et al., 2012, 2013). The neomycin sulfate stock (10 mg/mL in 0.9% NaCl, purchased from Sigma-Aldrich) was diluted in culture media to 1.5 mM. DAPT (catalogue number 565784,EMD Chemicals, Darmstadt, Germany) was diluted in 100%DMSO to a stock concentration of 10 mM and was used at a working concentration of 10 μM.
Tissue preparation and immuno fluorescence analysis
The cochlear explants were fixed with 4% paraformaldehyde for 30 minutes and then treated with 0.1% Triton X-100 plus 10% donkey serum for 30 minutes. This procedure was followed by incubation for 24 hours at 4°C with the following primary antibodies: rabbit-anti-myosin 7A (1:100; Proteus Biosciences Inc., Ramona, CA, USA) and goat-anti-Sox2(1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA).The preparation was washed three to five times with PBS and then incubated with secondary antibodies for 2 hours at 37°C in the dark. The secondary antibodies included donkey anti-mouse/rabbit Alexa Fluor 555 (1:1,000; Jackson ImmunoResearch, West Grove, Philadelphia, PA, USA) and donkey anti-mouse/rabbit/goat (H + L) Alexa Fluor 647 (1:1,000;Jackson ImmunoResearch). The specimens were incubated with rhodamine phalloidin (1:1,000; Life Technologies) for 1 hour at room temperature after the secondary antibody was washed out. The specimens were visualized with a Zeiss LSM 510 confocal laser scanning microscope (Zeiss, Germany).All of the images were digitally processed and evaluated using Adobe Photoshop CS5 software. Images were acquired with a pixel size of 0.03 μm × 0.03 μm × 0.30 μm following Nyquist sampling, with no pixel saturation to ensure that no structural information was lost.
Measurement of the specimens
We measured the distance from the outermost Sox2-immunoreactive cells to the innermost Sox2-immunoreactive cells in organ of Corti area 24 and 48 hours post FBS culture to determine the width of the sensory epithelium region. Each group included at least five different cochlear explants, and each explant was sampled in 10 randomly chosen areas.
To evaluate the number of GFP- and/or myosin 7A-immunoreactive cells, at least 10 cochleae in each experimental group were examined. Only the cells located in the mid-basal turn of the organ of Corti were counted. We counted the myosin 7A-immunoreactive cells in each 200 μm segment along the length of the cochlear sensory epithelium and randomly sampled five areas in each cochlea. Myosin 7A-immunoreactive cells were counted 8 days after the virus infection. When calculating the infection rate, we counted the Sox2-immunoreactive cells and GFP-Sox2 double-immunoreactive cells in the sensory epithelium. We pooled the number of Sox2-immunoreactive cells in the sensory epithelium in five areas of each cochlea. When calculating the conversion rate,we counted the GFP-immunoreactive cells and GFP-myosin 7A double-immunoreactive cells in the sensory epithelium.We pooled the number of GFP-immunoreactive cells in the sensory epithelium in five areas of the cochlea.
BrdU-incorporation assay to test cell proliferation
To test the proliferation of supporting cells, BrdU (Sigma)was used at a final concentration of 10 μM. We added the BrdU when we first changed the medium after Atoh1 infection and kept it in the medium until the end of the culture period. BrdU incorporation was detected at 7 days post infection using an mouse-anti-BrdU antibody at a concentration of 1:400 (Sigma). The cultured preparations were incubated with 2 M HCl for 30 minutes before permeabilization using Triton-X 100.
RNA extraction and quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR)
For qRT-PCR, RNA was purified from four explanted cochlea of each group 72 hours afterAd5-GFP-Atoh1infection using an RNeasy Plus Micro extraction Kit (Code No. 74034;Qiagen, Germany) and reverse-transcribed using a High Capacity RNA-to-cDNA kit (Code No. RR036A; TaKaRa,Japan). The qRT-PCR was performed in duplicate using a PreMix SYBR Green kit (Code No.RR420A; TaKaRa, Japan)on a 7500HT Fast Real-Time PCR System (Applied Biosystems, Waltham, MA, USA). All qRT-PCR reactions were performed in triplicate and relative quantification of gene expression was analyzed using the 2?ΔΔCTmethod (Livak and Schmittgen, 2001) with the house keeping gene β-actin as the endogenous reference.
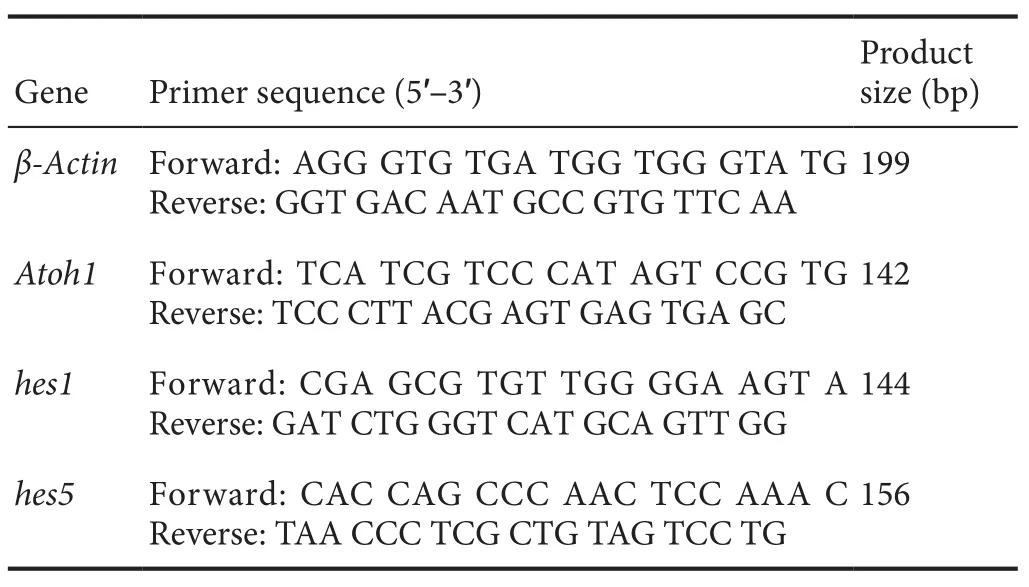
Primers used for genotyping are listed as follows:
Statistical analysis
All data are presented as the mean ± SEM. Statistical analyses were conducted using Microsoft Excel and GraphPad Prism 6.0 software (GraphPad Software, USA). One-way analysis of variance followed by least a signi ficant differencepost hoctest was used to analyze the data;P< 0.05 was considered statistically signi ficant.
Results
Atoh1 overexpression mainly induced the formation of ectopic hair cells
The explants were cultured in medium containing 5 % FBS for 24 hours before adenovirus infection. Eight days afterAd5-GFP-Atoh1infection, GFP-Myosin 7A double immunoreactive cells were observed to be restricted to the lesser epithelial ridge, which was external to the outer hair cells(Figure 1A). The greater epithelial ridge was also a main target of the adenovirus (data not shown), which was consistent with the findings of previous studies (Zheng and Gao, 2000;Woods et al., 2004; Yang et al., 2012, 2013). For the GFP alone control, no myosin 7A-GFP double-immunoreactive cells were detected in the lesser epithelial ridge (Figure 1C),suggesting that the formation of these hair cells was dependent on Atoh1 expression. TheGFP-Atoh1virus vector failed to infect the sensory epithelium, thus no new hair cells were found in situ(Figure 1A, white bracket). We also used neomycin to destroy the original hair cells to increase the virus infection rate, but this was not effective. Although the original hair cells were killed by neomycin, none of the supporting cells were immunoreactive for GFP after virus infection(Figure 2B, white bracket), indicating that cells in sensory epithelium were hard to infect with adenovirus.
Increasing the width of the sensory epithelium increased the adenovirus infection rate in the organ of Corti
The middle-basal turns of the cochlear explants were treated with 1.5 mM neomycin to kill all of the normal hair cells,as described in the methods section, and then were infected withAd5-GFP. In samples that were fixed 8 days after virus infection, few myosin 7A-immunoreactive cells were observed (Figure 2B–D), and Sox2-immunoreactive nuclei in supporting cells were present at lower levels (Figure 2A–D), indicating that treatment with 1.5 mM neomycin for 24 hours could destroy all of the normal hair cells of the middle-basal turn in culture. When the cultured explants were incubated with medium containing 5% FBS for 48 hours before adenovirus infection, GFP-immunoreactive cells were also seldom detected in the sensory epithelium (Figure 2C).When we increased the FBS concentration from 5% to 10%and prolonged the FBS application from 24 to 48 hours,many GFP-immunoreactive cells were observed in the sensory epithelium (Figure 2D).
We next investigated whether the adenovirus virus infection rate in the sensory epithelium depends on the width of sensory epithelium. In the 5% FBS for 24 hours group, the average width of organ of Corti was similar to that in the non-cultured explants (P> 0.05). There were no GFP-immunoreactive cells in the sensory epithelium. The average width of the organ of Corti in the 5% FBS for 48 hours group was signi ficantly wider than that in the no culture group (P< 0.05), and 28 ± 12% of the cells in the sensory epithelium were immunoreactive for GFP. In the 10% FBS for 48 hours group, the width of the sensory epithelium was signi ficantly wider than that in the other groups (P< 0.05). The supporting cells in the group were fully extended and 88 ± 10% of the cells in the sensory epithelium were immunoreactive for GFP. These results implied that increasing the FBS concentration and prolonging the duration of culture increased the width of sensory epithelium and this also increased the adenovirus infection rate.
DAPT enhanced Atoh1 transcription to increase the quantity of new hair cells in situ
To determine whether Atoh1 could induce the formation of new hair cellsin situ, 1.5 mM neomycin was applied to kill almost all of the normal inner and outer hair cells andAd5-GFPorAd5-GFP-Atoh1was applied after 48 hours of culture in 10% FBS medium. After infection with onlyAd5-GFP, few(Figure 3A, E) myosin 7A-immunoreactive cells were detected in the sensory epithelium and no myosin 7A-immunoreactive cells were detected in the lesser epithelial ridge. After infection withAd5-GFP-Atoh1, both the sensory epithelium and lesser epithelial ridge contained myosin 7A-immunoreactive cells (Figure 3C, E). Thus, the supporting cells in the sensory epithelium formed new hair cells when induced by Atoh1.
We also applied DAPT alone after the normal hair cells were destroyed by neomycin; however, no myosin 7A-immunoreactive cells were detected (per 200 μm), indicating that DAPT alone could not induce the formation of new hair cells (Figure 3B, E).
To determine whether DAPT could enhance Atoh1 activity and induce the formation of more new hair cellsin situ,we combined DAPT application withAtoh1infection and then counted the myosin 7A-immunoreactive cells in the sensory epithelium. When DAPT was applied afterAtoh1infection, the number of myosin 7A-immunoreactive cells per 200 μm in the sensory epithelium (Figure 3D, E) was two times higher than without DAPT (P< 0.05). The results demonstrated that DAPT enhanced the activity of Atoh1,leading to the formation of many more new hair cells, by increasing the rate of conversion of supporting cells to hair cells.
Meanwhile, we detected the transcriptional levels of Atoh1, hes1 and hes5 in the different groups using qRTPCR. DAPT significantly increased the Atoh1 expression level and decreased hes1 and hes5 levels in the DAPT alone and Atoh1-DAPT groups (P< 0.01; Figure 3F). The Atoh1 expression level in the DAPT group was signi ficantly lower than in the adenovirus-Atoh1 group, which was lower than in the adenovirus-Atoh1-DAPT group (P< 0.01; Figure 3F).
DAPT combined with Atoh1 induced new hair cell formation via transdifferentiation
To investigate whether DAPT or Atoh1 affected the cell cycle of the supporting cells or promoted the proliferation of the supporting cells to generate new hair cellsin situ, we administered BrdU from the time of DAPT and/orAd5-GFP-Atoh1application until the end of the culture, after neomycin insult. For the control group, we administered BrdU from the timeAd5-GFPinfection after neomycin insult (Figure 4A). There were no Sox2-BrdU double immunoreactive cells in the sensory epithelium, suggesting the supporting cells did not proliferate in the culture conditions.We also observed no Sox2-BrdU double-immunoreactive cells or myosin-BrdU double-immunoreactive cells in the sensory epithelium when there was new hair cell formationin situ(Figure 4B, C). This indicates that DAPT enhanced the Atoh1-mediated induction of new hair cell formationviatransdifferentiation without prior proliferation, which differs from the process that occurs in lower vertebrates.
Compared with the cells in lesser epithelial ridge, the supporting cells were quiescent even in the high-FBS medium for a prolonged duration of culture (Figure 4A–C); thus,none of the supporting cells in the sensory epithelium were immunoreactive for BrdU. Further, Atoh1 expression did not force the supporting cells in the sensory epithelium to proliferate, as none of the supporting cells in the sensory epithelium was immunoreactive for BrdU (Figure 4B).
Discussion

Figure 1 Atoh1 overexpression induced ectopic hair cell formation in the lesser epithelial ridge.

Figure 2 Adenovirus infection rate in the organ of Corti depends on width of the sensory epithelium.
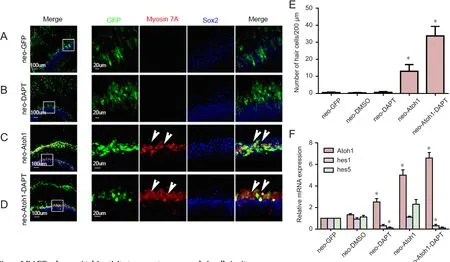
Figure 3 DAPT enhances Atoh1 activity to generate more new hair cells in situ.
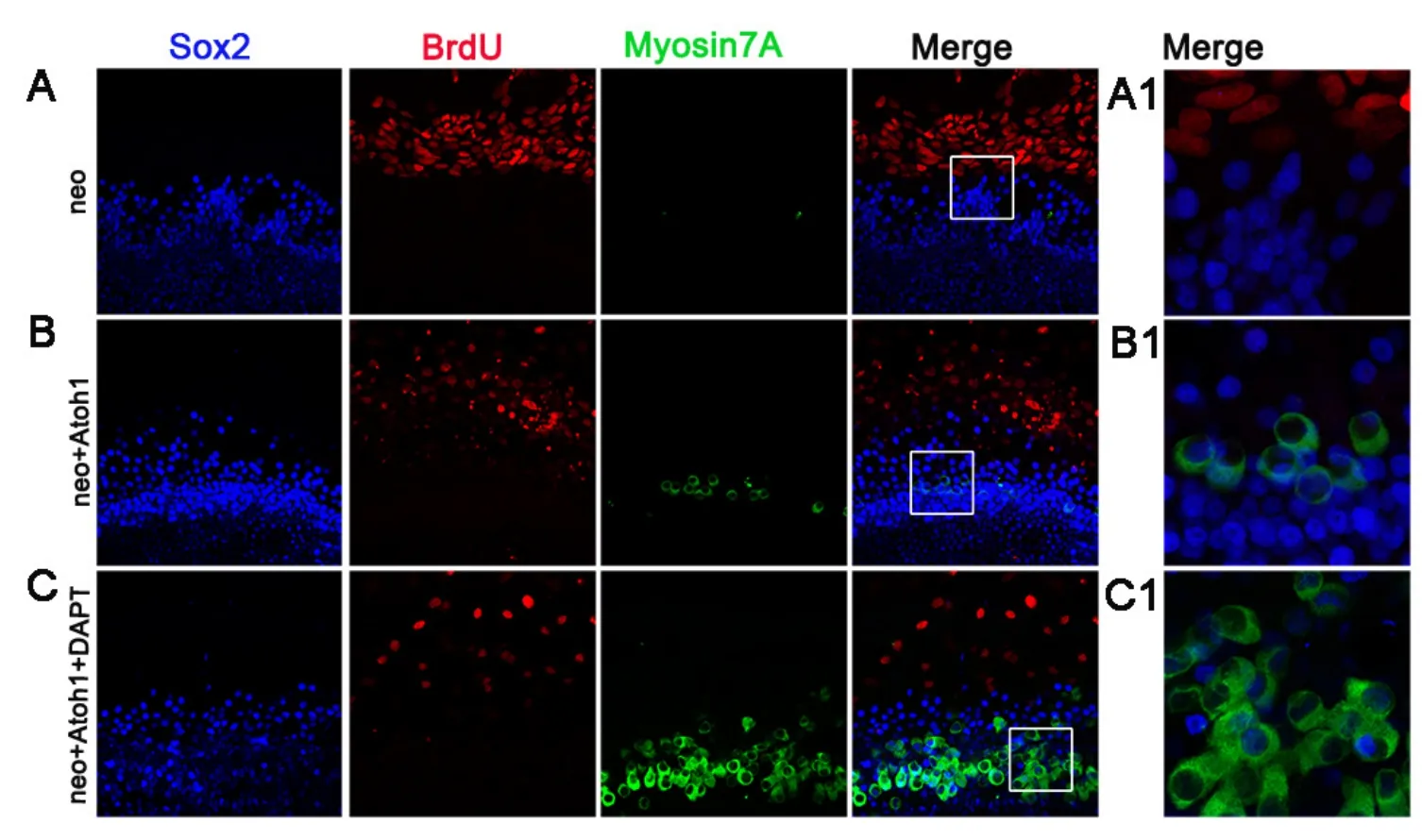
Figure 4 New hair cell formation in situ via transdifferentiation.
Previous studies have shown that Atoh1 overexpressionviaadenovirus vector infection is a powerful method of inducing hair cell formation in mammals (Zheng and Gao, 2000;Kawamoto et al., 2003; Woods et al., 2004; Izumikawa et al., 2005; Gubbels et al., 2008; Huang et al., 2009; Zhao et al., 2011; Chen et al., 2013). Adenovirus-mediated Atoh1 overexpression has mainly focused on the lesser epithelial ridge and the greater epithelial ridge, where it induces ectopic hair cell regeneration (Bermingham et al., 1999; Kelly et al., 2012; Liu et al., 2012; Jin et al., 2013; Luo et al., 2017).The supporting cells in the sensory region of the rat cochlea are tightly organized and difficult to transfect, thus new hair cells were hardly producedin situin rat cochlear explants(Bermingham et al., 1999; Luo et al., 2014, 2017). Inducing new hair cell formation in sensory epithelium after hair cell loss is an important approach to restore hearing, so the low adenovirus infection rate and low hair-cell transformation rate in the sensory epithelium are the problems that need to be solved.
In the present study, we chose the middle-basal turns of the cochlea and applied 1.5 mM neomycin for 24 hours to destroy almost all of the original hair cells. By enhancing the FBS concentration to 10% and prolonging the culture duration to 48 hours before virus infection, the width of sensory epithelium in the rat cochleae was significantly enhanced.Consequently, the adenovirus infection rate in the sensory region was greatly improved. The adenovirus serotype 5 vector that encoded Atoh1 infected the sensory regionin situin rat cochleae. We successfully generated new hair cells after viral gene transfer of Atoh1 in situ, but the number was insufficient.
When we added 10 μM DAPT in combination withAd5-GFP-Atoh1infection after neomycin treatment, many more GFP/myosin 7A double-immunoreactive cells were present than in cochleae without DAPT treatment. DAPT, a γ-secretase inhibitor, can block the Notch signaling pathway and reduce Hes1 and Hes5 expression, thereby eliminating their suppression of Atoh1 expression (Lanford et al., 1999; Zine et al., 2000; Zine et al., 2001; Zine and de Ribaupierre, 2002;Daudet and Lewis, 2005; Doetzlhofer et al., 2009; Mizutari et al., 2013). Concurrent application of DAPT with Atoh1 overexpression increased the rate of supporting cell conversion and produced a greater number of new hair cells in situ, which is a transdifferentiation process (Yamamoto et al.,2006; Kopan and Ilagan, 2009; Mizutari et al., 2013). Quantitative PCR results showed that DAPT enhanced the Atoh1 transcript levels as well as inhibiting Hes1 and Hes5 transcript levels. Thus, DAPT treatment signi ficantly enhanced Atoh1 activity and greatly improved the rate of new hair cell formation through the conversion of supporting cells to hair cells, producing many more hair cellsin situ.
The application of 10 μM DAPT alone could not induce new hair cell formation in the sensory epithelium and lesser epithelial ridge. According to our quantitative PCR results,although DAPT application increased the Atoh1 expression level, it was insufficient to induce hair cell formation. Thus,hair cell formation is Atoh1 level-dependent and Atoh1 expression is vital for hair cell formation.
In conclusion, ourin vitrostudy demonstrated that increasing the width of the sensory epithelium enhanced the adenovirus infection rate and supporting cells possess the ability to transdifferentiate into hair cellsin situ. DAPT alone did not induce hair cell formationin situor in the lesser epithelial ridge, as the Atoh1 expression level was not high enough. With Atoh1 infection, DAPT further enhanced the Atoh1 expression level to increase the conversion rate and promote the generation of more hair cellsviatransdifferentiation, which will lay foundation for the hair cell regenerationin vivo. Thus, the synergetic effect of Atoh1 overexpression and γ-secretase inhibitors is a promising approach for hearing recovery in the future.
Author contributions:WWL, ZH, DDR, and XWW performed experiments, and analyzed the data. FLC and JMY conceived and designed the study, and discussed the results. YJM directed and supervised the entire project. WWL, ZH, and JMY wrote the paper. All authors approved the final version of the paper.
Con flicts of interest:None declared.
Research ethics:The study protocol was approved by the Animal Ethics
Committee of Eye&ENT Hospital of Fudan University of China. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer reviewer:Chaoliang Tang, Wuhan University Renmin Hospital, China.
Atkinson PJ, Wise AK, Flynn BO, Nayagam BA, Richardson RT (2014)Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs. PLoS One 9:e102077.
Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N,Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY (1999) Math1:an essential gene for the generation of inner ear hair cells. Science 284:1837-1841.
Chen P, Johnson JE, Zoghbi HY, Segil N (2002) The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 129:2495-2505.
Chen Y, Yu H, Zhang Y, Li W, Lu N, Ni W, He Y, Li J, Sun S, Wang Z,Li H (2013) Cotransfection of Pax2 and Math1 promote in situ cochlear hair cell regeneration after neomycin insult. Sci Rep 3:2996.
Chonko KT, Jahan I, Stone J, Wright MC, Fujiyama T, Hoshino M,Fritzsch B, Maricich SM (2013) Atoh1 directs hair cell differentiation and survival in the late embryonic mouse inner ear. Dev Biol 381:401-410.
Corwin JT, Cotanche DA (1988) Regeneration of sensory hair cells after acoustic trauma. Science 240:1772-1774.
Daudet N, Lewis J (2005) Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development 132:541-551.
Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N (2009) Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti.Dev Cell 16:58-69.
Fuchs PA, Glowatzki E, Moser T (2003) The afferent synapse of cochlear hair cells. Curr Opin Neurobiol 13:452-458.
Fujikawa T, Petralia RS, Fitzgerald TS, Wang YX, Millis B, Morgado-Diaz JA, Kitamura K, Kachar B (2014) Localization of kainate receptors in inner and outer hair cell synapses. Hear Res 314:20-32.
Gao Z, Kelly MC, Yu D, Wu H, Lin X, Chi FL, Chen P (2016) Spatial and age-dependent hair cell generation in the postnatal mammalian Utricle. Mol Neurobiol 53:1601-1612.
Grant L, Yi E, Goutman JD, Glowatzki E (2011) Postsynaptic recordings at afferent dendrites contacting cochlear inner hair cells: monitoring multivesicular release at a ribbon synapse. J Vis Expdoi:10.3791/2442.
Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV (2008)Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature 455:537-541.
Han Z, Yang JM, Chi FL, Cong N, Huang YB, Gao Z, Li W (2010)Survival and fate of transplanted embryonic neural stem cells by Atoh1 gene transfer in guinea pigs cochlea. Neuroreport 21:490-496.
Huang Y, Chi F, Han Z, Yang J, Gao W, Li Y (2009) New ectopic vestibular hair cell-like cells induced by Math1 gene transfer in postnatal rats. Brain Res 1276:31-38.
Ikeda R, Pak K, Chavez E, Ryan AF (2015) Transcription factors with conserved binding sites near ATOH1 on the POU4F3 gene enhance the induction of cochlear hair cells. Mol Neurobiol 51:672-684.
Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y (2005) Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med 11:271-276.
Jin K, Ren DD, Chi FL, Yang JM, Huang YB, Li W (2013) Changes in ADF/destrin expression in the development of hair cells following Atoh1-induced ectopic regeneration. Exp Ther Med 6:177-183.
Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y (2003)Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci 23:4395-4400.
Kelly MC, Chang Q, Pan A, Lin X, Chen P (2012) Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci 32:6699-6710.
Kopan R, Ilagan MX (2009) The canonical Notch signaling pathway:unfolding the activation mechanism. Cell 137:216-233.
Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW (1999) Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet 21:289-292.
Li W, Wu J, Yang J, Sun S, Chai R, Chen ZY, Li H (2015) Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc Natl Acad Sci U S A 112:166-171.
Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, Zindy F,Gan L, Roussel MF, Zuo J (2012) Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci 32:6600-6610.
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T))Method. Methods 25:402-408.
Luo WW, Yang JM, Han Z, Yuan YS, Sheng HB, Liu X, Chi FL (2014)Atoh1 expression levels de fine the fate of rat cochlear nonsensory epithelial cells in vitro. Mol Med Rep 10:15-20.
Luo WW, Ma R, Cheng X, Yang XY, Han Z, Ren DD, Chen P, Chi FL,Yang JM (2017) Afferent synaptogenesis between ectopic hair-celllike cells and neurites of spiral ganglion induced by Atoh1 in mammals in vitro. Neuroscience 357:185-196.
Masuda M, Pak K, Chavez E, Ryan AF (2012) TFE2 and GATA3 enhance induction of POU4F3 and myosin VIIa immunreactive positive cells in nonsensory cochlear epithelium by ATOH1. Dev Biol 372:68-80.
Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge AS (2013) Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 77:58-69.
Ni W, Lin C, Guo L, Wu J, Chen Y, Chai R, Li W, Li H (2016) Extensive supporting cell proliferation and mitotic hair cell generation by in vivo genetic reprogramming in the neonatal mouse cochlea. J Neurosci 36:8734-8745.
Pan N, Jahan I, Kersigo J, Duncan JS, Kopecky B, Fritzsch B (2012) A novel Atoh1 “self-terminating” mouse model reveals the necessity of proper Atoh1 level and duration for hair cell differentiation and viability. PLoS One 7:e30358.
Ren H, Guo W, Liu W, Gao W, Xie D, Yin T, Yang S, Ren J (2016)DAPT mediates atoh1 expression to induce hair cell-like cells. Am J Transl Res 8:634-643.
Ryals BM, Rubel EW (1988) Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science 240:1774-1776.
Staecker H, Praetorius M, Baker K, Brough DE (2007) Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol 28:223-231.
Walters BJ, Coak E, Dearman J, Bailey G, Yamashita T, Kuo B, Zuo J(2017) In vivo interplay between p27Kip1, GATA3, ATOH1, and POU4F3 converts non-sensory cells to hair cells in adult mice. Cell Rep 19:307-320.
Woods C, Montcouquiol M, Kelley MW (2004) Math1 regulates development of the sensory epithelium in the mammalian cochlea.Nat Neurosci 7:1310-1318.
Yamamoto N, Tanigaki K, Tsuji M, Yabe D, Ito J, Honjo T (2006) Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. J Mol Med 84:37-45.
Yang J, Bouvron S, Lv P, Chi F, Yamoah EN (2012) Functional features of trans-differentiated hair cells mediated by Atoh1 reveals a primordial mechanism. J Neurosci 32:3712-3725.
Yang J, Cong N, Han Z, Huang Y, Chi F (2013) Ectopic hair cell-like cell induction by Math1 mainly involves direct transdifferentiation in neonatal mammalian cochlea. Neurosci Lett 549:7-11.
Yang SM, Chen W, Guo WW, Jia S, Sun JH, Liu HZ, Young WY, He DZ (2012b) Regeneration of stereocilia of hair cells by forced Atoh1 expression in the adult mammalian cochlea. PLoS One 7:e46355.
Zhao LD, Guo WW, Lin C, Li LX, Sun JH, Wu N, Ren LL, Li XX, Liu HZ, Young WY, Gao WQ, Yang SM (2011) Effects of DAPT and Atoh1 overexpression on hair cell production and hair bundle orientation in cultured Organ of Corti from neonatal rats. PLoS One 6:e23729.
Zheng JL, Gao WQ (2000) Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci 3:580-586.
Zine A, de Ribaupierre F (2002) Notch/Notch ligands and Math1 expression patterns in the organ of Corti of wild-type and Hes1 and Hes5 mutant mice. Hear Res 170:22-31.
Zine A, Van De Water TR, de Ribaupierre F (2000) Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development 127:3373-3383.
Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F (2001) Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci 21:4712-4720.
How to cite this article:Luo WW, Han Z, Ren DD, Wang XW, Chi FL, Yang JM (2017) Notch pathway inhibitor DAPT enhances Atoh1 activity to generate new hair cells in situ in rat cochleae. Neural Regen Res 12(12):2092-2099.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81420108010, 81271084, 81200740, 81371093.
Graphical Abstract

*Correspondence to:Fang-lu Chi or Juan-mei Yang,Ph.D., chifanglu@126.com or yangjuanmei1982@126.com.#These authors contributed
equally to this work.
orcid:0000-0002-3243-0393
(Fang-lu Chi)0000-0001-5125-3532(Juan-mei Yang)
10.4103/1673-5374.221169
2017-10-25
Copyedited by Turnley A, Robens J, Wang J, Li CH, Qiu Y, Song LP, Zhao M
- 中國神經(jīng)再生研究(英文版)的其它文章
- Roles of neural stem cells in the repair of peripheral nerve injury
- Quanti fication of intermuscular and intramuscular adipose tissue using magnetic resonance imaging after neurodegenerative disorders
- Long non-coding RNA NONMMUG014387 promotes Schwann cell proliferation after peripheral nerve injury
- Autologous transplantation with fewer fibers repairs large peripheral nerve defects
- Topiramate as a neuroprotective agent in a rat model of spinal cord injury
- Diffusion tensor imaging of spinal microstructure in healthy adults: improved resolution with the readout segmentation of long variable echo-trains

