Long non-coding RNA NONMMUG014387 promotes Schwann cell proliferation after peripheral nerve injury
Bin Pan, Zhong-ju Shi, Jia-yin Yan, Jia-he Li, Shi-qing Feng
Department of Orthopedics, Tianjin Medical University General Hospital, Tianjin, China
Long non-coding RNA NONMMUG014387 promotes Schwann cell proliferation after peripheral nerve injury
Bin Pan#, Zhong-ju Shi#, Jia-yin Yan, Jia-he Li, Shi-qing Feng*
Department of Orthopedics, Tianjin Medical University General Hospital, Tianjin, China
Schwann cells play a critical role in peripheral nerve regeneration through dedifferentiation and proliferation. In a previous study, we performed microarray analysis of the sciatic nerve after injury. Accordingly, we predicted that long non-coding RNA NONMMUG014387 may promote Schwann cell proliferation after peripheral nerve injury, as bioinformatic analysis revealed that the target gene of NONMMUG014387 was collagen triple helix repeat containing 1 (Cthrc1). Cthrc1 may promote cell proliferation in a variety of cells by activating Wnt/PCP signaling. Nonetheless, bioinformatic analysis still needs to be veri fied by biological experiment. In this study, the candidate long non-coding RNA, NONMMUG014387, was overexpressed in mouse Schwann cells by recombinant adenovirus transfection. Plasmid pHBAd-MCMV-GFP-NONMMUG014387 and pHBAd-MCMV-GFP were transfected into Schwann cells. Schwann cells were divided into three groups: control (Schwann cells without intervention), Ad-GFP (Schwann cells with GFP overexpression), and Ad-NONMMUGO148387 (Schwann cells with GFP and NONMMUGO148387 overexpression). Cell Counting Kit-8 assay was used to evaluate proliferative capability of mouse Schwann cells after NONMMUG014387 overexpression. Polymerase chain reaction and western blot assay were performed to investigate target genes and downstream pathways of NONMMUG014387. Cell proliferation was signi ficantly increased in Schwann cells overexpressing lncRNA NONMMUG014387 compared with the other two groups. Further, compared with the control group, mRNA and protein levels of Cthrc1, Wnt5a, ROR2, RhoA, Rac1, JNK, and ROCK were visibly up-regulated in the Ad-NONMMUGO148387 group. Our findings con firm that long non-coding RNA NONMMUG014387 can promote proliferation of Schwann cells surrounding the injury site through targeting Cthrc1 and activating the Wnt/PCP pathway.
nerve regeneration; peripheral nerve injury; Schwann cells; long non-coding RNAs; proliferation; Wnt/PCP pathway; Cell Counting Kit-8 assay; adenovirus overexpression; sciatic nerve; Cthrc1; neural regeneration
Introduction
Peripheral nerve injury is a common clinical problem that greatly compromises a patient’s quality of life (Kurtzke,1982; Dubuisson and Kline, 2002; Gordon et al., 2015). Although the peripheral nervous system shows regenerative ability following injury, functional outcome is often unsatisfactory, especially when axons are transected (Dubuisson and Kline, 2002; Bamba et al., 2016; Wang et al., 2016).Thus, understanding the mechanisms involved in peripheral nerve injury and regeneration is important. Schwann cells form the myelin sheath of the peripheral nervous system and are the first cells activated following peripheral nerve injury (Jessen and Mirsky, 2005). Schwann cells modulate their phenotype by rejecting myelin in response to injury (Freidin et al., 2009; Altun and Kurutas, 2016). Subsequently, through proliferation and migration, Schwann cells form Bungner bands to scavenge degenerated myelin and axon debris, and facilitate correct axonal regeneration (Reichert et al., 1994;Wang et al., 2012; Yao et al., 2013; Qian et al., 2016).
Long non-coding RNAs (lncRNAs) contain more than 200 nucleotides (Rinn and Chang, 2012). According to accumulating evidence, researchers have shown that lncRNAs regulate protein-coding gene expression at epigenetic, transcriptional, and post-transcriptional levels. Consequently,the role of lncRNAs in regulating diverse biological and pathological processes has gradually been recognized (Gupta et al., 2010; Min et al., 2016). Sense lncRNA loci are on the same DNA strand as the corresponding gene, but unlike other subgroups of lncRNAs, studies of sense lncRNAs are sparse(Wright, 2014). The sense lncRNA, Oct4P4, regulates Oct4P4 gene expression at the epigenetic level to impede mouse embryonic stem cell renewal (Scarola et al., 2015; Wongtrakoongate et al., 2015). Additionally, Wongtrakoongate et al. (2015)identified a sense lncRNA steroid receptor RNA activator that regulated gene expression at the transcriptional level.These findings indicate important roles of sense lncRNAs in regulation of gene expression. In a previous study, we performed microarray analysis of the sciatic nerve after injury.We identi fied a sense lncRNA, NONMMUG014387, that was upregulated after peripheral nerve injury. Bioinformatic analysis revealed that the target gene of NONMMUG014387 was collagen triple helix repeat containing 1 (Cthrc1) (Pan et al.,2017). Interestingly, we found that NONMMUGO14387 may promote Schwann cell proliferation in a Schwann cell line.LncRNA NONMMUGO14387 is located at chr15:39,076,900-39,079,473, and expressed in multiple tissues such as peripheral nerve, hippocampus, lung, spleen, and thymus, although its role is still unknown.
Cthrc1 is a highly conserved glycoprotein that was first identi fied by Pyagay et al. (2005) after comparing impaired and normal arteries. Cthrc1 promotes cell proliferation in a variety of cells by activating Wnt/ planar cell polarity(PCP) signaling (Yang et al., 2015). Cthrc1 is also involved in selective activation of Wnt pathways (Sang et al., 2016).Wnt signaling pathways are categorized into two types:the canonical Wnt/β-catenin pathway, and non-canonical pathways, which consist of Wnt/PCP and Wnt/Ca2+pathways (Yamamoto et al., 2008). Wnt pathway activation is the result of the interaction between Wnt proteins and Frizzled(Fzd) receptors and pathway-speci fic coreceptors (Habas and Dawid, 2005). The canonical pathway coreceptors include low-density lipoprotein receptor-related protein (LRP)-5 and LRP6 (Takada et al., 2005), while the non-canonical coreceptor is receptor tyrosine kinase like orphan receptor 2 (ROR2)(Tamai et al., 2000). Cthrc1 is a Wnt cofactor protein that selectively activates the Wnt/PCP pathway by forming the Cthrc1-Wnt-Fzd/Ror2 complex (Yamamoto et al., 2008).
To further examine the role of the lncRNA, NONMMUG014387, we isolated and cultured Schwann cells from C57BL6 mice. Next, we performed experiments to examine the effect of NONMMUG014387 on Schwann cell proliferation, and determine whether NONMMUGO14387 regulates Cthrc1 expression and participates in the Wnt/PCP pathway.
Materials and Methods
Animals
A total of 12 male speci fic-pathogen-free C57BL6 mice (aged 2 months old and weighing 18–22 g) were obtained from the Animal Lab Center of Academy of Military Medical Sciences of China (production license number: SCXK (Jun) 2012-0004; application license number: SYXK (Jin) 2014-0002).All mice were housed and maintained on 12-hour light/dark cycles, and allowed free access to food and water. Mice were kept in standard cages, with six animals per cage.
Schwann cells were isolated from the mice.
Mice with Schwann cells without intervention were used as the control group. The Ad-GFP and Ad-NONMMUGO148387 groups were transfected with Ad-GFP and NONMMUGO148387 overexpression vectors.
The study protocol was approved by the Animal Ethics Committee of Tianjin Medical University of China (approval number: TMUaMEC 2017008). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animal (NIH Publication No. 85-23, revised 1985).
Sciatic nerve injury model and Schwann cell culture
Twelve 2-month-old mice were anesthetized before bilateral ligation of the sciatic nerves. Mice were intraperitoneally anesthetized using 2% chloral hydrate (0.2 mL/10 g). The sciatic nerve was exposed, and a 10-0 suture used to tie a knot 1 cm distal to the ischial tuberosity to completely constrict the nerve for 60 seconds. This elicited a re flex response(Cobianchi et al., 2017), which allowed complete transection of neural fibers without breaking the epineurium. The suture was then carefully released and the lesion site marked with a 10-0 Ethilon suture (Boivin et al., 2007). After the muscles and skin were sutured, the mice were maintained for one week. Afterwards, mice were sacrificed by an overdose of intraperitoneal injection of 10% chloral hydrate (0.5 mL/10 g), and 10-mm long sciatic nerve sections distal to the ligations (1 cm distal to the ischial tuberosity) were collected.Schwann cells were isolated after digestion and filtration of sciatic nerve fragments.Schwann cell culture medium was prepared by supplementing DMEM with 10% fetal bovine serum, 2 μM forskolin, 10 ng/mL heregulin-β-1, and 50 ng/mL basic fibroblast growth factor according to a previous method (Wang et al.,2013). DMEM and fetal bovine serum were purchased from Hyclone (Shanghai, China). Forskolin was obtained from Sigma (St. Louis, MO, USA). Heregulin-β-1 and basic fibroblast growth factor were from Peprotech, Inc. (Rocky Hill,NJ, USA). Schwann cells were stained with an anti-S100 antibody for identi fication (Yu et al., 2017).
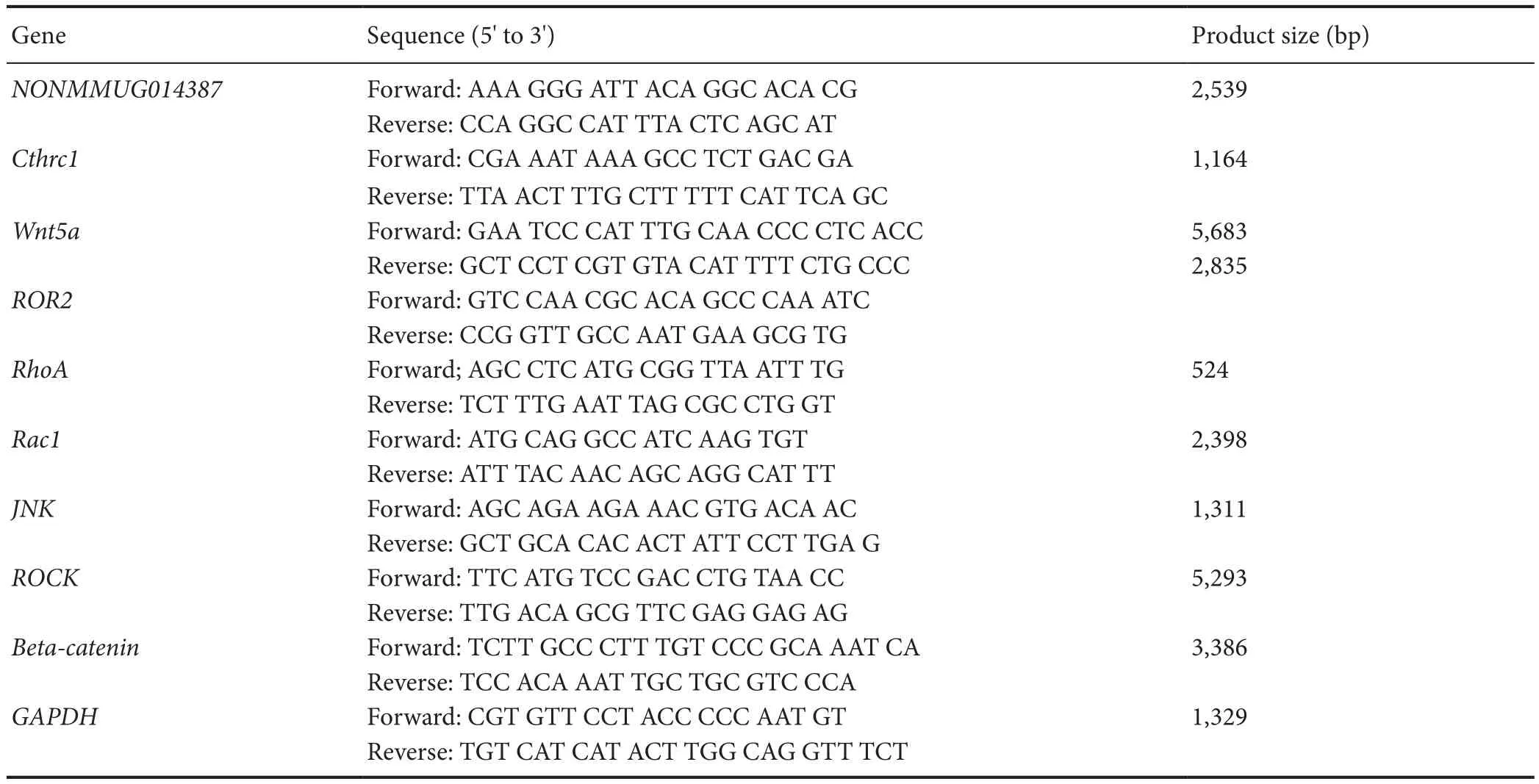
Table 1 Primer sequence
Construction and transfection of recombinant adenovirus vectors
For ectopic expression, plasmid pHBAd-MCMV-GFPNONMMUG014387 and pHBAd-MCMV-GFP were transfected into Schwann cells. Full-length NONMMUG014387(Hanbio Biotechnology Co., Shanghai, China) was subcloned into pHBAd/MCMV/GFP vector (Hanbio Biotechnology Co.) and regulated by the murine cytomegalovirus(MCMV) promoter. Green fluorescent protein (GFP) was regulated by the CMV promoter. After sequencing, adenoviral vector DNAs and packaging vectors were transfected into 293T cells (Hanbio Biotechnology Co.). Lipo fiterTM(Hanbio Biotechnology Co.) was used for transfection. Forty-eight hours after cotransfection, adenovirus in supernatants was collected and filtered through 0.45 μm filters. Finally, adenovirus was puri fied using ultracentrifugation and titer determination. The final concentration of recombinant Ad-GFP was 2 × 1010PFU/mL, and recombinant Ad-NONMMUGO148387 was 1 × 1010PFU/mL. Schwann cells were then transfected with Ad-GFP and Ad-NONMMUGO148387 for 36 hours, and subsequently reseeded for total RNA extraction. Polymerase chain reaction (PCR) was used to detect expression of NONMMUGO148387.
Cell proliferation assay
After Schwann cells were overexpressed with GFP and NONMMUGO148387, cell proliferation experiments were performed using a cell counting kit-8 (CCK-8) to determine Schwann cell proliferation. Cells in each group were inoculated in 96-well plates with 3 × 104cells per well and three repeated wells for each group. Each group was incubated for 0, 1, 2, 3, and 4 days at 37°C. Then, 10 μL CCK-8 solution was added to each well, and incubation terminated after 3 hours.The optical density (OD) value of each well was measured at 450 nm with a microplate reader (Hanbio Biotechnology Co.).The corresponding OD value represents cell proliferation.
Quantitative real-time PCR
After overexpression of Schwann cells with GFP and NONMMUGO148387, real-time PCR was performed. Total RNA was extracted using TRIzol, according to the manufacturer’s instructions. RNA was reverse transcribed into cDNA using a kit (Hanbio Biotechnology Co.). Quantitative PCR was performed using 20 μL reactions, which contained 1 μL cDNA template (1:100 dilution), 10 μL 2× Real-time PCR Master Mix, 0.8 μL forward primer (10 μM), 0.8 μL reverse primer(10 μM), and ddH2O. The reaction conditions were 95°C for 3 minutes, followed by 40 cycles of 95°C for 15 seconds,60°C for 15 seconds, and 72°C for 20 seconds. Glyceralde-hyde-3-phosphate-dehydrogenase (GAPDH) was used as the internal control. Relative changes in gene expression levels were analyzed using the 2?ΔΔCTmethod, as described previously (Schmittgen and Livak, 2008). Reverse transcriptase (Toyobo, Osaka, Japan), QPCR mix (Toyobo), BioTek ND5000(Toyobo), and LightCycler 96 (Roche, Basel, Switzerland)were used for real-time PCR. Gene expression in the control group was normalized to 1, while gene expression in the Ad-NONMMUGO148387 group was normalized to the control group (optical density ratio). All assays were performed in triplicate. The primer sequences are shown in Table 1.
Western blot assay
After Schwann cells were overexpressed with GFP and NONMMUGO148387, western blot assays were performed. Protein extracts were separated from Schwann cells using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDSPAGE) with 10% and 4% SDS polyacrylamide gels. Proteins were transferred to polyvinylidene fluoride membranes for 90 minutes. Membranes were then blocked using 5% non-fat dry milk for 1 hour at 37°C and incubated overnight at 4°C with a rabbit polyclonal antibody recognizing Cthrc1 (1:2,000; Abcam, Cambridge, UK), rabbit polyclonal antibody recognizing β-actin (1:2,000; Abcam), rabbit polyclonal antibody recognizing β-catenin (1:2,000; Abcam), rabbit polyclonal antibody recognizing Wnt family member 5A (Wnt5a) (1:2,000; Abcam), rabbit polyclonal antibody recognizing Ror2 (1:2,000;Abcam), rabbit polyclonal antibody recognizing ras homolog family member A (RhoA) (1:2,000; Abcam), rabbit polyclonal antibody recognizing RAS-related C3 botulinum substrate 1(Rac1) (1:2,000; Abcam), rabbit polyclonal antibody recognizing p-Jun N-terminal kinase (JNK) (1:2,000; Abcam), and rabbit polyclonal antibody recognizing Rho-associated coiledcoil containing protein kinase (ROCK) (1:2,000; Abcam).After washing with Tris-buffered saline with Tween, blots were incubated with horseradish peroxidase-labeled anti-rabbit secondary antibody (1:5,000; Santa Cruz Biotechnology,Santa Cruz, CA, USA) for 2 hours at room temperature. Densitometric analysis of resultant protein bands was performed using Quantity One software (Bio-Rad, Hercules, CA, USA).All grayscale values were normalized to β-actin (Wang et al.,2015). All assays were performed in triplicate.
Statistical analysis
All data are represented as the mean ± SD. SPSS 18.0 software(IBM, Armonk, IL, USA) was used for statistical analysis. Student’s t-test was used to compare samples from two different groups. One-way analysis of variance and Tukey’s post hoc test were used to compare samples from three different groups,and values of P < 0.05 considered statistically signi ficant.
Results
Successful construction of a lncRNA NONMMUG014387 overexpression model
To further investigate the function and role of the lncRNA NONMMUG014387 in nerve regeneration, we cultured Schwann cells from injured sciatic nerves. After digestion and filtration, the major cell components of sciatic nerve fragments included Schwann cells and fibroblasts. Schwann cells exhibited a spindle-like morphology and had ovoid nuclei with 2–3 projections from the cell body, while fibroblasts exhibited an irregular morphology and were larger than Schwann cells. Although Schwann cells were the major components, many fibroblasts remained after the first purification, whereas after the second puri fication, nearly all the cells were Schwann cells.
Subsequently, we overexpressed lncRNA NONMMUG014387 in Schwann cells using an adenovirus. The pHAd-MCMV-GFP overexpression carrier pro file is shown in Figure 1A. The target gene (i.e., NONMMUG014387)was inserted into the multiple cloning site (MCS) domain and regulated by the MCMV promoter. The carrier induced GFP expression, which was activated by the CMV promoter.After transfection, Schwann cells were divided into three groups: NONMMUG014387 overexpression group (Ad-NONMMUG014387-GFP), GFP group (Ad-GFP), and control group. As shown in Figure 1B, NONMMUG014387 expression in the Ad-NONMMUG014387-GFP group was markedly increased compared with the Ad-GFP and control groups, which demonstrates successful construction of the lncRNA NONMMUG014387 overexpression model.
NONMMUG014387 overexpression promoted Schwann cell proliferation
To determine the effect of lncRNA NONMMUG014387 overexpression on Schwann cell proliferation, we performed CCK-8 proliferation assays to determine the proliferation capacity of Schwann cells. As shown in Figure 1C, the proliferation ability of Schwann cells in the Ad-NONMMUG014387-GFP, Ad-GFP, and control groups were all significantly increased with increasing culture time. Indeed, no differences were observed among the three groups of Schwann cells at 1 day. However, after 1 day, cell proliferation was signi ficantly increased in Schwann cells overexpressing lncRNA NONMMUG014387 compared with cells in the other two groups.These data indicate that lncRNA NONMMUG014387 plays a role in promoting Schwann cell proliferation.
LncRNA NONMMUG014387 ampli fied Cthrc1 expression and activated the Wnt/PCP pathway
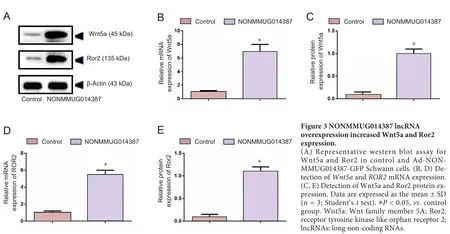
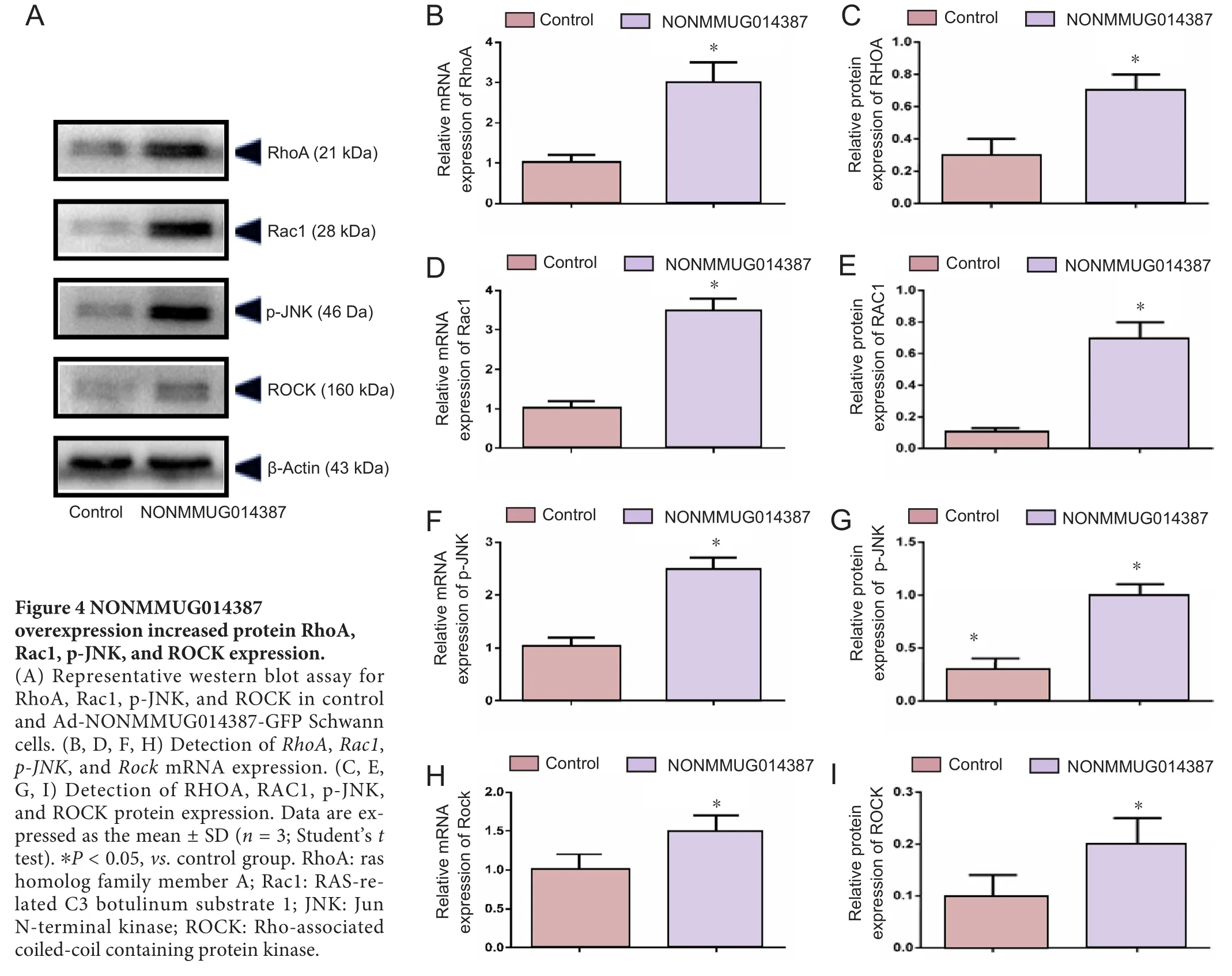
To further determine the mechanism of lncRNA NONMMUG014387-induced Schwann cell proliferation, we examined expression of Cthrc1 in Schwann cells overexpressing lncRNA NONMMUG014387. As shown in Figure 2A–C,mRNA and protein levels of Cthrc1 were up-regulated in Schwann cells overexpressing lncRNA NONMMUG014387,indicating that lncRNA NONMMUG014387 promotes Schwann cell proliferation by amplifying Cthrc1 expression.Because Cthrc1 is involved in selective activation of the Wnt pathway, we determined whether Wnt signaling is activated by Cthrc1 ampli fication in Schwann cells overexpressing NONMMUG014387. To examine activation of the Wnt/β-catenin pathway, we measured β-catenin expression. As shown in Figure 2D–F, compared with the control group,β-catenin protein was unchanged. To examine activation of Wnt/PCP signaling, we measured expression of critical proteins of the Wnt signaling pathway. The Wnt5a, ROR2, RhoA,Rac1, JNK, and ROCK proteins were significantly up-regulated in Schwann cells in the Ad-NONMMUG014387 group(Figures 3 and 4). This suggests that the Wnt/PCP pathway is induced by altered Cthrc1 expression in Schwann cells when lncRNA NONMMUG014387 is overexpressed.

Figure 1 NONMMUG014387 lncRNA overexpression promoted Schwann cell proliferation.
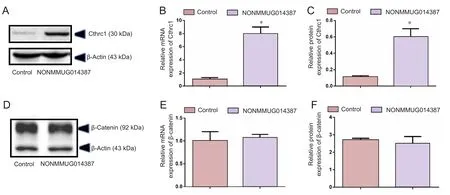
Figure 2 NONMMUG014387 lncRNA overexpression increased expression of Cthrc1.
Discussion
Peripheral nerve regeneration after injury requires dedifferentiation, proliferation, and migration of Schwann cells(Reichert et al., 1994; Rotshenker, 2011; Wang et al., 2012;Al-Zer and Kalbouneh, 2015). Our previous microarray results show that NONMMUGO14387 is up-regulated following peripheral nerve injury, while bioinformatic predictions show that lncRNA NONMMUG014387 plays a role in Schwann cells. Therefore, we performed experiments to examine the effect of lncRNA NONMMUGO14387 on Schwann cells. Through adenoviral overexpression of lncRNA NONMMUGO148387 in Schwann cells, we show that Schwann cell proliferation is significantly enhanced compared with control cells, indicating that lncRNA NONMMUG014398 promotes Schwann cell proliferation during nerve regeneration.
We further found that Cthrc1 expression levels are enhanced in Schwann cells overexpressing lncRNA NON-MMUGO14387. Cthrc1 is a multifunctional signaling molecule involved in a variety of cellular behavioral alterations.For example, Cthrc1 facilitates matrix extracellular signal-regulated kinase through extracellular signal-regulated kinase, which increases colon cancer cell invasiveness (Oishi et al., 2003). In addition, Cthrc1 activates angiopoietin-2 secretion to alter endothelial cell motility and facilitate angiogenesis during tumorigenesis (Kim et al., 2014). Studies have reported that Schwann cells exhibit active migration abilities upon Cthrc1 silencing (Lee et al., 2016). Cthrc1 plays a positive role in Schwann cell proliferation because Cthrc1 overexpression promotes Schwann cell proliferation, whereas Cthrc1 knockdown compromises Schwann cell proliferation(Zhou et al., 2014). In our experiments here, Schwann cells exhibit increased proliferation with increased Cthrc1 mRNA and protein levels, which is consistent with these findings.The mechanism of Cthrc1 regulation in Schwann cells is unknown, which led us to investigate the signaling pathways by which Cthrc1 promotes Schwann cell proliferation.
Cthrc1 selectively induces Wnt/PCP signaling by interacting with the extracellular Wnt-Fzd/Ror2 complex (Yamamoto et al., 2008; Sang et al., 2016). Selective activation of Wnt/PCP signaling by Cthrc1 has also been demonstrated in gastrointestinal stromal tumor cells (Apra et al.,2012). We further determined whether lncRNA NONMMUG014387-mediated Cthrc1 enhancement activates the Wnt/PCP pathway in Schwann cells. We found that Ror2,the coreceptor for the Wnt/PCP pathway, was enhanced with NONMMUGO14387 overexpression in Schwann cells,indicating that the Wnt/PCP pathway is activated by Cthrc1 in Schwann cells. Cthrc1 inhibits the canonical Wnt/β-catenin pathway by simultaneously suppressing Dishevelled2,without further modification of the downstream component, β-catenin (Yamamoto et al., 2008; Sang et al., 2016). In our experiment, no difference in β-catenin expression was observed between Schwann cells overexpressing lncRNA NONMMUGO14387 and the control group, which further re flects the reliability of our experiments.
The small GTPases, Rac and RhoA, are two parallel downstream components of Wnt/PCP signaling (Ma et al., 2014).These small GTPases regulate cytoskeletal dynamics, and are involved in cell division and modulating morphology (Habas et al., 2003; Nodari et al., 2007; Jung et al., 2011). JNK is an effector of Rac, while ROCK is an effector of RhoA (Murakoshi et al., 2011; Riou et al., 2013). ROCK protein is essential for epithelial cell phenotype alterations (Rosso et al.,2005). Researchers have shown that RhoA/ROCK and Rac1/JNK regulate epithelial cell morphogenesis in an antagonistic manner (Kalaji et al., 2012). In our experiments, elevated RhoA, Rac1, JNK, and ROCK protein levels were detected in Schwann cells overexpressing lncRNA NONMMUG014387,indicating that the downstream components of Wnt/PCP are activated by enhanced Cthrc1 expression to promote Schwann cell proliferation. However, whether RhoA/ROCK and Rac1/JNK antagonize protein function in Schwann cells requires further study.
It is intriguing that miR-9 inhibits Cthrc1 expression by direct binding to its 3′-UTR to alter Schwann cell behavior(Zhou et al., 2014; Lee et al., 2016). Moreover, we show that miR-9 expression is down-regulated in Schwann cells overexpressing lncRNA NONMMUG014387. These findings are consistent with the competing endogenous RNA (ceRNA)hypothesis. According to the ceRNA hypothesis, lncRNA and mRNA share the same microRNA response element to regulate each other by competitively binding to microRNA (Salmena et al., 2011; Martin et al., 2016). However,whether lncRNA NONMMUG014387 regulates Cthrc1 in a miR-9-dependent manner requires further investigation.
Our study provides new understanding of nerve regeneration after peripheral nerve injury. However, all our conclusions rely on lncRNA overexpression, with no loss-of-function assays. Accordingly, the detailed mechanism of lncRNA NONMMUG014387-elevated Cthrc1 expression remains to be determined. More experiments should be performed to validate our results.
Taken together, our study first reveals a critical interaction between lncRNA NONMMUG014387, Cthrc1, and the Wnt/PCP pathway in Schwann cells. After sciatic nerve injury, NONMMUG014387 expression was increased, and consequently, expression of Cthrc1 was increased. Increased Cthrc1 expression activated the Wnt/PCP pathway, which promoted Schwann cell proliferation. Our study provides new understanding of nerve regeneration and a potential mechanism for repairing peripheral nerve injury.
Author contributions:BP, JYY and SQF designed this study. BP, JYY and ZJS performed this study. BP, ZJS and JHL analyzed data. BP, JYY and JHL wrote the paper. BP and SQF provided critical revision of the paper. SQF and JYY provided the funding and supervision. All authors approved the final version of the paper.
Con flicts of interest:None declared.
Research ethics:The study protocol was approved by the Animal Ethics Committee of Tianjin Medical University of China (approval number:TMUaMEC 2017008). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Declaration of patient consent:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer reviewer:Luciana P, Cartarozzi, University of Campinas –UNICAMP, Campinas, Brazil.
Altun I, Kurutas EB (2016) Vitamin B complex and vitamin B12 levels after peripheral nerve injury. Neural Regen Res 11:842-845.
Al-Zer H, Kalbouneh H (2015) Dental pulp stem cells-derived schwann cells for peripheral nerve injury regeneration. Neural Regen Res 10:1945-1946.
Bamba R, Riley DC, Kelm ND, Does MD, Dortch RD, Thayer WP (2016) A novel technique using hydrophilic polymers to promote axonal fusion.Neural Regen Res 11:525-528.
Apra C, Richard L, Coulpier F, Blugeon C, Gilardi-Hebenstreit P, Vallat JM, Lindner V, Charnay P, Decker L (2012) Cthrc1 is a negative regulator of myelination in Schwann cells. Glia 60:393-403.
Boivin A, Pineau I, Barrette B, Filali M, Vallieres N, Rivest S, Lacroix S(2007) Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J Neurosci 27:12565-12576.
Cobianchi S, Jaramillo J, Luvisetto S, Pavone F, Navarro X (2017) Botulinum neurotoxin A promotes functional recovery after peripheral nerve injury by increasing regeneration of myelinated fibers. Neurosciencedoi:10.1016/j.neuroscience.2017.07.011.
Dubuisson AS, Kline DG (2002) Brachial plexus injury: a survey of 100 consecutive cases from a single service. Neurosurgery 51:673-682.
Freidin M, Asche S, Bargiello TA, Bennett MV, Abrams CK (2009) Connexin 32 increases the proliferative response of Schwann cells to neuregulin-1 (Nrg1). Proc Natl Acad Sci U S A 106:3567-3572.
Gordon T, Eva P, Borschel GH (2015) Delayed peripheral nerve repair:methods, including surgical ‘cross-bridging’ to promote nerve regeneration. Neural Regen Res 10:1540-1544.
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC,Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB,van de Vijver MJ, Sukumar S, Chang HY (2010) Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464:1071-1076.
Habas R, Dawid IB (2005) Dishevelled and Wnt signaling: is the nucleus the final frontier? J Biol 4:2.
Habas R, Dawid IB, He X (2003) Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev 17:295-309.
Jessen KR, Mirsky R (2005) The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 6:671-682.
Jung J, Cai W, Lee HK, Pellegatta M, Shin YK, Jang SY, Suh DJ, Wrabetz L, Feltri ML, Park HT (2011) Actin polymerization is essential for myelin sheath fragmentation during Wallerian degeneration. J Neurosci 31:2009-2015.
Kalaji R, Wheeler AP, Erasmus JC, Lee SY, Endres RG, Cramer LP, Braga VM (2012) ROCK1 and ROCK2 regulate epithelial polarisation and geometric cell shape. Biol Cell 104:435-451.
Kim HC, Kim YS, Oh HW, Kim K, Oh SS, Kim JT, Kim BY, Lee SJ, Choe YK, Kim DH, Kim SH, Chae SW, Kim KD, Lee HG (2014) Collagen triple helix repeat containing 1 (CTHRC1) acts via ERK-dependent induction of MMP9 to promote invasion of colorectal cancer cells. Oncotarget 5:519-529.
Kurtzke JF (1982) The current neurologic burden of illness and injury in the United States. Neurology 32:1207-1214.
Lee J, Song J, Kwon ES, Jo S, Kang MK, Kim YJ, Hwang Y, Bae H, Kang TH, Chang S, Cho HJ, Kim SC, Kim S, Koh SS (2016) CTHRC1 promotes angiogenesis by recruiting Tie2-expressing monocytes to pancreatic tumors. Exp Mol Med 48:e261.
Ma MZ, Zhuang C, Yang XM, Zhang ZZ, Ma H, Zhang WM, You H, Qin W, Gu J, Yang S, Cao H, Zhang ZG (2014) CTHRC1 acts as a prognostic factor and promotes invasiveness of gastrointestinal stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia 16:265-278.
Martin E, Ouellette MH, Jenna S (2016) Rac1/RhoA antagonism defines cell-to-cell heterogeneity during epidermal morphogenesis in nematodes. J Cell Biol 215:483-498.
Min W, Dai D, Wang J, Zhang D, Zhang Y, Han G, Zhang L, Chen C, Li X, Li Y, Yue Z (2016) Long noncoding RNA miR210HG as a potential biomarker for the diagnosis of glioma. PLoS One 11:e0160451.
Murakoshi H, Wang H, Yasuda R (2011) Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature 472:100-104.
Nodari A, Zambroni D, Quattrini A, Court FA, D’Urso A, Recchia A, Tybulewicz VL, Wrabetz L, Feltri ML (2007) Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol 177:1063-1075.
Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I,Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y (2003) The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8:645-654.
Pan B, Zhou HX, Liu Y, Yan JY, Wang Y, Yao X, Deng YQ, Chen SY, Lu L,Wei ZJ, Kong XH, Feng SQ (2017) Time-dependent differential expression of long non-coding RNAs following peripheral nerve injury. Int J Mol Med 39:1381-1392.
Pyagay P, Heroult M, Wang Q, Lehnert W, Belden J, Liaw L, Friesel RE,Lindner V (2005) Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res 96:261-268.
Qian TM, Zhao LL, Wang J, Li P, Qin J, Liu YS, Yu B, Ding F, Gu XS, Zhou SL (2016) miR-148b-3p promotes migration of Schwann cells by targeting cullin-associated and neddylation-dissociated. Neural Regen Res 11:1001-1005.
Reichert F, Saada A, Rotshenker S (1994) Peripheral nerve injury induces Schwann cells to express two macrophage phenotypes: phagocytosis and the galactose-speci fic lectin MAC-2. J Neurosci 14:3231-3245.
Rinn JL, Chang HY (2012) Genome regulation by long noncoding RNAs.Annu Rev Biochem 81:145-166.
Riou P, Kjaer S, Garg R, Purkiss A, George R, Cain RJ, Bineva G, Reymond N, McColl B, Thompson AJ, O’Reilly N, McDonald NQ, Parker PJ, Ridley AJ (2013) 14-3-3 proteins interact with a hybrid prenyl-phosphorylation motif to inhibit G proteins. Cell 153:640-653.
Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC (2005) Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci 8:34-42.
Rotshenker S (2011) Wallerian degeneration: the innate-immune response to traumatic nerve injury. J Neuroin flammation 8:109.
Salmena L, Poliseno L, Tay Y, Kats L, Pandol fiPP (2011) A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell 146:353-358.
Sang W, Zhu L, Ma J, Lu H, Wang C (2016) Lentivirus-mediated knockdown of CTHRC1 inhibits osteosarcoma cell proliferation and migration. Cancer Biother Radiopharm 31:91-98.
Scarola M, Comisso E, Pascolo R, Chiaradia R, Marion RM, Schneider C,Blasco MA, Schoeftner S, Benetti R (2015) Epigenetic silencing of Oct4 by a complex containing SUV39H1 and Oct4 pseudogene lncRNA. Nat Commun 6:7631.
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101-1108.
Takada R, Hijikata H, Kondoh H, Takada S (2005) Analysis of combinatorial effects of Wnts and Frizzleds on beta-catenin/armadillo stabilization and Dishevelled phosphorylation. Genes Cells 10:919-928.
Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F,Saint-Jeannet JP, He X (2000) LDL-receptor-related proteins in Wnt signal transduction. Nature 407:530-535.
Wang HB, Wang XP, Zhong SZ, Shen ZL (2013) Novel method for culturing Schwann cells from adult mouse sciatic nerve in vitro. Mol Med Rep 7:449-453.
Wang T, Zhang SJ, Cao SL, Guo WZ, Yan B, Fang HB (2015) Protective effects of salubrinal on liver injury in rat models of brain death. Chin Med J (Engl) 128:1523-1528.
Wang Y, Teng HL, Huang ZH (2012) Intrinsic migratory properties of cultured Schwann cells based on single-cell migration assay. PLoS One 7:e51824.
Wang Y, Tang Q, Zhu L, Huang R, Huang L, Koleini M, Zou D (2016)Effects of treatment of treadmill combined with electro-acupuncture on tibia bone mass and substance Pexpression of rabbits with sciatic nerve injury. PLoS One 11:e0164652.
Wongtrakoongate P, Riddick G, Fucharoen S, Felsenfeld G (2015) Association of the long non-coding RNA steroid receptor rna activator (SRA)with TrxG and PRC2 complexes. PLoS Genet 11:e1005615.
Wright MW (2014) A short guide to long non-coding RNA gene nomenclature. Hum Genomics 8:7.
Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S, Tarui H, Sasaki H (2008) Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell 15:23-36.
Yang XM, You HY, Li Q, Ma H, Wang YH, Zhang YL, Zhu L, Nie HZ, Qin WX, Zhang ZG, Li J (2015) CTHRC1 promotes human colorectal cancer cell proliferation and invasiveness by activating Wnt/PCP signaling. Int J Clin Exp Pathol 8:12793-12801.
Yao D, Li M, Shen D, Ding F, Lu S, Zhao Q, Gu X (2013) Expression changes and bioinformatic analysis of Wallerian degeneration after sciatic nerve injury in rat. Neurosci Bull 29:321-332.
Yu ZW, Men YZ, Dong P (2017) Schwann cells promote the capability of neural stem cells to differentiate into neurons and secret neurotrophic factors. Exp Ther Med 13:2029-2035.
Zhou S, Gao R, Hu W, Qian T, Wang N, Ding G, Ding F, Yu B, Gu X (2014)MiR-9 inhibits Schwann cell migration by targeting Cthrc1 following sciatic nerve injury. J Cell Sci 127:967-976.
How to cite this article:Pan B, Shi ZJ, Yan JY, Li JH, Feng SQ (2017) Long non-coding RNA NONMMUG014387 promotes Schwann cell proliferation after peripheral nerve injury. Neural Regen Res 12(12):2084-2091.
Funding: This work was supported by a grant from Student’s Platform for Innovation and Entrepreneurship Training Program in China,No. 201610062009; the National Natural Science Foundation of China (Key Program), No. 81330042; a grant from the Special Program for Sino-Russian Joint Research Sponsored by the Ministry of Science and Technology, China, No. 2014DFR31210; and a grant from the Key Program Sponsored by the Tianjin Science and Technology Committee of China, No. 13RCGFSY19000, 14ZCZDSY00044.
Graphical Abstract

*Correspondence to:Shi-qing Feng, M.D.,sqfeng@tmu.edu.cn.
#These authors contributed equally to this study.
orcid:0000-0001-9437-7674(Shi-qing Feng)
10.4103/1673-5374.221168
2017-11-24
Copyedited by JAMES R, Stow A, Yu J, Li CH, Qiu Y, Song LP, Zhao M
- 中國神經(jīng)再生研究(英文版)的其它文章
- Roles of neural stem cells in the repair of peripheral nerve injury
- Quanti fication of intermuscular and intramuscular adipose tissue using magnetic resonance imaging after neurodegenerative disorders
- Notch pathway inhibitor DAPT enhances Atoh1 activity to generate new hair cells in situ in rat cochleae
- Autologous transplantation with fewer fibers repairs large peripheral nerve defects
- Topiramate as a neuroprotective agent in a rat model of spinal cord injury
- Diffusion tensor imaging of spinal microstructure in healthy adults: improved resolution with the readout segmentation of long variable echo-trains

