Stand dynamics of the drought-affected floodplain forests of Araguaia River,Brazilian Amazon
Jürgen Homeier,Dariusz Kurzatkowski and Christoph Leuschner
Stand dynamics of the drought-affected floodplain forests of Araguaia River,Brazilian Amazon
Jürgen Homeier,Dariusz Kurzatkowski and Christoph Leuschner*
Abstract
Background:The floodplain forests of Araguaia River,a clear-water river in the southeastern Amazon(Tocantins State,Brazil),are characterized by seasonal flooding up to 3.5 m height,low nutrient levels in the water,and seasonal drought periods of 4–5 months.
Methods:We studied the forest dynamics(tree diameter growth,tree mortality and recruitment)of this unique forest ecosystem over a 5-year period by repeated censuses in 12 permanent plots established along a flooding gradient.
Results:The cumulative basal area in the plots increased by 0.84(±0.45)m2?ha–1?yr–1(mean ±SD)in the annually-flooded(AF)plots in lower terrain and by 0.69(±1.00)m2?ha–1?yr–1in the higher non-annually flooded(NAF)plots,corresponding to an aboveground biomass increase of 0.81(±0.57)and 0.69(±1.58)Mg?ha–1?yr–1in the AF and NAF plots,indicating a recent carbon sink in the biomass.Mean diameter growth rate was 1.8(±0.44)mm?yr–1in the AF and 2.0(±0.56)mm?yr–1in the NAF plots(corresponding to a coarse wood production of 1.53(±1.29)and 2.02(±0.52)Mg?ha–1?yr–1),indicating no flooding effect on radial growth.Mean mortality rates in the 5-year period were 1.9(±0.37)%?yr–1in the AF plots and 1.8(±0.87)%?yr–1in the NAF plots with no differences along the flooding gradient.Highest mortalities were registered in the AF plots for the 10–20 cm dbh class(2.4%?yr–1),likely as a consequence of flooding,and in the NAF plots for the 40–50 cm dbh class(3.0%?yr–1),probably mainly caused by ENSO-related droughts.
Conclusions:We conclude that these drought-affected tropical floodplain forests have a lower standing biomass and aboveground productivity than central Amazonian floodplain forests in more humid climates,and the imprint of the flooding gradient on stand dynamics is relatively weak,which may result from the lower flooding height and the interaction of flooding with low nutrient supply and periodic drought.
Keywords:Amazon,Diameter growth,Dry season,Flooding regime,Mortality rate,Productivity,Tree recruitment
Background
Amazonian freshwater floodplains cover an area of approximately 1.7 million km2(Junk et al.2011)and the majority of these wetlands are forested(Sch?ngart et al.2010;Wittmann et al.2010;Wittmann et al.2013).Many ecological processes in these unique semi-aquatic woodlands,such as nutrient cycling,decomposition and forest succession,are heavily influenced by the flood pulse,and plant species inhabiting these environments have developed specific morpho-anatomical and physiological adaptations to cope with the seasonal flooding(Junk 1989;Parolin et al.2004).The variable flooding regimes and differences in water and sediment chemistry determine tree species composition and forest dynamics in these systems(Junk 1989;Rosales et al.1999;Junk et al.2010;Wittmann et al.2010;Junk et al.2011).
Forests inundated seasonally by black-or clear-water rivers are collectively known as igapó and cover ca.180 000 km2of the Amazon Basin(Melack and Hess 2010).These rivers(e.g.Rio Negro,Tapajós,Tocantins and Araguaia)originate in Precambrian formations,notably the Guiana Shield and Central Brazilian Shield,with deeply weathered soils and thus carry black water and clear water,respectively(Irion et al.2010),with a reduced load ofsuspended inorganiccompoundscompared to white-water rivers.The fertility of igapó floodplains is therefore much lower than that of floodplains which are inundated by sediment-rich white water rivers(várzea floodplains).
The Araguaia floodplain covers the vast area of 58 600 km2in the southeast of the Amazon Basin(Melack and Hess 2010)and includes Bananal Island,the world’s largest fluvial island.This extended semi-aquatic landscape represents the fifth largest floodplain area in South America.Araguaia River is a clear-water river with large tracts of igapó(Junk et al.2011).The region is localized in the transition zone from the Amazonian moist lowland forest to the semi-humid to semi-arid Brazilian cerrado forest,two biomes with very high tree species richness.While the immediate study region is still covered by large tracts of natural forest vegetation wellpreserved in Cant?o State Park,the greater Araguaia floodplain is part of the so-called ‘Arc of deforestation’in southern Amazonia which is under heavy pressure by human expansion.
The forests in the study region are exposed to seasonal flood levels of up to 3.5 m height,corresponding to a submersion of the trees for up to 150 days per year.In this part of Amazonia with an extended dry season,the floodplain forest is not only exposed to long-lasting inundation but also to seasonal drought.Thus,the river banks in the transition from high to low terrain are characterized by two opposing stress gradients,increasing anoxia in direction of the river during the flooding season,and decreasing drought exposure in the dry season.We expected that the combination of these two stressors(i)has a negative effect on forest productivity and standing biomass,and(ii)represents an effective environmental filter,which largely determines the species composition of the floodplain forests at different heights on the river banks.In support of our first assumption,a biomass inventory found that canopy height and aboveground live tree biomass(AGB)are indeed lower in the Araguaia floodplain forest than in the less seasonal floodplain forests of the central Amazon(Kurzatkowski et al.2015).
In the present study,we measured tree radial growth and wood production and analyzed stand dynamics(tree mortality and recruitment)in the same plots along a flooding gradient in the Araguaia floodplain over a period of 5 years.We further expected(iii)that a higher flood level and longer inundation at the lowest sites should cause elevated tree turnover rates(greater mortality and also recruitment rates),while greater drought exposure at the highest sites should increase mortality at this end of the gradient as well but not recruitment.Our studies in this seasonally-dry tropical floodplain forest are among the first to analyze the dynamics of these unique forests.In the absence of comparable studies,we contrast our results with findings from floodplain forests in humid regions of Amazonia.
Methods
Study area
The study was conducted in the southern part of Cant?o State Park in the south-eastern Brazilian Amazon on the Araguaia river floodplain(Eiten 1985),that was already established in 1989.The region is located in Tocantins State,Brazil,in the transition zone between the Amazon forests in the west and the cerrado in the east(Fig.1).It is covered by large areas of intact moist forest and patches of cerrado on drier soil.
The soils of the study sites are quite variable ranging from upland red–yellow and yellow Oxisols and red–yellow Ultisols to alluvial soils with hydromorphic lateritic and gleyic characteristics(Departamento Nacional de Produ??o Mineral,DNPM 1978).The soils on the floodplain are acid and nutrient-poor Dystrophic Plinthosols and Gleysols(Martins and Kardec 2006).
The region has a hot,semi-humid tropical seasonal climate with 1755 mm average annual precipitation(Kurzatkowski et al.2015).The rainy period lasts from November to April/May,followed by a distinct dry season from May/June to September with monthly precipitation totals of 50 mm or less in these 4 to 5 months.Mean annual temperature is 26°C;the hottest months are August and September with means of 30°C,the coolest month is July(22°C).There is a strong interannual variability in the total amount and distribution of precipitation,and the year 2005 was particularly dry(Borma et al.2009).
Study plots
Twelve permanent plots of 0.25 ha size(50 m×50 m)were established in 2001 for studying forest dynamics processes in relation to variation in flood level height.The study plots are located on the right bank of Javaés River,a tributary of Araguaia river,in vicinity to Cangu?u Research Center(9°58′41′′S,50°02′12′′W)(Fig.1).The plots represent a gradient of flood levels and flooding duration with distances to Javaés River varying between 0.1 and 1.0 km.The distance between soil surface and the upper limit of the dark water mark visible on the tree trunks was used to define the maximum flood level in a plot.All plots were selected in forest areas without larger recent canopy gaps,thus avoiding patches with major disturbance or covered by early-successional stages.
The 12 plots were assigned to two groups differing in flood level height(Kurzatkowski et al.2015).In annually flooded(AF)stands(n=5),flooding occurred every year,reaching maximum water heights of 1.5 to 3.5 m with inundation lasting for 2 to 5 months.The second group contains non-annually flooded(NAF)stands(n=7)with inundation occurring only irregularly for periods of up to 2 months;flood levels reached only 1.3 m at the most.
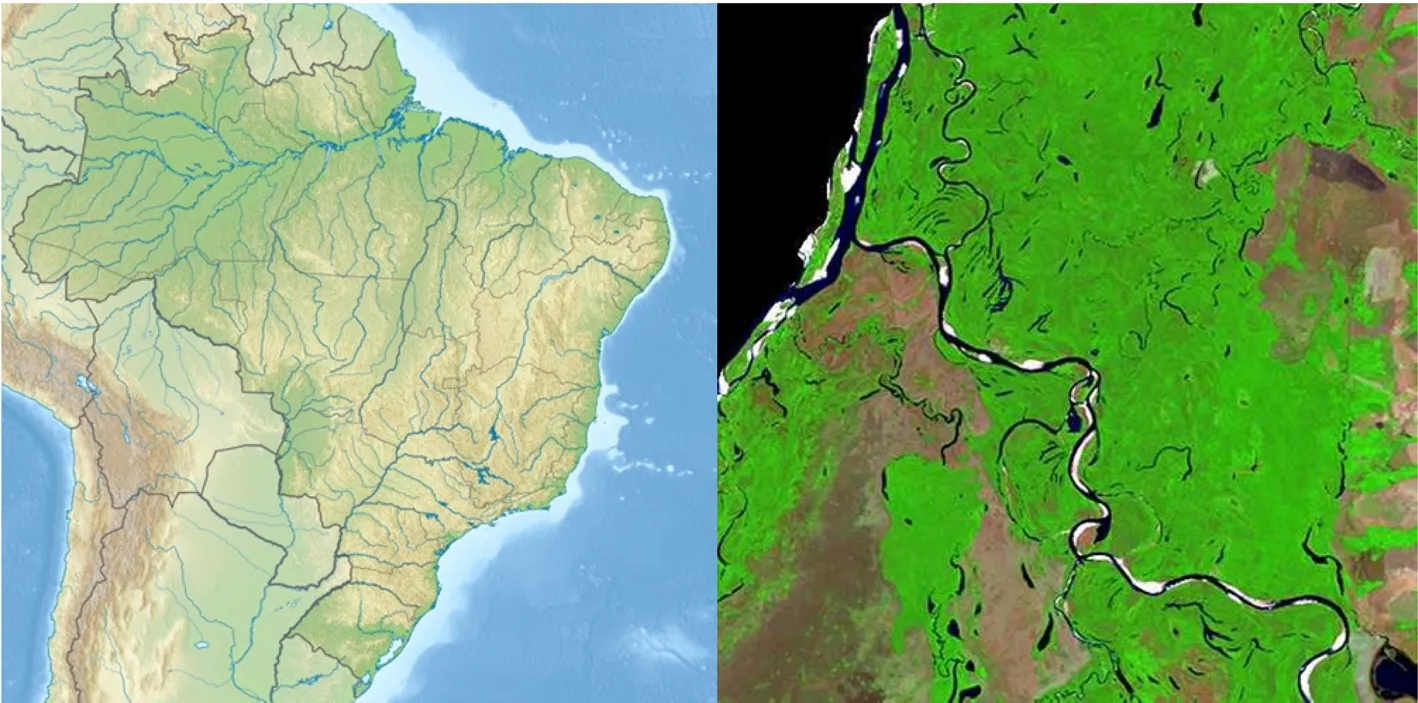
Fig.1 Location of the study area.Study site in Brazil along the Javaes river in the Cant?o State Reserve,Tocantins(Brasil Visto do Espa?o,http://www.cdbrasil.cnpm.embrapa.br)
Data collection and analysis
A census of the tree populations in all plots was conducted in 2001 and repeated in 2006.The diameter at breast height(dbh)of all living trees with dbh≥5 cm was recorded at 1.3 m.Diameters of buttressed trees were measured immediately above the buttresses to avoid overestimation(Condit 1998).Tree heights were measured with a telescope pole of 15 m length;the heights of taller trees were estimated.Palms and lianas were not included in the inventory.Tree species identification was conducted by a local field assistant with knowledge of the tree flora.In addition,plant samples were collected and compared to herbarium collections at the Federal University of Tocantins in Palmas.Phylogenetic classification was based on Angiosperm Phylogeny Group III(AGP III,2009).The scientific species names were checked in the Tropicos database(www.tropicos.org).
Following Bellingham and Sparrow(2009),we calculated annual rates of tree recruitment and mortality.The annualized tree mortality rate(M;in%)was derived from the expression:
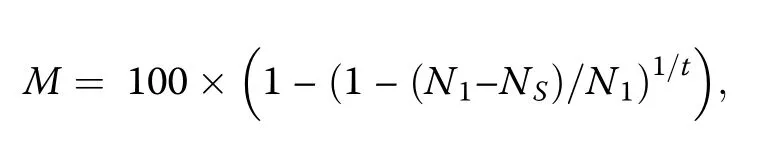
whereN1is the number of individuals in the first census,NSis the number of surviving individuals(excluding new recruits)in the second census,andtis the time in years.The annualized tree recruitment rate(R;in%)was calculated as:
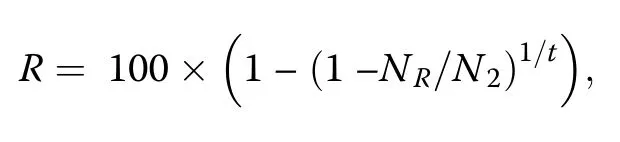
whereNRis the number of recruited trees(all trees that reached the 5 cm dbh threshold between 2001 and 2006)between censuses,andN2is the total number of live individuals(including new recruits)in the second census.
Upright standing stems were considered dead,if their inner bark was dry and easily separated from the sapwood.For the 12 most common tree species on our plots,the rates of recruitment and mortality were also analyzed separately.
To estimate aboveground coarse woody biomass(AGB),we applied the allometric equation for tropical wet forests proposed by Chave et al.(2005),with wood specific gravity(WSG),dbh and tree height as parameters.WSG data for the tree species were obtained from the Global Wood Density Database (http://datadryad.org/handle/10255/dryad.235)(Chave et al.2009;Zanne et al.2009).In cases where species-specific information was missing,we used means of WSG calculated from the values of the species of the same genus.For not identified trees we used the average of WSD of the respective plots.
The aboveground coarse wood biomass production(WP)of all trees,which had survived in 2006,was calculated as the AGB difference between the censuses in 2006 and 2001.Means of parameters were compared withT-tests,using Statistica 12(StatSoft,Tulsa,OK,USA)and regression analyses were performed with Xact 8.0 software(SciLab,Hamburg,Germany).
Due to the avoidance of large recent gaps or patches with early regrowth,we expect our AGB estimates to be in the upper range of forest biomass figures.On the other hand,our selection criteria should result in belowaverage tree growth rates and WP rates.
Results
Stem density,coarse wood production and forest dynamics
In the surveyed area of the 12 plots(3 ha in total)in the Araguaia floodplain forest,average stem density(dbh≥5 cm)in 2001 was 1005(±155)(mean ±SD)ha–1in the NAF plots and 1182(±367)ha–1in the AF plots(Table 1).During the 5-year study period,we registered only marginal changes in stem density,as no major disturbance event apart from drought occurred.Stem density increased to 1007(±157)ha–1in the NAF plots and decreased to 1180(±361)ha–1in the AF plots.In 2001,basal area and tree aboveground biomass(AGB)means of 20.9(±3.4)m2?ha–1and 146.6(±43.9)Mg?ha–1were calculated for the NAF plots,while 17.1(±2.4)m2?ha–1and 100.5(±20.3)Mg?ha–1were recorded in the AF plots,respectively.
The cumulative basal area in the plots increased by 0.84(±0.45)and 0.69(±1.00)m2?ha–1?yr–1in the AF and NAF plots,corresponding to an AGB increase of 0.81(±0.57)Mg?ha–1?yr–1in the AF plots and 0.69(±1.58)Mg?ha–1?yr–1in the NAF plots.The surviving trees had a mean diameter growth of 1.8(±0.44)(AF plots)and 2.0(±0.56)mm?yr–1(NAF plots)in the 5 years,summing up to a coarse wood production(WP)of 1.53(±0.20)in the AF plots and 2.02(±0.52)Mg?ha–1?yr–1in the NAF plots during the period 2001–2006(Fig.2).
Mean tree mortality rates in the 5-year period were 1.9%in the AF plots and 1.8%in the NAF plots,corresponding to a dead wood production of 1.01(±0.34)and 1.57(±1.29)Mg?ha–1?yr–1.Highest mortality rates were registered in the AF plots for the 10–20 cm dbh class with 2.4%,and in the NAF plots for the 40–50 cm class(3.0%,Fig.3).The mean recruitment rates were 1.8%in the AF plots and 2.0%in the NAF plots,resulting in a mean ingrowth of 0.29(±0.17)in the AF plots and 0.23(±0.09)Mg?ha–1?yr–1in the NAF plots.
None of the means of aboveground coarse wood production(p=0.06),mortality(p=0.37)and recruitment(p=0.39)differed significantly atp<0.05 between the two forest types.Neither mortality nor recruitment rate showed significant linear correlations with the maximum flood level height observed in the study plots(Fig.4).
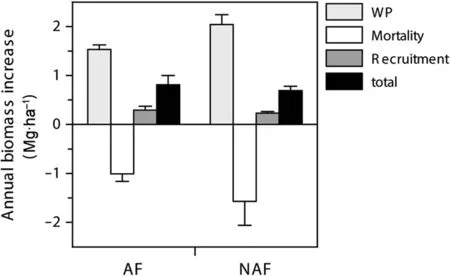
Fig.2 Biomass dynamics in permanent plots.Fractions of biomass of trees with dbh≥5 cm in annually flooded forest(AF;five plots)and non-annually flooded forest(NAF;seven plots)during the study period from 2001–2006.Shown are means(±SE)of coarse wood production(WP),production of dead tree mass(mortality),tree recruitment and the net change in live biomass(total;WP+recruitment–mortality).The two forest types show no significant differences
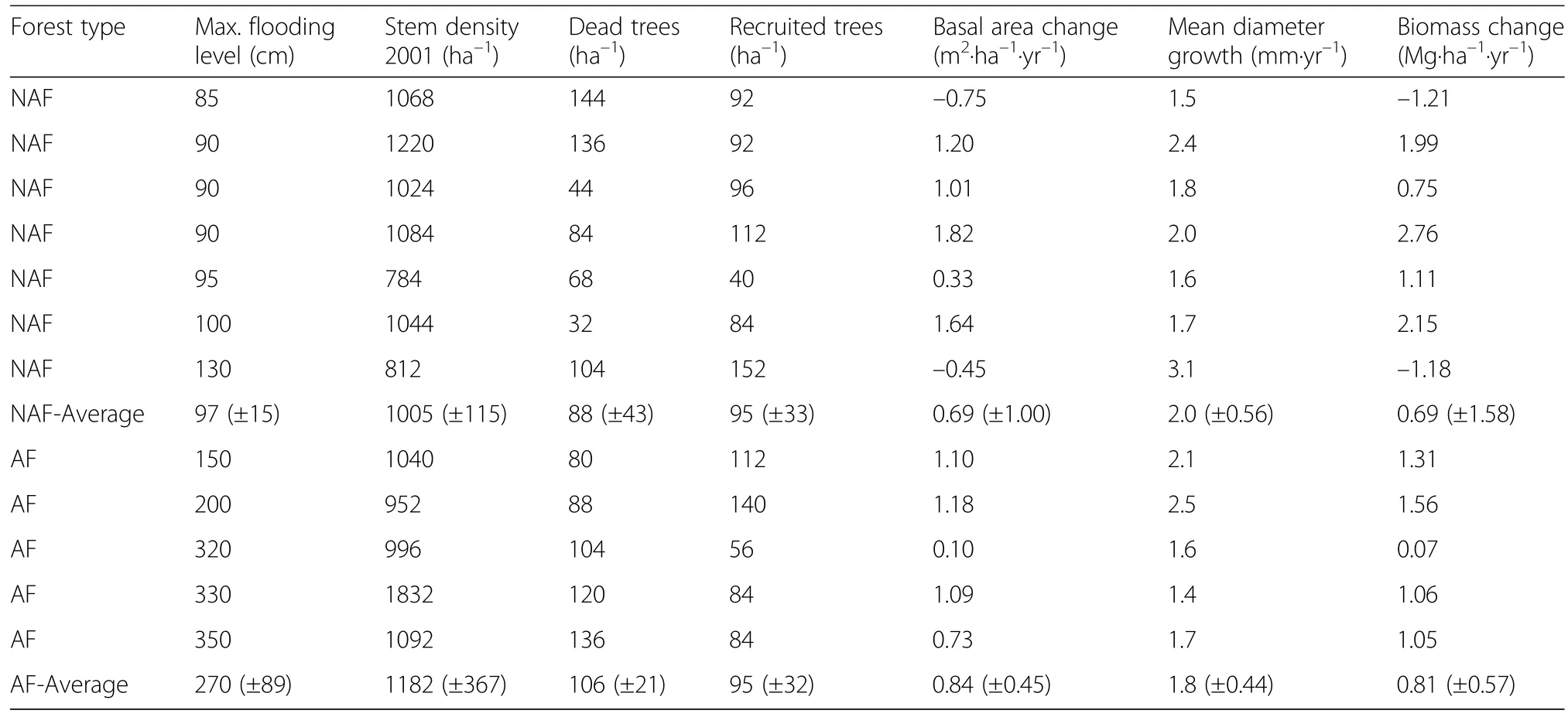
Table 1 Maximum flood levels and changes in stem density,biomass and growth for all permanent plots
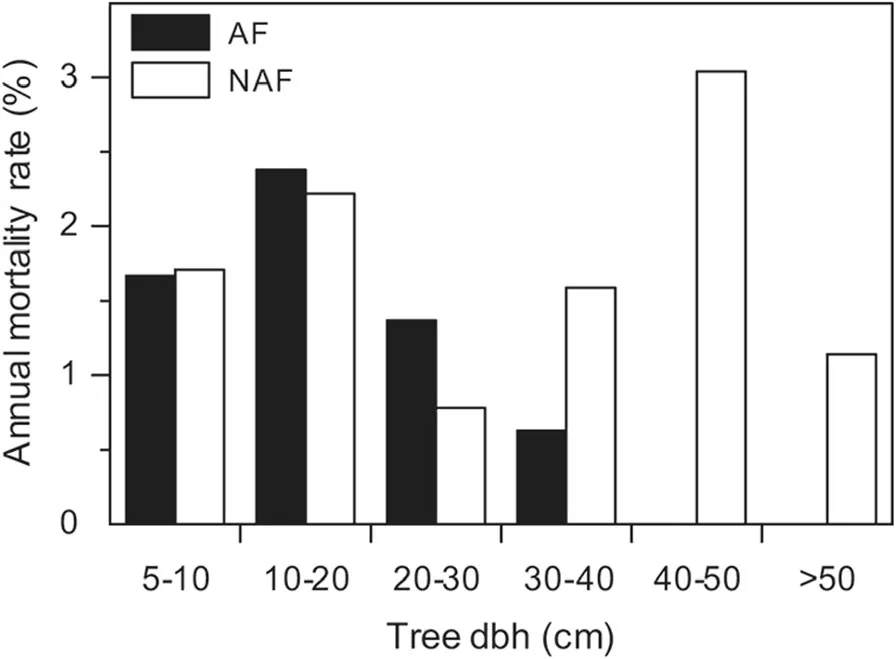
Fig.3 Tree mortality.Annual tree mortality rates in different diameter classes in the two forest categories AF(annually flooded,black bars)and NAF(non-annually flooded,white bars)during the study period from 2001–2006.Of the 3224 stems registered in 2001(AF:1465,NAF:1759)285 died during the 5-year period until 2006(AF:132,NAF:153)
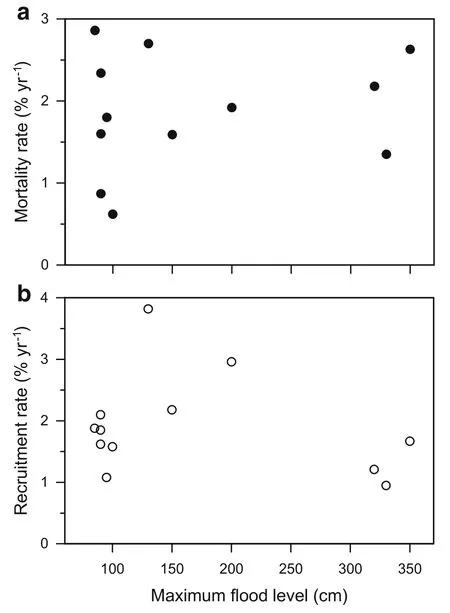
Fig.4 Annual mortality and recruitment in permanent plots.Annual tree mortality rate(a)and annual tree recruitment rate(b)in relation to the maximum flooding level of the study plots.None of the relations were significant at p<0.05
Population dynamics of selected species
The 12 most abundant tree species in the study plots,all present with stem densities >15 ha–1,were included in a species-specific demographic analysis(Table 2).The most frequently occurring species in the AF plots(all with more than 147 stems?ha–1)wereAlibertia edulis,Inga marginataandMouriri guainensisand in the NAF plots(>91 stems?ha–1)Protium heptaphyllum,Brosimum rubescensandProtium paniculatum.
While 11 of the 12 species occurred in both forest types and could be compared with respect to flooding tolerance,Cordia sellowianawas abundant in the NAF plots but completely absent from the AF plots.Alibertia edulis,Piranhea trifoliateandSaccellium brasiliensehad significantly higher densities in the AF plots,whereasProtium heptaphyllumwas more common in the NAF plots.
Average annual mortality and recruitment of the 12 species during the 5-year study period varied markedly between species and forest types.The highest annual mortality rates in the NAF plots were observed forCombretum leprosum,Inga marginata,Protium heptaphyllumandXylopia frutescens(2.1–2.6%).In the annually flooded plots,the species with highest mortality rates wereProtium paniculatum,Combretum leprosum,Xylopia frutescensandProtium heptaphyllum(3.5–21.4%).The same four tree species with highest mortality had also the largest recruitment rates in the AF plots(4.0–22.9%).
When the tree species were grouped by their forest type preference(Fig.5),the five AF species showed almost no differences in dynamic properties between forest types;they had slightly higher mortality rates than recruitment rates in both forest types.In contrast,the seven NAF species had higher recruitment than mortality rates in both forest types.The average recruitment rates as well as the mortality rates of the latter species were conspicuously higher in the AF plots than in the NAF plots.Mean stem diameter growth rates typically varied between 1.23 and 1.97 mm?yr–1in the AF and NAF plots and were relatively uniform among the species(Table 2).Particularly high growth rates were recorded in both forest types for the pioneer treeXylopia frutescens(1.94 mm?yr–1in AF and 2.44 mm?yr–1in NAF plots).OnlyCombretum leprosumand the twoProtiumspecies showed higher growth rates in the AF than in the NAF plots.
Discussion
The two stand inventories in 2001 and 2006 produced the unexpected result that mean stem diameter growth was very similar in the annually-flooded and nonannually flooded plots(1.8 and 2.0 mm?yr–1)and thus apparently independent of flooding height and duration.Cumulative basal area in the plots increased on average by 0.69 m2?ha–1?yr–1in the NAF plots,and byeven 0.84 m2?ha–1?yr–1in the AF plots(difference not significant).This related to an average aboveground biomass increase of 2.02 and 1.53 Mg?ha–1?yr–1in the NAF and AF plots.As with stem radial growth,aboveground productivity apparently was not reduced by higher and longer flooding.This matches the biomass inventory data,which also showed no effect of flood height and duration on the standing aboveground biomass stocks(Kurzatkowski et al.2015).
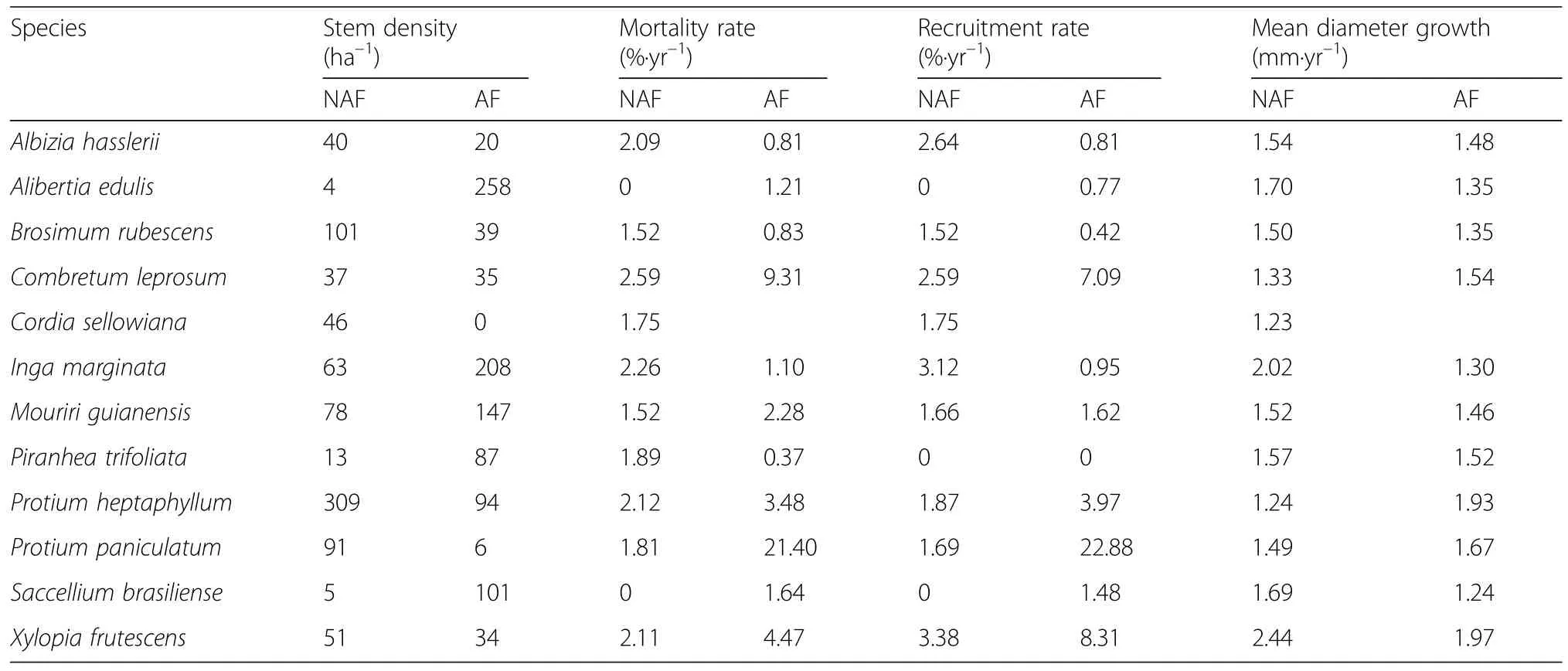
Table 2 Abundant species'mortality and recruitment
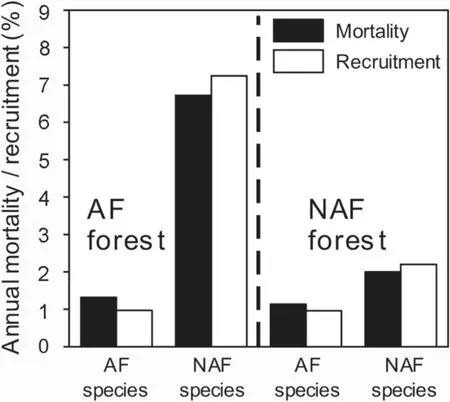
Fig.5 Mortality and recruitment of species grouped by habitat preference.Mean annual mortality and recruitment rates of the twelve common tree species(see Table 2)in the AF and NAF plots.The species are categorized according to their forest type preference into AF-species(Alibertia edulis,Inga marginata,Mouriri guianensis,Piranhea trifoliata and Saccelium brasiliense)and NAF-species(Albizia hassleri,Brosimum rubescens,Combretum leprosum,Cordia sellowiana,Protium heptaphyllum,Protium paniculatum and Xylopia frutescens).There are no significant differences between compared species groups or between forest types
The apparently low effect of flooding height and duration on tree diameter growth in our plots comes as a surprise.Other studies found a negative relation between diameter growth and the height and duration of annual inundations,e.g.in the igapó forests along Rio Negro(Sch?ngart et al.2010),Amazonian várzea forests(Nebel et al.2001a,2001b;Sch?ngart et al.2010)and riparian forests in the Brazilian Pantanal(Wittmann et al.2008;Sch?ngart et al.2011).Worbes(1997)confirmed with dendrochronological methods that the floodplain trees typically reduce wood growth during the aquatic phase and then remain in a state of cambial dormancy.Flooding height and duration perhaps were in the Araguaia system not as extreme as in other floodplain studies,where closed forest occurs up to inundation depths of 9 m(black water rivers)or 7.5 m(white water rivers)(Junk et al.2011).In addition,the relatively extreme environment(low fertility and seasonal drought)could have masked a flooding effect on cambial activity in this region.
In our study,a significant flooding effect was however detected on wood density,which increased significantly with increasing flood height and duration,and for maximum tree height of the stands,which decreased significantly in direction of the river.We interpret these relationships as a hint that the flooding regime and associated soil anoxia acted as an environmental filter selecting for more flood-tolerant tree species with higher wood density close to the river.The production of wood with higher density compensated for the smaller maximum tree height in the AF stands with the consequencethat standing biomass and also biomass production were not lower than in the NAF plots.Thus,the productivity data suggest that higher wood density was not related to lower diameter growth in our species sample.With respect to the causes of these patterns,our dbh and tree height data suggest that the average AF tree must either be shorter-lived or grow slower in height than the average NAF tree.As trees with dbh>50 cm were more abundant in the NAF plots,we assume that the greater maximum tree height in these stands is a consequence of greater age and not intrinsically higher height growth rates.In two of the three highly flooded plots,the mortality rate in the five study years was indeed high with >2%?yr–1,which may suggest that trees in the AF plots do no reach larger sizes and ages due to reduced survival under high floods.
Except for two plots,the net woody biomass change was positive in the period 2001–2006 in the AF and NAF plots,indicating that the Araguaia floodplain forests function currently as a carbon sink with a net storage of 0.81 and 0.69 Mg?ha–1?yr–1in the AF and NAF plots.In secondary floodplain forests in humid Amazonia,Lucas et al.(2014)found an average net biomass increase of 6.4 Mg?ha–1?yr–1,for old-growth Amazonian forests Phillips et al.(2009)estimated a mean biomass increase for the trees with dbh ≥10 cm of 0.90 ± 0.66 Mg?ha–1?yr–1.Baker et al.(2004)gave a mean long-term biomass increase for Amazonian lowland forests of 1.21 ± 0.43 Mg?ha–1?yr–1.This is slightly higher than our values,but may easily be explained by the more stressful conditions(more pronounced dry season combined with low nutrient availability)in the igapó floodplains and the lower canopy height.A long-term biomass increase in recent decades has also been found in other tropical forests,e.g.in those of Africa(Lewis et al.2009),but studies reporting no biomass change in recent decades do also exist(Laurance et al.2004;Chave et al.2008).The causes of an apparent long-term forest biomass increase in the tropics are debated and refer to climate change or recovery from past disturbances(Clark 2002;Wright 2005;K?rner 2006,Chave et al.2008;Lewis 2006,Lewis et al.2009;Fisher et al.2008).Since we do not have soil carbon inventory data,it remains unclear whether the ecosystem C balance in Araguaia floodplain is also positive or not.
The Araguaia floodplain forest differs from other Amazonian igapó forests by its extended dry season which may represent an additional stressor for the trees.While plot-level tree diversity was not markedly lower than in central or western Amazonian várzea and igapó forests,mean stand-level basal area(19.4 m2?ha–1)and aboveground biomass(117 Mg?ha–1)were markedly smaller than in other Neotropical floodplains,including fertility of the clear waters of Araguaia river and the 4–5 months of dry season.While mean stem diameter growth was apparently not negatively affected by temporal water shortage in the NAF plots on higher terrain,we found a particularly high mortality rate,mostly of larger trees,in the highest NAF plots.A likely explanation is the extended drought in 2005(Phillips et al.2009),which may have killed several of the larger trees especially in the NAF plots.It appears that drought affected the AF plots less severely,probably because of the higher ground water level in the dry season.Even in nonflooded terra firme forests of moister tropical regions,diameter growth was found to be strongly related to the seasonality of precipitation(Brienen and Zuidema 2005)and severe droughts negatively affect forest dynamics,decreasing wood biomass productivity and increasing tree mortality(Phillips et al.2009;Corlett 2011;Lewis et al.2011).
Stand dynamics(recruitment and mortality)
The mortality rates observed in our study(averages of 1.93%?yr–1in the AF and 1.83%?yr–1in the NAF plots)are comparable or lower than values reported from other floodplains in the Amazon(Brazil:1.56–1.99%,Campbell et al.1992;Ecuador:3.01,Korning and Balslev 1994;Peru:1.79–3.16,Gentry and Terborgh 1990;Phillips et al.1994;Nebel et al.2001a,2001b).In terra firme forests near Manaus,Rankin-de-Merona et al.(1990)reported lower values of 1.13%and Korning and Balslev(1994)calculated values of 1.04–1.88%for Ecuadorian terra firme forests.Elevated average mortality rates in floodplain forests as compared to terra firme stands are to be expected,but our record is too short to draw sound conclusions.
Highest mortality was observed in large trees(dbh class> 40–50 cm)of the NAF plots on higher terrain.As large trees were hit most,we assume that the severe ENSO-related 2005 drought in the Amazon may have contributed to the elevated death rate(Williamson et al.2000;Condit et al.2004;van Nieuwstadt and Sheil 2005),as it has been reported by Phillips et al.(2009)from other regions of the Amazon basin.The 2005 drought was also detected in the study region and resulted in strongly reduced soil moisture in the upper 2 m of soil during the dry season(Borma et al.2009).In the course of climate change,Olivares et al.(2015)documented extended drought periods at the margins of the Amazon basin.While the growth response to climate change may be quite difficult to detect,increased mortality due to more severe droughts can have rapid and conspicuous effects on stand structure through the death
Tree species differences in growth and mortality
Kubitzki(1989)assumed that many Amazonian floodplain tree species are ecotypes of taxa originating from the surrounding terra firme forests.When the species immigrate into the floodplains,they gradually develop adaptations to the periodic inundation.From the overall registered 54 tree species in the 12 plots,we classified 12 species by their habitat preference:seven could be categorized as species preferring the NAF forest due to higher stem densities here,and five as AF species.This suggests that the species differed in their degree of adaptation to this extreme environment.As an example of an AF species,Inga marginatahad four times higher stem densities in the AF forest than in the NAF plots.This observation supports the assumption that this species colonizes low-lying,highly flooded river margins and uses the water for seed dispersion.However,this species likely has its ancestors not in the semi-deciduous drier forests of the south-eastern Amazon surrounding the Araguaia floodplain,but it probably has migrated along the large rivers from the central Amazon.Yet,other species likely have colonized the floodplain from the surrounding drier forests.For example,Protium heptaphyllumis an abundant species in semi-deciduous forests and the cerrado,which was three times more frequent in the NAF plots.Cordia sellowianais a typical element of the Cerrado vegetation;it occurred exclusively in the NAF plots.This suggests that these species from higher terrain do not yet have developed adequate adaptations to the high flood levels in the AF plots or,alternatively,may physiologically not be capable of doing so because they have adapted to drought.However,the majority of species found in these floodplain forests seems to have closer relations to the more humid central Amazon.
The species with NAF plot preference can indeed be considered as more flooding-sensitive,as their mortality rate was four times larger in the AF plots than that of the co-occurring AF species(Fig.5).In contrast,both tree categories had similar mortalities in the drier NAF plots.This indicates that the AF species are rarer in the drier NAF plots due to other reasons than a particularly high drought sensitivity.In fact,abundance in a plot,mortality rate and diameter growth in the two forest categories were in most species not related to each other(see Table 2).This excludes a simple ecophysiological explanation for the presence or absence of certain species along the flooding gradient.
While a decline in tree species richness with increasing flooding height and duration seems to be typical for manyAmazonian floodplain forests(Terborgh and Andresen 1998;Nebel et al.2001a,2001b;Parolin et al.2004),we found only a very slight(non-significant)increase in plot-level species numbers from the AF to the NAF plots(Kurzatkowski et al.2015).The missing diversity gradient in the Araguaia floodplain may again be a consequence of the low nutrient concentration of the clear waters and the action of drought in this region.
In Central Amazonian floodplain forests,Worbes et al.(1992)found a clear differentiation of the average diameter growth rate among the dominant trees according to their successional status:pioneer species(9.4 mm?yr–1)> earlysecondary species(4.3 mm?yr–1) > late-secondary species(3.0 mm?yr–1) > climax species(2.0 mm?yr–1).Our species had in most cases growth rates < 2 mm?yr–1.This,however,does not necessarily classify them all as climax species.Comparing the average diameter increment of three commercial tree species in igapó and várzea floodplain forests,Sch?ngart et al.(2005)measured significantly lower rates in the igapó forests,which may explain our relatively low rates.
The natural seasonal flood pulse is a critical mediator of tree regrowth,as floods facilitate seed dispersal,deposit sediments and promote the connectivity between different forest sites(Ward and Wiens 2001)which could enhance tree regeneration.However,we found no systematic difference in recruitment rates between AF and NAF plots,and for the 12 most abundant species no clear pattern emerged indicating a more successful offspring establishment in the plot category which was preferred by the adults.This suggests that other factors than flooding regime which affect seedling establishment and survival,must be equally important in our stands.
Conclusions
Ourforestdynamicsdata from the seasonally-dry Araguaia floodplain show that the standing aboveground biomass and ANPP are relatively low compared to central Amazonian várzea floodplain forests,in support of our first assumption.In contrast to other Amazonian igapó and várzea floodplains,the flooding gradient imprinted only weakly on most stand-level structural and dynamic properties,while it influenced mean wood density and maximum stand height.As expected,tree mortality rates were relatively high in both annually and not-annually flooded plots,probably due to different causes.The high mortality observed in large NAF plot trees likely is a consequence of ENSO-related droughts which may threaten seasonally-dry floodplain forests at the edge of the Amazon more than in the humid regions of central Amazonia.Contrary to our expectation,we found no decrease in recruitment rate from low to high terrain suggesting that flooding is only one factor controlling tree regeneration in these floodplain forests.We conclude that the seasonally-dry floodplain forests of Araguaia are unique forest ecosystems which are at many places threatened by transformation.When intact they still seem to function as a net carbon sink,but this may change with increasing severity of climate warming-related droughts.
Acknowledgements
We are grateful for support to the Instituto Ecológica,Palmas,and the Federal University of Tocantins(UFT).We thank Antonio Morales for tree identification and numerous students from UFT for assistance with field work.
Funding
This research was conducted as a component in the“Carbon Sequestration Project in Bananal Island”sponsored by AES Barry Foundation.DK received scholarships from CNPq(Brazilian National Council for Scientific and Technological Development)and from KAAD(Catholic Academic Exchange Service,Germany)which is gratefully acknowledged.
Availability of data and materials
After acceptance data will be made publicly available as an additional supporting file.
Authors’contributions
JH,DK and CL developed the concept of the study;DK recorded field data;JH&DK analyzed the data;JH,DK&CL wrote the manuscript.All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Baker TR,Phillips OL,Malhi Y,Almeida S,Arroyo L,Di Fiore A,Erwin T,Higuchi N,Killeen TJ,Laurance SG,Laurance WF,Lewis SL,Monteagudo A,Neill DA,Vargas Nunez P,Pitman NCAN,Vasquez Martinez R(2004)Increasing biomass in Amazonian forest plots.P Roy Soc Lond B Bio B 359:353–365
Bellingham PJ,Sparrow AD(2009)Multi-stemmed trees in montane rain forests:their frequency and demography in relation to elevation,soil nutrients and disturbance.J Ecol 97:472–483
Borma LDS,Da Rocha HR,Cabral OM,Von Randow C,Collicchio E,Kurzatkowski D,Brugger PJ,Freitas H,Tannus R,Oliveira L,Rennó CD,Artaxo P(2009)Atmosphere and hydrological controls of the evapotranspiration over a floodplain forest in the Bananal Island region.Amazonia.J Geophys Res 114,G01003
Brienen RJW,Zuidema PA(2005)Relating tree growth to rain-fall in Bolivian rain forests:a test for six species using tree ring analysis.Oecologia 146:1–12
Campbell DG,Stone JL,Rosas A(1992)A comparison of the phytosociology and dynamics of three floodplain(Várzea)forests of known ages,Rio Juruá,western Brazilian Amazon.Bot J Linn Soc 108(3):213–237
Chave J,Andalo C,Brown S,Cairns MA,Chambers JQ,Eamus D,Folster H,Fromard F,Higuchi N,Kira T,Lescure JP,Nelson BW,Ogawa H,Puig H,Riera B,Yamakura T(2005)Tree allometry and improved estimation of carbon stocks and balance in tropical forests.Oecologia 145:87–99
Chave J,Condit R,Muller-Landau H,Thomas S,Ashton P,Bunyavejchewin S,Co L,Dattaraja H,Davies S,Esufali S,Ewango C,Feeley K,Foster R,Gunatilleke N,Gunatilleke S,Hall P,Hart T,Hernandez C,Hubbell S,Itoh A,Kiratiprayoon S,LaFrankie J,Lao S,Makana J,Noor M,Rahman Kassim A,Samper C,Sukumar R,Suresh H,Tan S,Thompson J,Tongco M,Valencia R,Vallejo M,Villa G,Yamakura T,Zimmerman J,Losos E(2008)Assessing evidence for a pervasive alteration in tropical tree communities.PLoS Biol 6,e45
Chave J,Coomes DA,Jansen S,Lewis SL,Swenson NG,Zanne AE(2009)Towards a worldwide wood economics spectrum.Ecol Lett 12(4):351–366
Clark DA(2002)Are tropical forests an important carbon sink?Reanalysis of the long-term plot data.Ecol Appl 12:3–7
Condit R(1998)Tropical Forest Census Plots:Methods and Results from Barro Colorado Island,Panama and a Comparison with other Plots.Springer,Berlin
Condit R,Aguilar S,Hernandez A,Perez R,Lao S,Angehr G,Hubbell SP,Foster RB(2004)Tropical forest dynamics across a rainfall gradient and the impact of an El Nino dry season.J Trop Ecol 20:51–72
Coomes DA,Burslem DF,Simonson WD(2014)Forests and Global Change Cambridge University Press
Corlett RT(2011)Impact of warming on tropical lowland forests.Trends Ecol Evol 26:606–613
Departamento Nacional de Produ??o Mineral,DNPM(1978)Projecto RADAMBRASIL.Folha SC.20 Porto Velho;geologia,geomorfologia,pedologia,vegeta??o e uso potencial da terra.Anexo:Analise estistitica de dados(Vegetac?o).Departamento Nacional de Produ??o Mineral,Rio de Janeiro
Eiten G(1985)Vegetation near Santa Teresinha,NE Mato Grosso.Acta Amazon 15(3/4):275–301
Fisher JB,Tu KP,Baldocchi DD(2008)Global estimates of the land–atmosphere water flux based on monthly AVHRR and ISLSCP-II data,validated at 16 FLUXNET sites.Remote Sens Environ 112(3):901–919
Gentry AH,Terborgh J(1990)Composition and dynamics of the Cocha Cashu mature floodplain forest.In:Gentry AH(ed)Four Neotropical Rainforests.Yale University Press,New Haven and London,pp 542–563
Irion G,de Mello JASN,Morais J,Piedade MTF,Junk WJ,Garming L(2010)Development of the Amazon valley during the Middle to Late Quaternary:sedimento-logical and climatological observations.In:Junk WJ,Piedade MTF,Wittmann F,Sch?ngart J,Parolin P(eds)Central Amazonian floodplain forests:ecophysiology,biodiversity and sustainable management.Springer,Berlin,pp 27–42
Junk WJ(1989)Flood tolerance and tree distribution in central Amazonian floodplains.In:Holm-Nielsen LB,Nielsen IC,Balslev H(eds)Tropical Forests:Botanical Dynamics.Speciation and Diversity.Academic Press,London,pp 47–64
Junk WJ,Piedade MTF,Wittmann F,Sch?ngart J,Parolin P(2010)Amazonian Floodplain Forests:Ecophysiology,biodiversity and sustainable management.Springer,Berlin,Ecological Studies,Vol.210.pp 62–101
Junk WJ,Piedade MTF,Sch?ngart J,Cohnhaft M,Adeney JM,Wittmann F(2011)A classification of major naturally occurring Amazonian lowland wetlands.Wetlands 31:623–640
K?rner C(2006)Forests,biodiversity and CO2:surprises are certain.Biologist 53:82–90
Korning J,Balslev H(1994)Growth and mortality of trees in Amazonian tropical rain forest in Ecuador.J Veg Sci 4:77–86
Kubitzki K(1989)The ecogeographical differentiation of Amazonian inundation forests.Plant Syst Evol 162:285–304
Kurzatkowski D,Leuschner C,Homeier J(2015)Effects of flooding on trees in the semi-deciduous transition forests of the Araguaia floodplain,Brazil.Acta Oecol 69:21–30
Laurance WF,Oliveira AA,Laurance SG,Condit R,Nascimento HEM,Sanchez-Thorin AC,Lovejoy TE,Andrade A,D’Angelo S,Ribeiro JE,Dick CW(2004)Pervasive alteration of tree communities in undisturbed Amazonian forests.Nature 428:171–175
Lewis SL(2006)Tropical forests and the changing earth system.P Roy Soc Lond B Bio B 361:195–210
Lewis SL,Lopez-Gonzalez G,Sonké B(2009)Increasing carbon storage in intact African tropical forests.Nature 477:1003–1006
Lewis SL,Brando P,Phillips OL,van der Heijden GMF,Nepstad D(2011)The 2010 Amazon drought.Science 331:554
Lucas CMJ,Sch?ngart J,Sheikh P,Wittmann F,Piedade MT,McGrath DG(2014)Effects of land-use and hydroperiod on aboveground biomass storage and productivity in secondary Amazonian floodplain forests.Forest Ecol Manag 319:116–127
Martins A,Kardec E(2006)Rela??es solo-geoambiente em áreas de ocorrências de Ipucas na planície do Médio Araguaia-Estado de Tocantins.Rev árvore 30(2):297–310
Melack JM,Hess LL(2010)Remote sensing of the distribution and extent of wetlands in the Amazon basin.In:Junk WJ,Piedade MTF,Wittmann F,Sch?ngart J,Parolin P(eds)Central Amazonian floodplain forests:ecophysiology,biodiversity and sustainable management.Springer,Berlin,pp 43–59
Nebel G,Kvist LP,Vanclay JK,Christensen H,Freitas L,Ruíz J(2001a)Structure and floristic composition of flood plain forests in the Peruvian Amazon.I.Overstorey.Forest Ecol Manag 150:27–57
Nebel G,Kvist LP,Vanclay JK,Vidaurre H(2001b)Forest dynamics in flood plain forests in the Peruvian Amazon:effects of disturbance and implications for management.Forest Ecol Manag 150(1):79–92
Olivares I,Svenning JC,von Bodegan PM,Balsev H(2015)Effect of warming and drought on the vegetation and plant diversity in the Amazon basin.Bot Rev 81:42–69
Parolin P,Ferreira LV,Albernaz AL,Almeida SS(2004)Tree species distribution in varzea forests of Brazilian Amazonia.Folia Geobotanica 39:371–383
Phillips OL,Hall P,Gentry AH,Sawyer SA,Vasquez R(1994)Dynamics and species richness of tropical rain forests.P Natl Acad Sci USA 91:2805–2809
Phillips OL,Aragao LE,Lewis SL,Fisher JB,Lloyd J,Lopez-Gonzalez G,Malhi Y,Monteagudo A,Peacock J,Quesada CA,van der Heijden G,Almeida S,Amaral I,Arroyo L,Aymard G(2009)Drought sensitivity of the Amazon rainforest.Science 323:1344–1347
Rankin-de-Merona JMRW,Hutchings H,Lovejoy TE(1990)Tree mortality and recruitment over a five-year period in undisturbed upland rainforest of the central Amazon.In:Gentry AH(ed)Four Neotropical Forests,vol Chapter 29.Yale University Press,New Haven,pp 573–584
Rosales J,Petts G,Salo J(1999)Riparian flooded forests of the Orinoco and Amazon basins:A comparative review.Biodiversity Conservation 8:551–586
Sch?ngart J,Wittmann F,Piedade MTF,Junk WJ,Worbes M(2005)Wood growth patterns of Macrolobium acaciifolium(Benth.)Benth.(Fabaceae)in Amazonian black-water and white-water floodplain forests.Oecologia 145:454–461
Sch?ngart J,Wittmann F,Worbes M(2010)Biomass and net primary production of Central Amazonian floodplains forests.In:Junk WJ,Piedade MTF,Wittmann F(eds)Amazonian floodplain forests:ecophysiology,biodiversity and sustainable management.Springer,Berlin,pp 347–388
Sch?ngart J,Arieira J,Felfili Fortes C,Cezarine de Arruda E,Nunes da Cunha C(2011)Age-related and stand-wise estimates of carbon stocks and sequestration in the aboveground coarse wood biomass of wetland forests in the northern Pantanal,Brazil.Biogeosciences 8:3407–3421
Terborgh J,Andresen E(1998)The composition of Amazonian forests:patterns at local and regional scales.J Trop Ecol 14:645–664
Van Nieuwstadt MGL,Sheil D(2005)Drought,fire and tree survival in a Borneo rain forest,East Kalimantan,Indonesia.J Ecol 93:191–201
Ward JV,Wiens JA(2001)Ecotones of riverine ecosystems:role and typology,spatio-temporal dynamics,and river regulation.Ecohydrol Hydrobiol 1:25–36
Williamson GB,Laurance WF,Oliveira AA,Delamonica P,Gascon C,Lovejoy TE,Pohl L(2000)Amazonia tree mortality during the 1997 El Ni?o drought.Cons Biol 14:1538–1542
Wittmann F,Zorzi BT,Tizianel FAT,Urquiza MVS,Faria RR,Sousa NM,Módena éS,Gamarra RM,Rosa ALM(2008)Tree species composition,structure and aboveground wood biomass of a riparian forest of the lower Miranda River,Southern Pantanal,Brazil.Folia Geobot 43:397–411
Wittmann F,Junk WJ,Sch?ngart J(2010)Phytogeography,species diversity,community structure and dynamics of central Amazonian floodplain forests.In:Junk WJ,Piedade MTF,Parolin P,Wittmann F,Sch?ngart J(eds)Central Amazonian Floodplain forests:ecophysiology,biodiversity and sustainable management.Springer,Ecological Studies
Wittmann F,Householder E,Piedade MTF,Assis RL,Sch?ngart J,Parolin P,Junk WJ(2013)Habitat specificity,endemism and the neotropical distribution of Amazonian white-water floodplain trees.Ecography 36:690–707
Worbes M(1997)The forest ecosystem of the floodplains.In:Junk W(ed)The Central Amazon Floodplain:Ecology of a Pulsating System.Ecological Studies 126,Springer,Berlin
Worbes M,Klinge H,Revilla JD,Martius C(1992)On the dynamics,floristic subdivision and geographical distribution of varzea forests in Central Amazonia.J Veg Sci 3:553–564
Wright SJ(2005)Tropical forests in a changing environment.Trends Ecol Evol 20:553–560
Zanne AE,Lopez-Gonzalez G,Coomes DA,Ilic J,Jansen S,Lewis SL,Miller RB,Swenson NG,Wiemann MC,Chave J(2009)Data from:Towards a worldwide wood economics spectrum.Dryad Digital Repository.doi:10.5061/dryad.234
*Correspondence:cleusch@gwdg.de
Plant Ecology,Albrecht von Haller Institute for Plant Sciences,University of Goettingen,Untere Karspüle 2,37073 Goettingen,Germany
?The Author(s).2017 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0
International License(http://creativecommons.org/licenses/by/4.0/),which permits unrestricted use,distribution,and
reproduction in any medium,provided you give appropriate credit to the original author(s)and the source,provide a link to the Creative Commons license,and indicate if changes were made.
Received:1 March 2017 Accepted:24 June 2017
- Forest Ecosystems的其它文章
- Development and evaluation of robust tree biomass equations for rubber tree(Hevea brasiliensis)plantations in India
- Climate change effects in the Western Himalayan ecosystems of India:evidence and strategies
- Dead standing pine trees in a boreal forest landscape in the Kalevala National Park,northern Fennoscandia:amount,population characteristics and spatial pattern
- Small mammal community response to early meadow–forest succession
- Tree species diversity and utilities in a contracting lowland hillside rainforest fragment in Central Vietnam
- Conspecific negative density dependence in American beech

