Expression changes of nerve cell adhesion molecules L1 and semaphorin 3A aTher peripheral nerve injury
Qian-ru He, Meng Cong, Qing-zhong Chen, Ya-feng Sheng Jian Li, Qi Zhang Fei Ding Yan-pei Gong,
1 Jiangsu Key Laboratory of Neuroregeneration, Co-Innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China
2 Afliated Hospital of Nantong University, Nantong, Jiangsu Province, China
Expression changes of nerve cell adhesion molecules L1 and semaphorin 3A aTher peripheral nerve injury
Qian-ru He1,#, Meng Cong1,#, Qing-zhong Chen2, Ya-feng Sheng1, Jian Li2, Qi Zhang1, Fei Ding1, Yan-pei Gong2,*
1 Jiangsu Key Laboratory of Neuroregeneration, Co-Innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China
2 Afliated Hospital of Nantong University, Nantong, Jiangsu Province, China
How to cite this article:He QR, Cong M, Chen QZ, Sheng YF, Li J, Zhang Q, Ding F, Gong YP (2016) Expression changes of nerve cell adhesion molecules L1 and semaphorin 3A aTher peripheral nerve injury. Neural Regen Res 11(12):2025-2030.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:This study was supported by the National Natural Science Foundation of China, No. 81371389, 31500927, 31300942, 81201017; the Collegiate Natural Science Foundation of Jiangsu Province of China, No. 13KJB180018; the Natural Science Foundation of Nantong University of China, No. 14ZY013.
Graphical Abstract

Nerve cell adhesion molecules L1 and semaphorin 3A (SEMA 3A) show diferent expression patterns at the proximal and distal ends of injured sensory and motor nerves
The expression of nerve cell adhesion molecule L1 in the neuronal growth cone of the central nervous system is strongly associated with the direction of growth of the axon, but its role in the regeneration of the peripheral nerve is still unknown. This study explored the problem in a femoral nerve section model in rats. L1 and semaphorin 3A mRNA and protein expressions were measured over the 4-week recovery period. Quantitative polymerase chain reaction showed that nerve cell adhesion molecule L1 expression was higher in the sensory nerves than in motor nerves at 2 weeks aTher injury, but vice versa for the expression of semaphorin 3A. Western blot assay results demonstrated that nerve cell adhesion molecule L1 expression was higher in motor nerves than in the sensory nerves at the proximal end aTher injury, but its expression was greater in the sensory nerves at 2 weeks. Semaphorin 3A expression was higher in the motor nerves than in the sensory nerves at 3 days and 1 week aTher injury. Nerve cell adhesion molecule L1 and semaphorin 3A expressions at the distal end were higher in the motor nerves than in the sensory nerves at 3 days, 1 and 2 weeks. Immunohistochemical staining results showed that nerve cell adhesion molecule L1 expression at the proximal end was greater in the sensory nerves than in the motor nerves; semaphorin 3A expression was higher in the motor nerves than in the sensory nerves at 2 weeks aTher injury. Taken together, these results indicated that nerve cell adhesion molecules L1 and semaphorin 3A exhibited diferent expression patterns at the proximal and distal ends of sensory and motor nerves, and play a coordinating role in neural chemotaxis regeneration.
nerve regeneration; neural cell adhesion molecule L1; semaphorin 3A; sensory nerve; motor nerve; peripheral nerve injury; chemotaxis regeneration; neural regeneration
Introduction
The recovery of injured peripheral nerve depends on the exact connection between the regenerating sensory and motor nerves, i.e., chemotaxis regeneration. Recognition molecules such as the immunoglobulin superfamily of cell adhesion molecules are expressed on the surface of nerve cells. Interstitial cells synthesize and secrete a variety of neurotransmitters that attract or repel neuronal axons to guide axon extension; thereby regenerating axons enter the correct sensory or motor pathway (Maness and Schachner, 2007; Gordon et al., 2015).
Nerve cell adhesion molecule L1 (L1cam, L1), which belongs to the immunoglobulin superfamily cell adhesion molecule (Pollerberg et al., 2013), is mainly expressed in the developing or regenerating peripheral nervous system, promotes axon growth, and participates in myelination (Manessand Schachner, 2007; Tonosaki et al., 2014). L1 knockout mice presented axon loss and abnormal axon projection in sensory nerves, suggesting that L1 exerted an important effect on axon-Schwann cell interaction (Law et al., 2008). Our previous study showed that L1 expression in the sensory nerves of normal rats was 5.8 times than that of motor nerves, as measured by comparative proteomics technology (He et al., 2012b). Numerous studies on the central nervous system demonstrated that the interaction of L1 in the neuronal growth cone and semaphorin 3A (SEMA 3A) was strongly associated with the direction of growth of the axon (Castellani et al., 2000; Piper et al., 2009; Dang et al., 2012).The efects of L1 and SEMA 3A on peripheral nerve regeneration remain poorly understood, therefore, we established rat models of sensory and motor nerve injuries. We analyzed the changes in L1 and SEMA 3A expression at the proximal and distal ends of nerves at various time points aTher injury.This provided a foundation for investigating the L1 efect on peripheral nerve chemotaxis regeneration.
Materials and Methods
Ethics statement
Rats received humane care in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health). All procedures were approved by the Jiangsu Experimental Animal Management Committee in China. Precautions were taken to minimize sufering and the number of animals used in each experiment.
Preparation of models of sensory and motor nerve injuries
A total of 159 healthy male specific-pathogen-free Sprague-Dawley rats aged 2 months and weighing 180-200 g were provided by the Experimental Animal Center of Nantong University of China (animal license No. SCXK (Su) 2008-0010). Of them, 150 rats were randomly allocated to a normal control group (n = 30) and a nerve injury group (n = 120). In the nerve injury group, rats were intraperitoneally anesthetized with sodium pentobarbital (30 mg/kg) (Merck, Darmstadt, Germany). Saphenous nerves (sensory nerves) and muscular branches (motor nerves) innervated by the femoral nerve were dissociated on the medial side of the right hind limb. A 0.5-1 cm nerve defect was made by cutting the middle of the saphenous nerves and the muscular branches. The wound was washed and the skin was sutured then sterilized. The rats were returned to their cages (H?ke et al., 2006). In the normal control group, the saphenous nerves and muscular branches were only dissociated.
Sample collection
Nerves at the proximal and distal ends of the injury site were harvested at 3 days, 1, 2 and 4 weeks in the nerve injury group. Approximately 1 cm-long sections of the saphenous nerve and a muscular branch innervated by the femoral nerve were taken from rats in the normal control group. Experimental rats were sacrifced by cervical dislocation.
Real-time quantitative polymerase chain reaction (PCR)Normal saphenous nerves and muscular branches were collected from fve rats in the normal control group. The proximal and distal ends of injured saphenous nerves and muscular branches were collected from fve rats at each of the various time points in the nerve injury group. RNA was extracted by the Trizol method (He et al., 2012a). cDNA was synthesized in accordance with the instructions of PCR kit (Qiagen, Valencia, CA, USA). The primers were synthesized by the Shanghai Generay Biotech Co., Ltd., China. All primer sequences are listed in Table 1. SYBR Green (Roche, Mannheim, Germany) was added for PCR amplification. Amplification conditions were as follows: 95°C for 6 minutes, 44 cycles of 95°C for 10 seconds, 60°C for 30 seconds and 70°C for 10 seconds. In the real-time PCR amplifcation process, the fuorescence signals are collected and transformed into amplifcation and melting curve. ATher amplifcation, the melting curves were analyzed. PCR products were quantified with the 2-ΔCT. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal reference. Experiments were performed in triplicate.
Western blot assay
Normal saphenous nerve and muscular branch nerve tissues were collected from five rats in the control group. Samples of injured saphenous nerves and muscular branches at the proximal and distal ends were collected from fve rats at each of the various time points in the nerve injury group. Total protein was extracted by using T-PER Tissue Protein Extraction Reagent (Pierce, Rockford, IL, USA). Total protein concentration was measured using the Bradford method (He et al., 2012b). After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), proteins were transferred onto the membrane. Membranes were blocked, and incubated with primary antibody mouse monoclonal anti-neural cell adhesion molecule L1 (1:500; Abcam, Cambridge, MA, USA), rabbit polyclonal anti-semaphorin 3A (1:1,000; Abcam), and mouse monoclonal to β-actin (1:10,000; Abcam) at 4°C overnight. The next day, membranes were rinsed in Tris-buffered saline with Tween-20 and incubated with secondary antibody anti-rabbit IgG (H&L) (DONKEY) antibody (1:5,000; Rockland Immunochemicals, Inc., Pottstown, PA, USA) or anti-mouse IgG (H&L) (DONKEY) antibody (1:5,000; Rockland Immunochemicals, Inc.) at room temperature for 1.5 hours. Phosphate bufered saline (PBS), 0.01 M, instead of primary antibody, served as negative control. ATher visualization, the result was conducted with an Odyssey Infrared Imaging System (Odyssey, Lincoln, NE, USA). Optical density values were analyzed with the PDQuest 7.2.0 software (Bio-Rad, Richmond, CA, USA). β-Actin was used as the internal reference. Experiments were conducted in triplicate.
Immunohistochemical staining
The remaining nine Sprague-Dawley rats were used to establish models of sensory and motor nerve injuries by the method mentioned above. Two weeks aTher injury, rats were anesthetized and perfused with 4% paraformaldehyde.Saphenous nerves and muscular branches at the proximal and distal ends of the injury site were harvested and postfxed overnight. The tissues were then dehydrated with 30% sucrose solution, embedded, and sliced into sections with a freezing microtome. ATher blocking with a solution containing 10% goat serum, 3% bovine serum albumin, and 0.1% Triton-X100, sections were incubated with primary antibody mouse monoclonal anti-rat neural cell adhesion molecule L1 (1:500; Abcam), rabbit polyclonal anti-semaphorin (anti-SEMA) 3A (1:500; Abcam) at 4°C overnight. After three washes with PBS, sections were incubated with secondary antibody FITC-labeled goat anti mouse IgG (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Cy3-labeled goat anti-rabbit IgG (1:200; Santa Cruz Biotechnology) at room temperature in the dark for 2 hours. Primary antibody was not added in blank controls. The samples were mounted with fuorescent mounting medium (Biyuntian, Haimeng, China), photographed and recorded with laser scanning confocal microscope (TCS SP5, Leica Microsystems, Germany). Ten felds were randomly selected from each group. Images were semi-quantitatively analyzed with Image Pro Plus 7C soThware (Media Cybernetics, Silver Spring, MD, USA). Mean fuorescence intensity (mean density) was calculated.
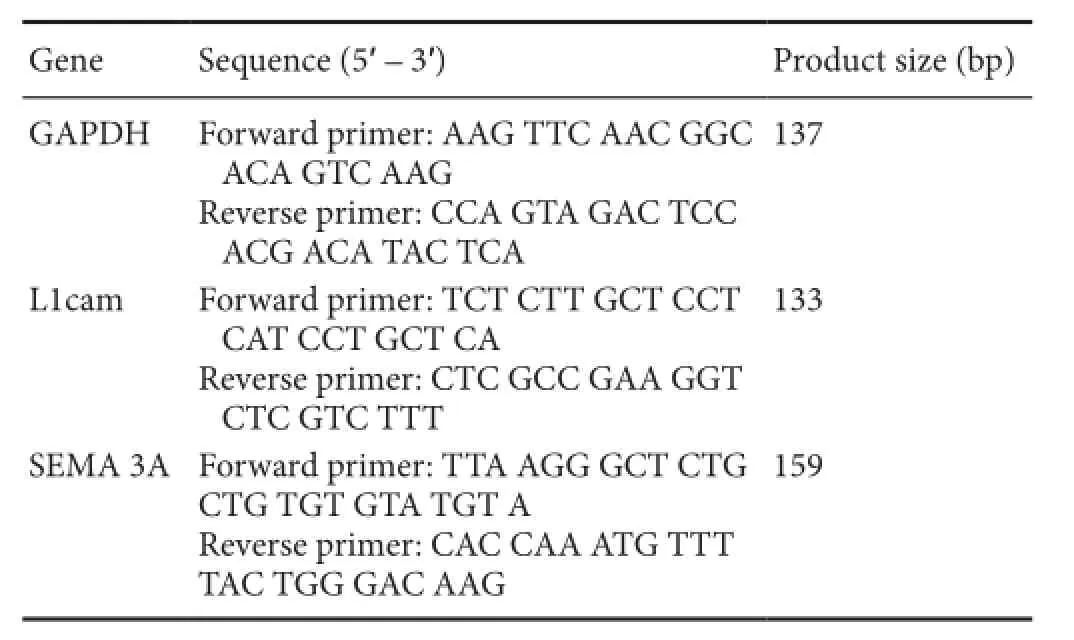
Table 1 Primer sequences for polymerase chain reaction
Statistical analysis
All data, expressed as the mean ± SD, were analyzed with SPSS 17.0 soThware (SPSS Inc., Chicago, IL, USA). Mean between the two groups was compared using two-sample t-test. A value of P < 0.05 was considered statistically signifcant.
Results
Changes in mRNA expression aTher sensory and motor nerve injuries
PCR results revealed that L1 mRNA expression was higher in sensory nerves than in motor nerves in the normal control group (P < 0.01). As post injury time increased, L1 mRNA expression in the proximal ends of sensory nerves decreased and then increased. By 2 weeks, L1 mRNA expression was signifcantly higher in sensory nerves than in motor nerves (P< 0.01; Figure 1A). L1 mRNA expression at the distal ends was higher in the motor nerves than in the sensory nerves at 2 weeks (P < 0.05; Figure 1B).
SEMA 3A mRNA expression in the injured sensory and motor nerves
PCR results showed that SEMA 3A mRNA expression was signifcantly higher in the motor nerves than in the sensory nerves in the normal control group (P < 0.05). SEMA 3A mRNA expression at the proximal end of sensory nerves gradually increased aTher injury. The diference in SEMA 3A mRNA expression between sensory and motor nerves lessened gradually. At 2 weeks aTher injury, SEMA 3A mRNA expression of motor nerves was 6.5 times than that of sensory nerves at the distal end (P < 0.01; Figure 2).
Changes in L1 and SEMA 3A expression in sensory and motor nerves aTher injury
Western blot assay results showed that L1 protein expression was higher in sensory nerves than in motor nerves in the normal control group (P < 0.05). L1 expression was diminished in the sensory and motor nerves at the proximal end to diferent degrees aTher injury. L1 expression was higher in motor nerves than in sensory nerves at the proximal end at 3 days and 1 week (P < 0.05), but the diference in expression was reversed at 2 weeks (P < 0.05; Figure 3B).
SEMA 3A expression was greater in motor nerves than in sensory nerves in the normal control group (P < 0.05). SEMA 3A expression was higher in motor nerves than in sensory nerves at the proximal end at 3 days and 1 week (P < 0.05), but SEMA 3A expression levels were similar in sensory and motor nerves at both 2 and 4 weeks (P > 0.05; Figure 3C).
At 3 days, 1 and 2 weeks aTher injury, L1 and SEMA 3A expressions at the distal ends were greater in the motor nerves than in the sensory nerves (P < 0.05), but were similar at 4 weeks (P > 0.05; Figure 4).
Immunohistochemical staining further verified that at 2 weeks after injury, L1 expression at the proximal ends was higher in sensory nerves than in motor nerves (P < 0.01), but at the distal end, SEMA 3A expression was greater in motor nerves than in sensory nerves (P < 0.05; Figure 5).
Discussion
During development and regeneration of the nervous system, a neurite grows along a specific pathway; and the chemotaxis-induced extension of the neurite is due to the complex and precise interaction between neurons, neurons and glial cells and extracellular matrices (Allodi et al., 2012). To maintain these specifc interactions, neural cells must express a series of recognition molecules, such as the immunoglobulin superfamily cell adhesion molecules, integrins and receptor tyrosine kinase. Moreover, neural interstitial cells contain a variety of guiding factors for attraction or repulsion, such as semaphorins, slit, netrins and ephrins (Bielle et al., 2011; Wang et al., 2011).
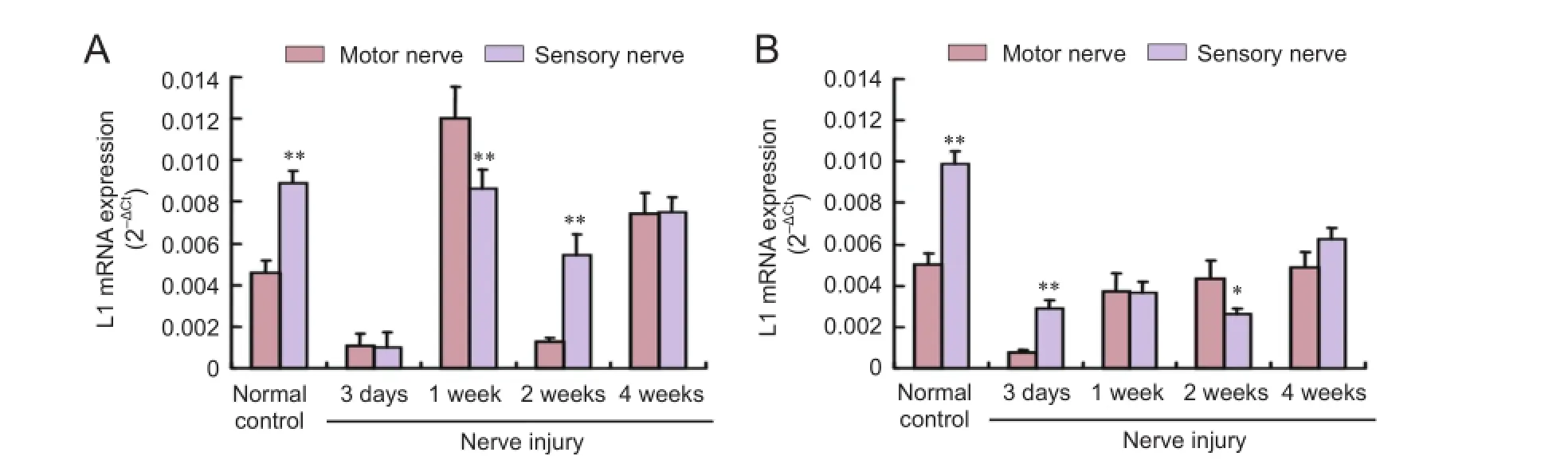
Figure 1 L1 mRNA expression in injured sensory and motor nerves.
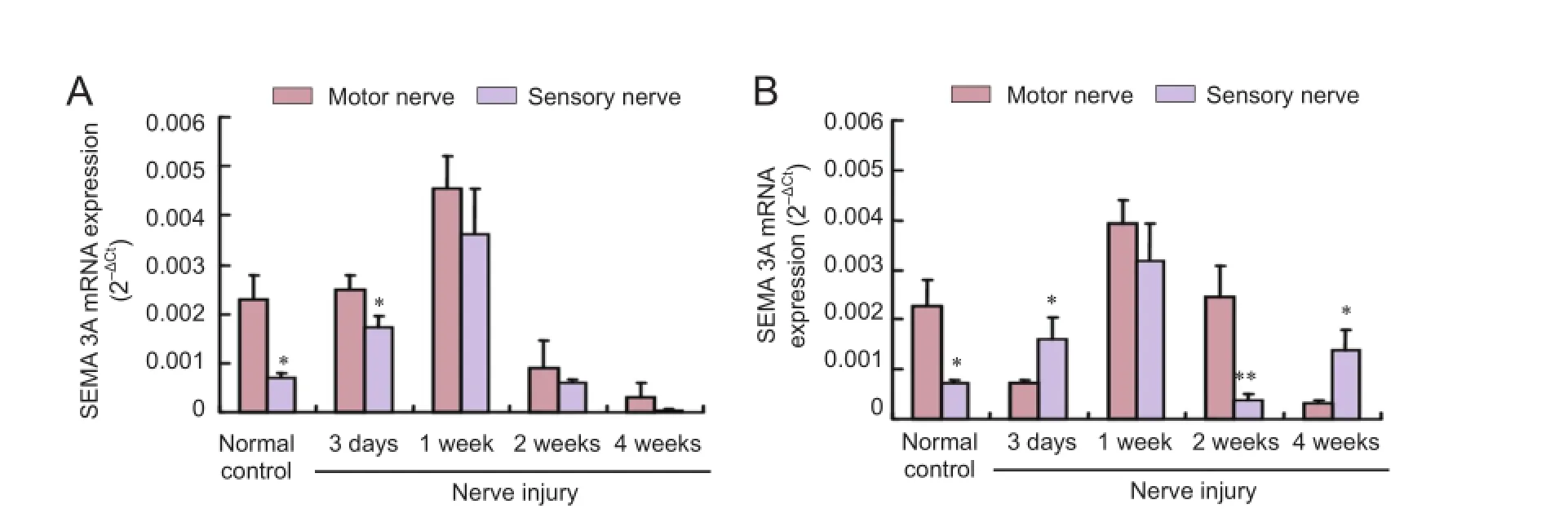
Figure 2 SEMA 3A mRNA expression in injured sensory and motor nerves.
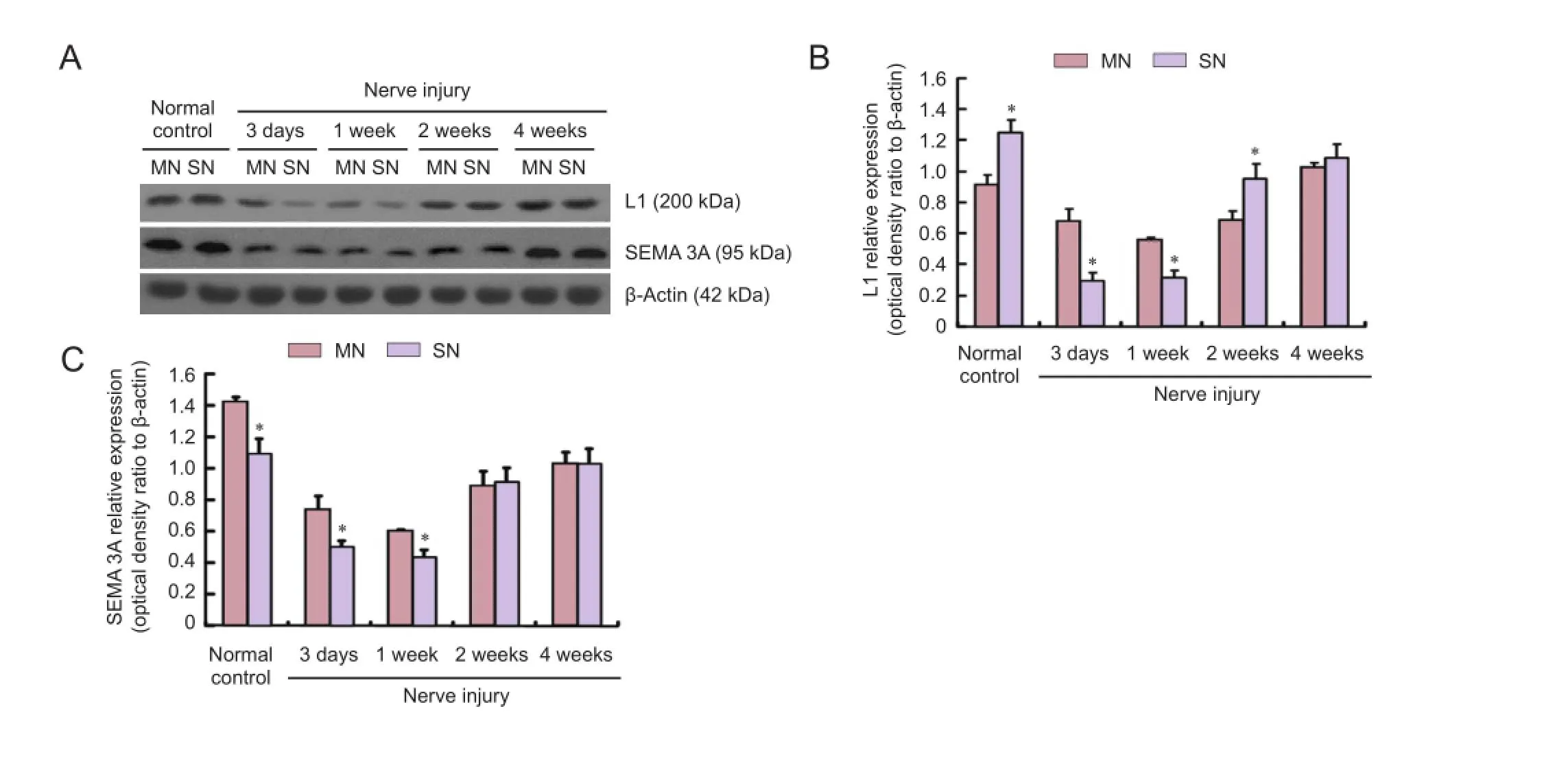
Figure 3 Western blot assay of L1 and SEMA 3A protein expressions at the proximal end of injured sensory and motor nerves.
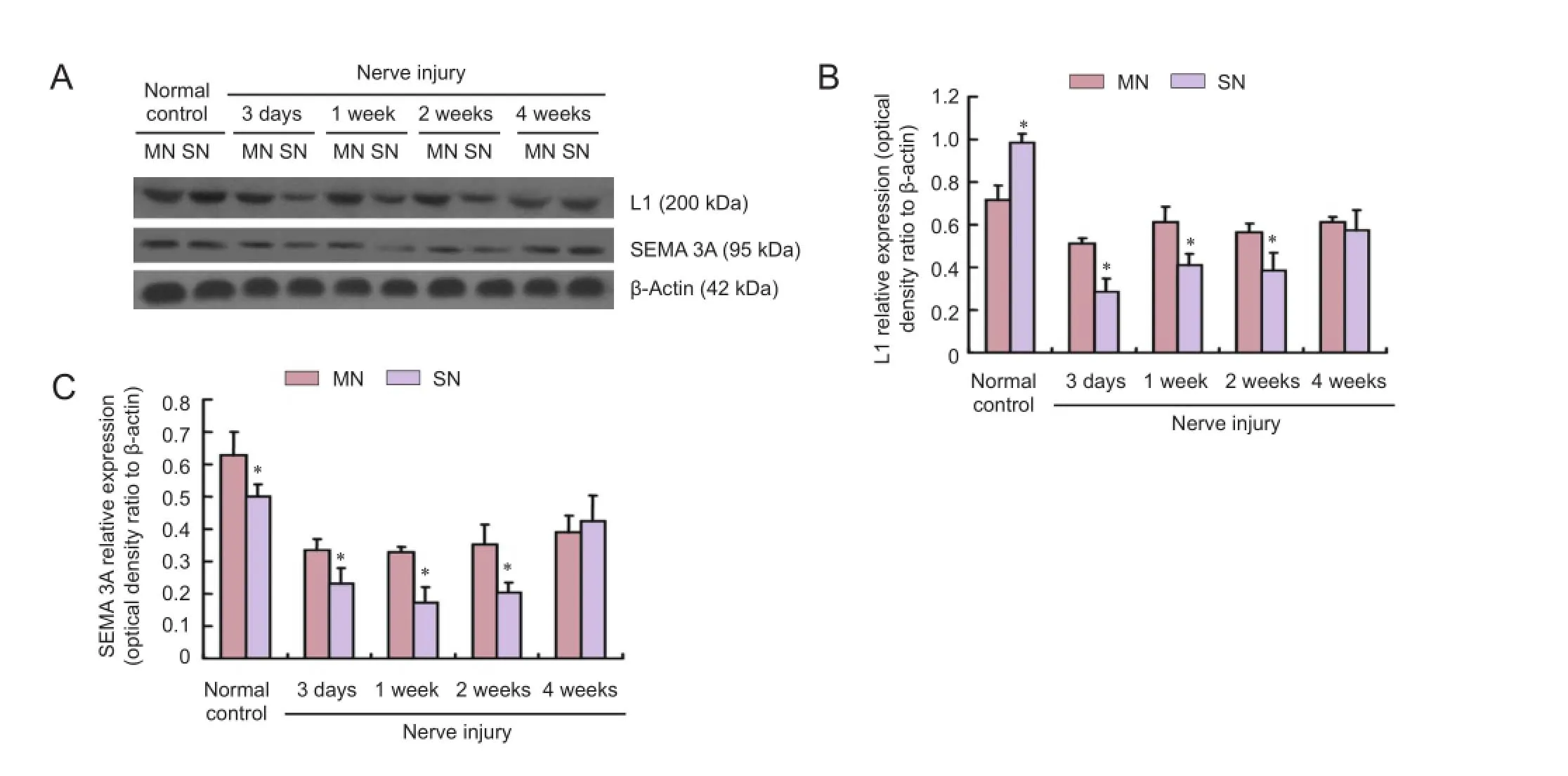
Figure 4 L1 and SEMA 3A protein expressions at the distal end of injured sensory and motor nerves (western blot assay).
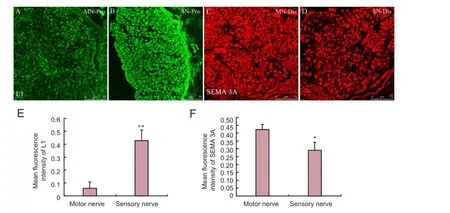
Figure 5 L1 and SEMA 3A protein expressions in sensory and motor nerves at 2 weeks aTher injury.
Previous studies suggested that L1 knockout mice presented with a loss of sensory nerve axons and lacked myelin sheaths, leading to a decrease in sensory function. The sensory nerve axons projected prematurely to the dorsal horn of the spinal cord during the development of L1 deletion mice, indicating that L1 played an important role in sensory nerve axon-Schwann cell interactions (Haney et al., 1999; Law et al., 2008). Our results confrmed that L1 mRNA and protein expressions were high in the sensory nerves of the normal control group, which were consistent with our previous study on sensory and motor nerve proteomics (He et al., 2012b). In the early stage of injury (3 days and 1 week), L1 expression decreased at the proximal end of sensory and motor nerves to diferent degrees. The decrease in protein expression was probably because of retrograde degeneration, cell injury, and axonal retraction at the proximal end of nerves aTher nerve transection. Nevertheless, L1 protein decreased to diferent degrees in diferent nerve types. At 3 days and 1 week aTher injury, L1 expression was high at the proximal end of motor nerves. At 2 weeks, L1 expression was high in the sensory nerves. Immunohistochemical results also verified that L1 protein expression was high at the proximal end of sensory nerves at 2 weeks. Thus, the second week is a key period of chemotaxis regeneration. The high expression of L1 at the proximal end of sensory nerves suggests that L1 is closelylinked to chemotaxic regeneration.
Semaphorins are a class of secreted and membrane proteins that were originally identifed as axonal growth cone guidance molecules, and their family members are mainly considered medium- and short-range repulsive guidance molecules in axonal growth (Cheng et al., 2001; Zhang et al., 2014). Many neurons, such as dorsal root ganglion neurons, motor neurons, hippocampal neurons, and sympathetic neurons, are afected by semaphorins (Gu et al., 2003; Carcea et al., 2010). L1 and SEMA 3A interactions in neuronal growth cone are strongly associated with axon guidance. The L1-SEMA3A signaling pathway plays a key role in the normal projection of nerve fber axons in the corpus callosum and cortical neurodevelopment (Gu et al., 2003; Law et al., 2008). Castellani et al. (2000) confirmed that SEMA3A secreted from the anterior horn of the spinal cord repelled cortical neurons and the axons of the dorsal root ganglion, also that transmembrane L1 expression in a growth cone played an important role in rejection. Our results showed that SEMA 3A mRNA and protein expression was high in motor nerves of the normal control group. At 2 weeks after injury, i.e., the key stage of chemotaxis regeneration, SEMA 3A mRNA and protein expressions at the distal end were remarkably higher in motor nerves than in sensory nerves. During nerve regeneration, Schwann cells were regularly arranged after proliferation and formed a pathway for axon regeneration, simultaneously secreted a large number of neurotrophic factors and nerve guidance factors to precisely guide the regenerating axon. Therefore, we presumed that highly expressed SEMA 3A at the distal end of motor neurons rejected the regenerating sensory nerve axons away from the wrong motor nerve pathway. Instead they entered the correct sensory pathway, probably through interacting with the highly expressed L1 at the proximal end of the sensory nerves.
Bechara et al. (2008) demonstrated that SEMA3A-induced atrophy and collapse of neuronal growth cones aTher acting on L1 and transmembrane protein 1 is mainly mediated by activating the FAK-MAPK signaling pathway. Moreover, transient expression of axonal glycoprotein 1 plays a key role in endocytosis and transport aTher regulating the interaction of L1 and transmembrane protein 1 with SEMA 3A (Dang et al., 2012). The complicated molecular mechanisms of L1 and SEMA 3A on chemotaxis regeneration of sensory and motor nerves deserve further investigation.
In summary, L1 and SEMA 3A present diferent expression patterns at the proximal and distal ends of injured sensory and motor nerves over time and possibly play a coordinating role in the chemotaxic regeneration of peripheral nerves.
Author contributions:QRH conceived and designed the study, performed the experiments, analyzed data and wrote the paper. MC conducted experiments and analyzed data. YFS and JL prepared animal models. QZ participated in statistical analysis and critical revision of the paper for important intellectual content. QZC was responsible for proofreading. FD served as a principle investigator and obtained funding. YPG served as a principle investigator, was in charge of paper authorization and statistical analysis. All authors approved the fnal version of the paper.
Conficts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:This paper was double-blinded and stringently reviewed by international expert reviewers.
Allodi I, Udina E, Navarro X (2012) Specifcity of peripheral nerve regeneration: Interactions at the axon level. Prog Neurobiol 98:16-37.
Bechara A, Nawabi H, Moret F, Yaron A, Weaver E, Bozon M, Abouzid K, Guan JL, Tessier-Lavigne M, Lemmon V, Castellani V (2008) FAKMAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. EMBO J 27:1549-1562.
Bielle F, Marcos-Mondéjar P, Leyva-Díaz E, Lokmane L, Mire E, Mailhes C, Keita M, García N, Tessier-Lavigne M, Garel S, López-Bendito G (2011) Emergent growth cone responses to combinations of Slit1 and Netrin 1 in thalamocortical axon topography. Curr Biol 21:1748-1755.
Carcea I, Ma’ayan A, Mesias R, Sepulveda B, Salton SR, Benson DL (2010) Flotillin-mediated endocytic events dictate cell-type specifc responses to Semaphorin 3A. J Neurosci 30:15317-15329.
Castellani V, Chédotal A, Schachner M, Faivre-Sarrailh C, Rougon G (2000) Analysis of the L1-defcient mouse phenotype reveals crosstalk between Sema3A and L1 signaling pathways in axonal guidance. Neuron 27:237-249.
Cheng HJ, Bagri A, Yaron A, Stein E, Pleasure SJ, Tessier-Lavigne M (2001) Plexin-A3 mediates semaphorin signaling and regulates the development of hippocampal axonal projections. Neuron 32:249-263.
Dang P, Smythe E, Furley AJ (2012) TAG1 regulates the endocytic trafcking and signalling of the Semaphorin3A receptor complex. J Neurosci 32:10370-10382.
Gordon T, Eva P, Borschel GH (2015) Delayed peripheral nerve repair: methods, including surgical ‘cross-bridging’ to promote nerve regeneration. Neural Regen Res 10:1540-1544.
Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD (2003) Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell 5:45-57.
H?ke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM (2006) Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci 26:9646-9655.
Haney CA, Sahenk Z, Li C, Lemmon VP, Roder J, Trapp BD (1999) Heterophilic binding of L1 on unmyelinated sensory axons mediates Schwann cell adhesion and is required for axonal survival. J Cell Biol 146:1173-1184.
He Q, Man L, Ji Y, Ding F (2012a) Comparison in the biological characteristics between primary cultured sensory and motor Schwann cells. Neurosci Lett 521:57-61.
He Q, Man L, Ji Y, Zhang S, Jiang M, Ding F, Gu X (2012b) Comparative proteomic analysis of diferentially expressed proteins between peripheral sensory and motor nerves. J Proteome Res 11:3077-3089.
Law CO, Kirby RJ, Aghamohammadzadeh S, Furley AJ (2008) The neural adhesion molecule TAG-1 modulates responses of sensory axons to difusible guidance signals. Development 135:2361-2371.
Maness PF, Schachner M (2007) Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci 10:19-26.
Piper M, Plachez C, Zalucki O, Fothergill T, Goudreau G, Erzurumlu R, Gu C, Richards LJ (2009) Neuropilin 1-Sema signaling regulates crossing of cingulate pioneering axons during development of the corpus callosum. Cereb Cortex 19 Suppl 1:i11-21.
Pollerberg GE, Thelen K, Theiss MO, Hochlehnert BC (2013) The role of cell adhesion molecules for navigating axons: Density matters. Mech Dev 130:359-372.
Tonosaki M, Itoh K, Umekage M, Kishimoto T, Yaoi T, Lemmon VP, Fushiki S (2014) L1cam is crucial for cell locomotion and terminal translocation of the Soma in radial migration during murine corticogenesis. PLoS One 9:e86186.
Wang L, Klein R, Zheng B, Marquardt T (2011) Anatomical coupling of sensory and motor nerve trajectory via axon tracking. Neuron 71:263-277.
Zhang L, Kaneko S, Kikuchi K, Sano A, Maeda M, Kishino A, Shibata S, Mukaino M, Toyama Y, Liu M, Kimura T, Okano H, Nakamura M (2014) Rewiring of regenerated axons by combining treadmill training with semaphorin3A inhibition. Mol Brain 7:14.
Copyedited by Ann Dawes E, Frenchman B, Yu J, Qiu Y, Li CH, Song LP, Zhao M
*Correspondence to: Yan-pei Gong, M.D., gonghand@hotmail.com.
#These authors contributed equally to this study.
orcid: 0000-0002-4847-9669 (Yan-pei Gong)
10.4103/1673-5374.197148
Accepted: 2016-06-16
- 中國神經(jīng)再生研究(英文版)的其它文章
- Injury of the arcuate fasciculus in a patient with progressive bulbar palsy
- “Three Methods and Three Points” regulates p38 mitogen-activated protein kinase in the dorsal horn of the spinal cord in a rat model of sciatic nerve injury
- Biodegradable magnesium wire promotes regeneration of compressed sciatic nerves
- Electroacupuncture at Dazhui (GV14) and Mingmen (GV4) protects against spinal cord injury: the role of the Wnt/β-catenin signaling pathway
- Application of a paraplegic gait orthosis in thoracolumbar spinal cord injury
- Fine motor skill training enhances functional plasticity of the corticospinal tract aTher spinal cord injury