Effect of surface ligand density on cytotoxicity and pharmacokinetic profle of docetaxel loaded liposomes
Shenyang Pharmaceutical University,Shenyang,China
Effect of surface ligand density on cytotoxicity and pharmacokinetic profle of docetaxel loaded liposomes
Chun Chu,Ping Xu,Haoyue Zhao,Qing Chen,Dawei Chen, Haiyang Hu,Xiuli Zhao,Mingxi Qiao*
Shenyang Pharmaceutical University,Shenyang,China
A R T I C L EI N F O
Article history:
Received 16 March 2016
Received in revised form 21 April 2016
Accepted 22 April 2016
Available online 10 May 2016
Docetaxel
Liposomes
Biotin
Ligand density
Tumor targeting
Various biotin-modifed liposomes incorporated with docetaxel(DTX)were prepared to study the effect of surface biotin density on the pharmacokinetic profle of the liposome.Four types of liposomes such as PEG modifed liposome(PDL),0.5%(mol)biotin modifed liposome (0.5BDL),1%(mol)biotin modifed liposome(1BDL)and 2%(mol)biotin modifed liposome (2BDL)were prepared using thin flm dispersion method.The prepared liposomes were characterized by measuring encapsulation effciency(EE),particle size,Zeta-potential,physical stability and drug release proflesin vitro.MTT assay was performed to elevate the cytotoxicity of liposomes on MCF-7 cells.In vivoevaluation was further performed to investigate the effect of biotin surface density on the pharmacokinetic profles.All the prepared liposomes exhibited high encapsulation effciency,small particle size,narrow particle distribution and sustained release proflesin vitro.In MTT assay,0.5BDL showed largest tumor cell toxicity,compared with DTX solution.All liposomes containing DTX showed prolonged blood circulationin vivo,and 0.5BDL showed the longest circulation time among the biotin modifed liposome.Surface modifcation of liposome had a negative impact on the circulation of liposomes in the blood,which needs to be considered when designing the ligand mediated targeting delivery systems.A proper amount of biotin liposome with 0.5%molar ratio is expected to produce the best anti-tumor effect.
?2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Docetaxel,a second-generation semi-synthetic taxane derivative has shown dramatic antitumor activities,mostly against various human cancers such as ovarian carcinoma,advanced breast cancer,lung cancer and head/neck cancer[1].Currently,docetaxel is formulated using Tween80 and ethanol (50:50,v/v)as solvent;however,its clinical use is limited due to its side effects related to formulation,such as neutropenia,peripheral neuropathy and hypersensitivity reactions[1,2]. Therefore,there is a strong rationale for reformulating docetaxel using a safer vehicle than Tween80.
Recently,many carriers have been studied,such as nanoparticle-aptamer,bioconjugates[3],DTX loaded liposomes[4],pegylated liposomes[4,5],N-palmitoyl chitosan anchored DTX liposomes[6],and pegylated immunoliposomes [7].Liposomes with spherical lipid bilayer structures are one of the most successful drug carriers in drug delivery systems; however,they are prone to be taken up by reticuloendothelial system(RES)cells in liver and spleen[8,9].As is well known that PEG modifed liposomes exhibit a long circulation property in the blood and accumulate in tumorviapassive targeting [4,10,11],increasing evidence has suggested that the selectivity of PEG modifed liposomes is far from satisfaction.Therefore, many researchers have been focusing on developing active targeting drug delivery systems,in which many ligands have been introduced to the surface of drug carrier,such as folic acid[12], antibody[13]and integrin αvβ3[14].Biotin,a member of the vitamin family(vitamin H),is a growth promoter of cells.Its ligand in cancerous tumors is higher than in normal tissue[15] because rapid proliferation of cancer cells requires extra biotin. Some cancer cells including JC,Colo-26,P815 and MCF-7 overexpress biotin-specifc receptors[16,17],which are responsible for the uptake of essential nutrients such as biotin,lipoate,pantothenate[18]and peptides[19].Biotin has been considered as a promising ligand for active targeting[16,20–22];Yang et al. prepared biotin–dendrimer conjugate,which exhibited much higher cellular uptake into Hela cells than the dendrimer without biotin modifcation[23].Biotinylated pullulan acetate nanoparticles was prepared by Na,which has been shown strong adsorption to the HepG2 cells[24].Biotin-conjugated polymeric micelles could effectively release doxorubicin in acidic tumor cells compared to that without biotin[25].
However,the effect of biotin modifcation and density on cytotoxicity and pharmacokinetic profles of the carriers has not been explored to date.Previous studies have shown that the cellular uptake increased with the increase of ligand density on the surface of particles[26–28].However,dense surface coverage of ligand may not produce expected improvements in cellular uptake[29,30].Moreover,some studies have shown that the insertion of ligand resulted in faster clearance of liposomes from plasma,which compromised the accumulation of liposomes in tumor via EPR effect[31,32].It was reported that the 2.56 mol%NGR(asparagine–glycine–arginine amino acid sequence)resulted in lower total tumor accumulation than the formulation with only 0.64 mol%NGR[33].
As known,the success of an active targeting strategy relied heavily on the accumulation of the carriers at tumor site via passive targeting.If the drug loaded carriers were quickly eliminatedin vivo,the total tumor accumulation of carriers will be decreased.The pharmacokinetic profles of the active targeting formulation are important factors that need to be considered to achieve successive active targeting.
The researches on the effect of biotin density of liposome on cytotoxicity and pharmacokinetic profles were absent.In this study,biotin was conjugated to PEG chains on the surface of liposomes containing DTX,in an attempt to improve cancer targeting.DTX loaded liposomes modifed with different biotin density were prepared to investigate the effect of biotin density on the cytotoxicity and the pharmacokinetic profles of liposomes in blood.This study will lay a foundation for optimization of liposome formulation for furtherin vivoevaluation.
2.Materials and methods
2.1.Materials
Docetaxel was purchased from Jiansu Hengrui Medicinal Co., Ltd(Jiangsu,China).Cholesterol(Chol)was purchased from Tianjin Bodi Chemical Industry Co.(Tianjin,China).Soy phosphatidylcholine(Spc)was purchased from Shanghai Taiwei Pharmacetical Co.(Shanghai,China).Dichloromethane was purchased from Tianjin Jingxi Chemical Industry Co.(Tianjin, China).Disteroylphophatidyl ethanolamine methoxypolyethylene glycol conjugate(DSPE-PEG2000)was purchased from Nippon Fine Chemical Co.,Ltd(Kobe,Japan)and Biotin-PEG2000-DSPE was purchased from Creative PEG Works Co.(Winston-Salem,NC,USA).Methanol,acetonitrile and tert-butyl methyl ether were of high-performance liquid chromatography(HPLC) grade,all other regents and solvents used were of analytical grade.Sprague–Dawley rats(200±20 g)were supplied by the Laboratory Animal Center of Shenyang Pharmaceutical University(Shenyang,China).All animal procedures were approved by the Animal Ethics Committee of Shenyang Pharmaceutical University.
2.2.Preparation of liposomes
Liposomes were prepared by thin-flm hydration method.Briefy, to prepare PEG modifed liposome(PDL),Spc Chol(Spc:Chol, 9:2,molar ratio)and DSPE-PEG(4%mol)as well as DTX(1:18, mass ratio)were frst dissolved in dichloromethane solutions.The mixture was placed in a round-bottomed fask and the solvent was removed by rotary evaporation at 37°C under vacuum to obtain the dry flm.Afterward,the flm was hydrated with pH 7.4 PBS buffer in a water bath at 40°C with stirring for 20 min until a homogenous liposome suspension was obtained.The suspension was then sonicated with a ultrasonic cell disruptor,and the pulse function was 220w,on 1 s and off 1 s for 2 min.Polycarbonate flters,with a pore size of 220 nm were used to decrease the size of liposomes.The liposomes were purifed by centrifugating at 15 000×gfor 15 min.Biotin modifed liposomes(0.5BDL,1BDL,2BDL)were prepared using the same method mentioned above except that 0.5%,1%and 2%(mol)of DSPE-PEG2000were used respectively.
2.3.Characterization of liposomes
The DTX concentration was determined by high performance liquid chromatography(HPLC).In detail,the analysis was performed with a Hitachi HPLC system(UV Detector L-2400, Pump L-2130,Hitachi,Tokyo,Japan),equipped with a 20 μl loop and a reversed-phase column(Hypersil,ODS,4.6×250 mm, 5 μm).The mobile phase was made up of acetonitrile and water (60:40;v/v)at a fow rate of 1 ml/min,and the DTX was detected at a wavelength of 228 nm.Methanol was used before analysis as a demulsifer.The encapsulation effciency(EE%)and drug loading content(LC%)were calculated using equation 1 and equation 2:

where,Weis the amount of drug encapsulated,Waddis the amount of drug used andWtis total amount of encapsulated drug and liposome.
The diameter and Zeta potential of the liposomes were determined by Zetasizer3000(Malven Instruments Ltd.,UK). Intensity autocorrelation was measured at a scattering angle of 90 degrees at room temperature.
The morphological feature of the liposomes was observed with a transmission electron microscope(TEM)(JEM-1200EX, JEOL Ltd.,Japan)at 80 kV.A drop of liposomal sample was placed on a copper grid,excess water was blotted with a piece of flter paper and a drop of 1%phosphotungstic acid was added for negative staining.
Initial stability of liposomes was evaluated by detecting leakage of DTX from liposomes at 4°C.Encapsulation effciency of four liposomes was detected by HPLC at 1,2,3,4,5, 7,10,13 and 15 days after preparation.The leakage rate(LR%) was calculated using equation 3:

whereEEnis the encapsulation effciency detected at n day,andEE1is the encapsulation effciency detected at the frst day.
In vitrorelease of DTX from liposomes was determined using dialysis method with a pharmaceutical dissolution tester at 37°C.The samples were put into dialysis bags(COMW:8000–14,000),and PBS(80 ml,PH7.4)containing 0.5%(v/v)Tween80 was used as the release medium.Tween80 was added to ensure the sink condition.The stirring speed is 50 rpm.At each time point,2 ml release medium was withdrawn and replaced with fresh PBS.The concentration of DTX in the release medium was determined using HPLC.The mobile phase was made up of acetonitrile and water(52:48,v/v)at a fow rate of 1 ml/min.
2.4.Cytotoxicityin vitro
The human breast cell line MCF-7(purchased from Shanghai Fuleibao Bio-Tech Co.,Ltd)was maintained in Dulbecco’s modifed Eagle’s medium(DMEM)containing 10%fetal bovine serum and 0.1%antibiotics(penicillin streptomycin)in a 5%CO2humidifed atmosphere at 37°C.The MTT assay was used to test the cytotoxicityin vitro;the cells were seeded at 8×103cells/ well in 96 well plates and incubated for 24 h.The cells were then incubated in 96 well plates at 37°C for 24 h,48 h,and 72 h in the presence of a series of concentration of formulations, including DTX solution(FD),PDL,0.5BDL,1BDL,and 2BDL.The cells incubated in medium without any drug or liposomes were used as controls.20 μl of MTT(5 mg/ml)were added at the end of incubation period.The plates were incubated for an additional 4 h.DMSO(150 μl)were added to dissolve the formazan crystals and the absorbance value was determined at wavelengths of 490 nm.
2.5.Pharmacokinetic studies
SD rats were divided into fve groups randomly(three rats per group).PDL,0.5BDL,1BDL,and 2BDL were i.v.administration at a dose of 10 mg/kg and DTX in tween80(FD)was chosen as control group.Blood samples were collected at 5 min,10 min, 15 min,30 min,45 min,1 h,2 h,4 h,8 h,12 h and 24 h.The samples were then centrifuged at 5000 rpm for 10 min to separate the plasma;10 μl of paclitaxel(10 μg/ml)used as internal standard was added into 200 μl of plasma.The mixture was vortexed for 30 s twice to mix well,and then 2 ml of tertbutyl methyl ether was added.Then the mixture was sonicated for 3 min,and vortexed for 5 min.Clear supernatant was obtained by centrifugating at 10,000 rpm for 5 min.The supernatant was dried with nitrogen gas and reconstituted in 100 μl of methanol.The concentration of DTX in blood was measured using HPLC.A reversed-phase column(Hypersil,ODS, 4.6×250 mm,5 μm)was used.The mobile phase was made up of acetonitrile and water(57:43,v/v)at a fow rate of 1 ml/min. The DTX detection was performed at a wavelength of 228 nm. Pharmacokinetic parameters were determined using the software DAS2.0.The signifcance of the difference was analyzed by ANOVA models with Statistical Program for Social Sciences(SPSS 11.0)and the signifcant level was set at 0.05.
3.Results and discussion
3.1.Characterization of liposomes
Encapsulation effciency(EE%),drug loading content(LC%), mean diameter with poly dispersion index(PDI)and zeta potential of four liposomes were displayed in Table 1.It could be seen that incorporation of Biotin-PEG2000-DSPE into the membrane increased the mean diameter and slightly decreased the Zeta potential of the liposomes.Morphology of four liposomes observed by TEM is shown in Fig.1.The doublemembrane of the liposomes could be seen clearly and no drug crystal was visible.The leakage rate(Fig.2)of all liposomes was less than 25%at 4°C in the hydrated state for 15 days.The release behavior of DTX from four liposomes and free DTX in the release medium were shown in Fig.3.The four liposomes (PDL,0.5BDL,1BDL,2BDL)released 69.12±9.98%,57.44±0.37%, 54.49±6.58%and 52.37±3.62%of DTX respectively at 96 h in comparison with free DTX(96.15±0.48%at 6 h),indicating that four liposomes had a sustained release profle.
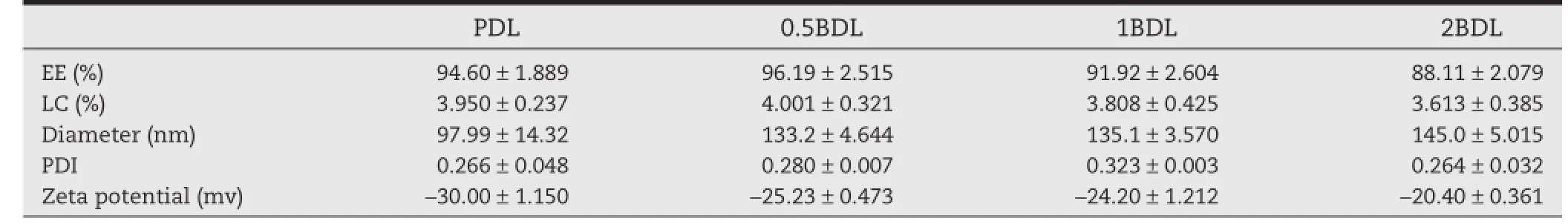
Table 1–Characterization of four kinds of liposomes(n=3).
Docetaxel is a potent anticancer drug and its use is restricted by its poor aqueous solubility where addition ofTween80 to enhance DTX solubility was associated with its side effects [1].Liposomes are capable of increasing the aqueous solubility of DTX.Moreover,biotin was used to modify the liposomes with an aim to increase the DTX accumulation in tumor site. All the prepared liposomes had high encapsulation effciency and drug loading rate.Mean diameter of liposomes increased with the incorporation of Biotin-PEG2000-DSPE due to the interaction of Biotin at the end of Biotin-PEG2000-DSPE and the swelling resulting from the hydrophilic property of biotin [24].The particulate carrier systems with a diameter larger than 200 nm are known to induce nonspecifc scavenging by monocytes and the reticuloendothelial system(RES)[34,35].It was reported that some tumor vessels could cause extravasation of particulates with a diameter less than 400 nm[5,36].The liposomes with a diameter around 100 nm extravasated much easier than bigger ones with a diameter ranging from 200 to 400 nm[37,38].The liposomes prepared in this study were found to have a diameter between 97.99±14.32 nm and 145.0±5.015 nm,which were not expected to be removed by the RES.Biotinylation of liposome did not markedly change the Zeta potential;although biotin is known to possess a positive charge,the result is in agreement with an earlier study [39].
The therapeutic effect of drug in carriers is highly dependent on the release rate of the drug from the carrier.If the drug leaks from the carrier too rapidly,the carrier will lose most of the loaded drug before it reaches the diseased site,leading to the compromised therapeutic effect.In our study,we compared the DTX release behavior of FD and all liposomes.As shown in Fig.3,four liposomes released DTX slower than FD. The sustained release of DTX from liposomes was probably attributed to the encapsulation by the bilayer membrane of liposomes.

Fig.1–Transmission electron microscopy of PDL(A); 0.5BDL(B);1BDL(C)and 2BDL(D)(×40,000).
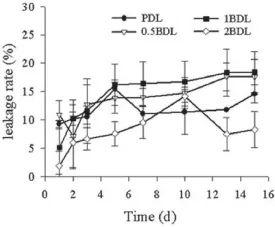
Fig.2–Leakage rate of four kinds of liposomes at 4°C (n=3).
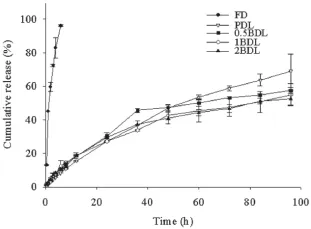
Fig.3–Drug release from FD and four kinds of liposomes in PBS(PH 7.4)containing 0.5%Tween80(n=3).
3.2.MTT assay
Cell viability of 24 h,48 h,and 72 h after adding empty liposome(EL),FD,PDL,0.5BDL,1BDL and 2BDL are shown in Fig.4(A, B,C).IC50was calculated and the results were shown in Table 2. The IC50of liposomes were higher than FD(P<0.01)after 24 h and 48 h incubation.However,0.5BDL showed lower IC50than FD at 72 h(P<0.01).Compared with 0.5BDL,both 1BDL and 2BDL showed signifcantly higher IC50(P<0.01)and there is no signifcant difference between 1BDL and 2BDL(P>0.05).The result suggested that empty liposome has no inhibition effect on cell growth,and cell viability decreased with increasing of the concentration of DTX.For all liposomes and FD,IC50decreased with the increasing of incubation time.
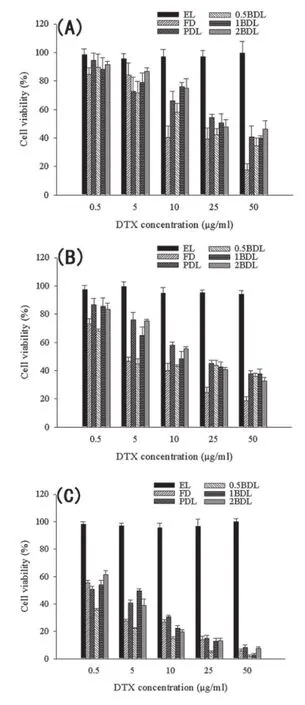
Fig.4–Viability of MCF-7 after incubation with FD and four kings of liposomes.Data are presented as Mean±SD (n=5–6).
Itwasfoundthatdrugconcentrationandexposuretimewere closely related to cytotoxicity of all formulations.This result was in agreement with previous research[40,41].The higher cell viability of liposomes than FD at 24 h and 48 h is probably relatedtothedoublemembraneofliposomeandthestericeffect of PEG chains,which,frst,can inhibit the release of drug from carriers,and second,can prevent liposomes from interacting with cells.In addition,liposomes may delay internalization of drug in cells due to the negative charge on the surface of liposomes.Liposomes with negative charge generally exhibit stronger binding than neutral ones because of the existence of a membrane receptor recognizing negatively charged particles[42].Fig.3 suggested that among liposomes,PDL had the highest drug release rate,which induced the highest cytotoxicity at 24 h.The biotin receptor on the surface of MCF-7 may play a role in uptaking the liposomes into the cells and causing thecytotoxicity.ItwasfoundthatIC50of1BDLand2BDLshowed no signifcant difference in the cytotoxicity.But IC50value of 0.5BDL is nearly two times and three times lower.Higher biotin density on the liposomes failed to show higher cytotoxicity. This is consistent with the fndings reported by other research groups[43,44].Thismightbeattributedtotheinternalizedligand molecules leading to a down-regulation or‘shut-off’of the receptorrecyclingsystem.Theligandsconjugatedliposomesmay contributetotheintracellularligandconcentrationandaretherefore responsible for the saturation and‘shut-off’of the receptor uptake pathway.Liposomes with more targeting ligands would lead to more intracellular ligand content than those with less targeting ligands.This could result in a decreased cytotoxicity when more ligands are utilized[43].An alternative explanation is the possible existence of DSPE-PEG2000-Biotin micelles formed at higher number of targeting ligand.The biotin modifed micelles would compete with the receptors and prevent biotin modifed liposomes binding to the receptors.
3.3.Pharmacokinetic studies
Mean plasma concentration–time profles of DTX after i.v.administration of DTX solution and four liposomes at a dose of 10 mg/kg was shown in Fig.5.The main pharmacokinetic parameters were summarized in Table 3.When DTX was encapsulated in liposomes,the pharmacokinetic parameters were clearly different from those of FD.The AUC and the MRT were signifcantly increased,and the plasma clearance(CL)was reduced signifcantly.The AUC of liposomes(PDL;0.5BDL;1BDL and 2BDL)were 10.86 times,4.456 times,2.689 times and 3.976 times higher than FD(3.791±1.375 mg/l·h).The MRT of liposomes(PDL,0.5BDL,1BDL and 2BDL)were 14.89 times,4.755 times, 1.973 times and 1.851 times higher than FD(3.573±2.121h).In comparison with FD,CL of liposomes were 0.087 times,0.208 times,0.234 times and 0.352 times smaller.Among liposomes PDL presented the longest MRT,the largest AUC and the lowest CL,indicating a long circulation time.Compared with PDL,the biotin modifed liposomes with different density exhibited a relatively smallerAUC,MRT and larger CL.Among the biotin modifed liposomes,0.5BDL showed best stability in blood circulation and a higher chance to exert biotin mediated endocytosis.
The pharmacokinetic data are shown in Table 3.It can be observed from Table 3 that the biotin modifed liposomes presented shorter circulation time and smaller AUC and MRT. Among the biotin modifed liposomes,0.5BDL showed relatively longer circulation time,largerAUC and MRT.Biotin-PEG2000-DSPE chains may cover parts of DSPE-PEG2000,leading to less protection effect of PEG.In addition,biotin as a ligand at the endof the PEG chains may be recognized by cell membrane receptor in blood and result in faster elimination from blood. Previous studies suggested that nanoparticles should be small, slightly negatively charged and covered with a protective PEG layer to achieve passive targeting[11].Our study further indicates that the surface density of ligand will have a negative impact on the blood circulation of nanocarriersin vivo.Studies on tumor-bearing rat need to be further investigated to elucidate the active targeting effciency.
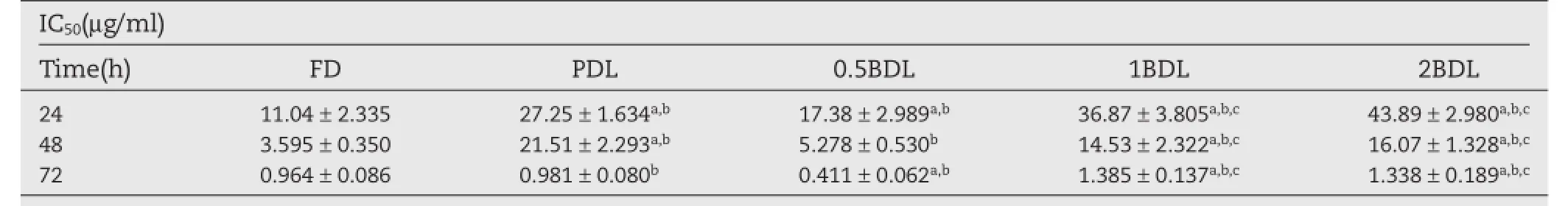
Table 2–IC50value of FD and four kinds of liposomes(n=5–6).
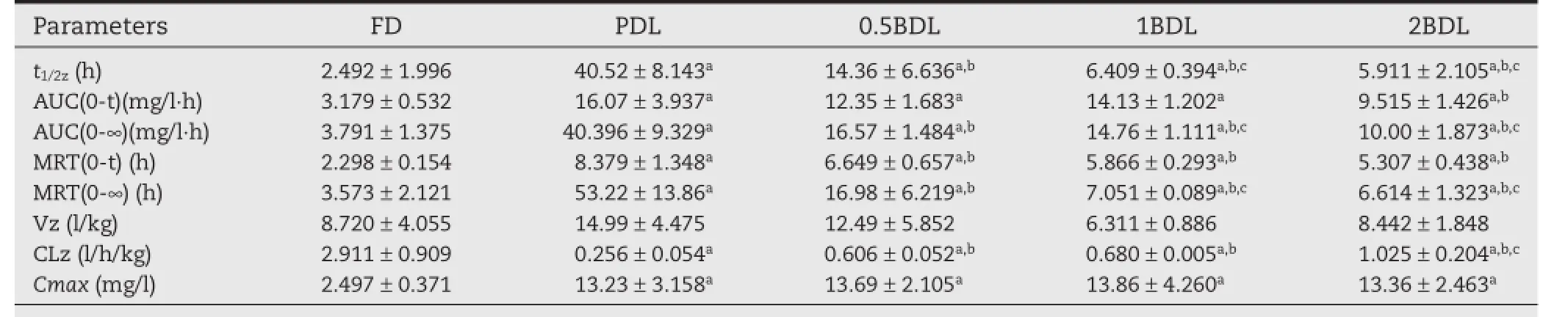
Table 3–Summary of the pharmacokinetic parameters of FD and four kinds of liposomes after i.v.administration in rats at dosage of 10 mg/kg(n=3).
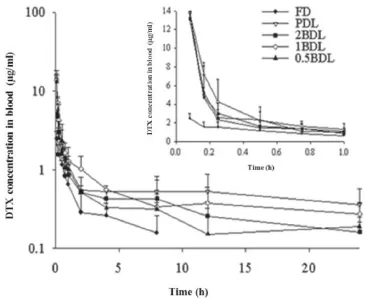
Fig.5–Mean plasma concentration–time profles of DTX after i.v.administration of DTX solution and four liposomes at a dose of 10 mg/kg(n=3).
4.Conclusions
The biotin ligand density on the surface of liposome has an impact on the cytotoxicity and pharmacokinetic of liposomes.The cytotoxicity of biotin conjugated liposomes decreased with an increase in biotin surface density.The elimination of biotin conjugated liposomes from blood was increased with increasing of biotin surface density.The ligand density of the active targeting liposomes needs to be optimized to achieve successful targeting.
R E F E R E N C E S
[1]Rowinsky EK.The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents.Annu Rev Med 1997;48:353–374.
[2]Weiss RB,Donehower RC,Wiernic PH,et al.Hypersensitivity reactions from taxol.J Clin Oncol 1990;8:1263–1268.
[3]Ellington AD,Szostak JW.In vitro selection of RNA molecules that bind specifc ligands.Nature 1990;346(6287):818–822.
[4]Immordino ML,Brusa P,Arpicco S,et al.Preparation, characterization,cytotoxicity and pharmacokinetics of liposomes containing docetaxel.J Control Release 2003;91(3):417–429.
[5]Maeda H,Wu J,Sawa T,et al.Tumor vascular permeability and the EPR effect in macromolecular therapeutics:a review.J Control Release 2000;65(1–2):271–284.
[6]Ge L,Zhu JB,Xiong F,et al.Preparation,characterization and pharmacokinetics of N-palmitoyl chitosan anchored docetaxel liposomes.J Pharm Pharmacol 2007;59:661–667.
[7]Wang QW,Lu HL,Song CC,et al.Radiosensitivity of human colon cancer cell enhanced by immunoliposomal docetaxel. World J Gastroenterol.2005;11:4003–4007.
[8]Allen TM,Hansen C,Martin F,et al.Liposomes containing synthetic lipid derivatives of poly(ethylene glycol)show prolonged circulation half-lives in vivo.Biochim Biophys Acta.1991;1066(1):29–36.
[9]Lasic DD,Martin FJ,Gabizon A,et al.Sterically stabilized liposomes:a hypothesis on the molecular origin of the extended circulation times.Biochim Biophys Acta. 1991;1070(1):187–192.
[10]Sadzuka Y,Nakade A,Hirama R,et al.Effects of mixed polyethyleneglycol modifcation on fxed aqueous layer thickness and antitumor activity of doxorubicin containing liposome.Int J Pharm 2002;238(1–2):171–180.
[11]Dadashzadeh S,Mirahmadi N,Babaei MH,et al.Peritoneal retention of liposomes:effects of lipid composition,PEG coating and liposome charge.J Control Release 2010;148(2):177–186.
[12]Gosselin MA,Lee RJ.Folate receptor-targeted liposomes as vectors for therapeutic agents.Biotechnol Annu Rev 2002;8: 103–131.
[13]Allen TM,Brandeis E,Hansen CB,et al.A new strategy for attachment of antibodies to sterically stabilized liposomes resulting in effcient targeting to cancer cells.Biochim Biophys Acta.1995;1237(2):99–108.
[14]Kibria G,Hatakeyama H,Ohga N,et al.Dual-ligand modifcation of PEGylated liposomes shows better cell selectivity and effcient gene delivery.J Control Release 2011;153(2):141–148.
[15]Budavari S.The Merck Index,drug development research. 12th ed.Branchburg,NJ:Merck;1996.
[16]Russell-Jones G,McTavish K,McEwan J,et al.Vitaminmediated targeting as a potential mechanism to increase drug uptake by tumours.J Inorg Biochem 2004;98(10):1625–1633.
[17]Lee ES,Na K,Bae YH.Super PH-sensitive multifunctional polymeric micelle.Nano Lett 2005;5:325–329.
[18]Stella VJ,Borchardt RT,Hageman MJ,et al.Prodrugs challenges and rewards part 1.Berlin:Springer Ebooks;2007.
[19]Ramanathan S.Targeting the sodium-dependent multivitamin transport(SMVT)for improving the oral absorption properties of a retro-inverso Tat nonapetide. Pharm Res 2001;18(7):950–956.
[20]Minko T.Enhancing the anticancer effciency of camptothecin using biotinylated poly(ethylene glycol) conjugates in sensitive and multidrug-resistant human ovarian carcinoma cells.Cancer Chemother Pharmacol. 2002;50(2):143–150.
[21]Phillips WT,Medina LA,Klipper R,et al.A novel approach for the increased delivery of pharmaceutical agents to peritoneum and associated lymph nodes.J Pharmacol Exp Ther 2002;303(1):11–16.
[22]Mishra PR,Jain NK.Biotinylated methotrexate loaded erythrocytes for enhanced liver uptake.‘A study on the rat’. Int J Pharm 2002;231(2):145–153.
[23]Yang W,Cheng Y,Xu T,et al.Targeting cancer cells with biotin–dendrimer conjugates.Eur J Med Chem 2009;44(2):862–868.
[24]Na K,Bum Lee T,Park K-H,et al.Self-assembled nanoparticles of hydrophobically-modifed polysaccharide bearing vitamin H as a targeted anti-cancer drug delivery system.Eur J Med Chem 2003;18(2):165–173.
[25]Kim JH,Li Y,Kim MS,et al.Synthesis and evaluation of biotin-conjugated pH-responsive polymeric micelles as drug carriers.Int J Pharm 2012;427(2):435–442.
[26]Yeeprae W,Kawakami S,Yamashita F,et al.Effect of mannose density on mannose receptor-mediated cellular uptake of mannosylated O/W emulsions by macrophages.J Control Release 2006;114(2):193–201.
[27]Garg A,Tisdale AW,Haidari E.Targeting colon cancer cells using PEGylated liposomes modifed with a fbronectinmimetic peptide.Int J Pharm 2009;366:201–210.
[28]Gu F,Zhang L,Teply BA,et al.Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers.Proc Natl Acad Sci U S A. 2008;105:2586–2591.
[29]Fakhari A,Baoum A,Siahaan TJ,et al.Controlling ligand surface density optimized nanoparticles binding to ICAM-1.J Pharm Pharm Sci 2010;100(3):1045–1056.
[30]Olivier V,Meisen I,Meckelein B,et al.Infuence of targeting ligand fexibility on receptor binding of particulate drug delivery systems.Bioconjug Chem.2003;14:1203–1208.
[31]Shmeeda H,Amitay Y,Gorin J,et al.Delivery of zoledronic acid encapsulated in folate-targeted liposome results in potent in vitro cytotoxic activity on tumor cells.J Control Release 2010;146(1):76–83.
[32]Gabizon A,Horowitz AT,Goren D,et al.In vivo fate of folatetargeted polyethylene-glycol liposomes in tumor-bearing mice.Clin Cancer Res 2003;9(17):6551–6559.
[33]Dunne M,Zheng J,Rosenblat J,et al.APN/CD13-targeting as a strategy to alter the tumor accumulation of liposomes.J Control Release 2011;154(3):298–305.
[34]Litzinger DC,Buiting AMJ,van Rooijen N,et al.Effect of liposome size on the circulation time and intraorgan distribution of amphipathic poly(ethylene glycol)-containing liposomes.Biochim Biophys Acta.1994;1190(1):99–107.
[35]Gabizon A,Price DC,Hberty J,et al.Effect of liposome composition and other factors on the targeting of liposomes to experimental tumors:biodistribution and imaging studies.Cancer Res 1999;263:6371–6378.
[36]Yuan F,Dellian M,Fukumura D,et al.Vascular permeability in a human tumor xenograft:molecular size dependence and cutoff size.Cancer Res 1995;55:3752–3756.
[37]Charrois GJR,Allen TM.Rate of biodistribution of STEALTH?liposomes to tumor and skin:infuence of liposome diameter and implications for toxicity and therapeutic activity.Biochim Biophys Acta.2003;1609(1):102–108.
[38]Seynhaeve ALB,Hoving S,Schipper D,et al.Tumor necrosis factor α mediates homogeneous distribution of liposomes in murine melanoma that contributes to a better tumor response.Cancer Res 2007;67(19):9455–9462.
[39]Pulkkinen M,Pikkarainen J,Wirth T,et al.Three-step tumor targeting of paclitaxel using biotinylated PLA-PEG nanoparticles and avidin–biotin technology:formulation development and in vitro anticancer activity.Eur J Pharm Biopharm 2008;70(1):66–74.
[40]Crosasso P,Ceruti M,Brusa P,et al.Preparation, characterization and properties of sterically stabilized paclitaxel-containing liposomes.J Control Release 2000;63:19–30.
[41]Chervinsky DS,Brecher ML,Hoelcle MJ.Cremophor-EL enhances taxol effcacy in a multi-drug resistant C1300 neuroblastoma cell line.Anticancer Res.1993;13:93–96.
[42]Woodle MC.Surface-modifed liposomes:assessment and characterization for increased stability and prolonged blood circulation.Chem Phys Lipids 1993;64:249–262.
[43]Justin MS,Annapragada A,Natarajan JV,et al.Controlled targeting of liposomal doxorubicin via the folate receptor in vitro.J Control Release 2003;92:49–67.
[44]Tyagi N,Ghosh PC.Folate receptor mediated targeted delivery of ricin entrapped into sterically stabilized liposomes to human epidermoid carcinoma(KB)cells:effect of monensin intercalated into folate-tagged liposomes.Eur J Pharm Sci 2011;43:343–353.
*< class="emphasis_italic">Corresponding author.
.Shenyang Pharmaceutical University,103 WenHua Road,ShenHe District,110016 Shenyang,China.Tel.:+86 24 23986306.
E-mail address:qiaomingxi@163.com(M.Qiao).
http://dx.doi.org/10.1016/j.ajps.2016.04.001
1818-0876/?2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年5期
Asian Journal of Pharmacentical Sciences2016年5期
- Asian Journal of Pharmacentical Sciences的其它文章
- Quantitative determination of metaxalone in human plasma by LC-MS and its application in a pharmacokinetic study
- Effects of duration of phenytoin administration on mRNA expression of cytochrome P450 and P-glycoprotein in the liver and small intestine of rats
- Stability of freeze-dried pH-responsive dextrin nanogels containing doxorubicin
- Evaluation of pharmacokinetics underlies the collaborated usage of lamivudine and oxymatrine in beagle dogs
- A hydrophobic peptide fraction that enhances the water dispersibility of curcumin
- An intravenous clarithromycin lipid emulsion with a high drug loading,H-bonding and a hydrogen-bonded ion pair complex exhibiting excellent antibacterial activity
