A hydrophobic peptide fraction that enhances the water dispersibility of curcumin
Department of Applied Chemistry,Faculty of Engineering,University of Miyazaki,1-1 Gakuen Kibanadai Nishi, Miyazaki 889-2192,Japan
A hydrophobic peptide fraction that enhances the water dispersibility of curcumin
Risa Yamashita,Tatsuya Oshima*,Yoshinari Baba
Department of Applied Chemistry,Faculty of Engineering,University of Miyazaki,1-1 Gakuen Kibanadai Nishi, Miyazaki 889-2192,Japan
A R T I C L EI N F O
Article history:
Received 28 January 2016
Received in revised form 19 April 2016
Accepted 5 May 2016
Available online 9 May 2016
Curcumin
Peptide
Dispersibility
Complexation
Hydrocolloid
The present study describes the complexation between curcumin(Cur)and a peptide mixture (Pep).Pep was prepared by enzymatic hydrolysis of casein and used as an excipient for poorly water-soluble Cur.An aqueous solution of Pep and an acetone solution of Cur were mixed and lyophilized to obtain a white-yellow powder of the peptide and Cur complex(Cur-Pep).The water dispersibility of Cur was enhanced by the complexation with Pep.Pep was fractionated using ammonium sulfate precipitation and ultrafltration to identify which peptides preferentially interact with Cur.Relatively hydrophobic peptides with high molecular weights(>5 kDa)were more effective in enhancing the water dispersibility of Cur than other fractions.Cur-Pep dispersed under acidic and neutral conditions,at which amphoteric Pep is positively or negatively charged.Cur-Pep exists as a hydrocolloid with particle size 160–330 nm in aqueous media.
?2016 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1.Introduction
Curcumin(1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione,Cur,Fig.1)is a polyphenol compound derived from turmeric root(Curcuma longa)and has attracted attention as a potential chemotherapeutic agent[1,2].It has a wide range of biological and pharmacological effects including antioxidant,antitumor,anti-infammatory,and anticancer properties [3–5].However,absorption of Cur in the gastrointestinal tract is poor because it is not highly soluble[6].Its maximum solubility is reportedly 11 ng/ml in a pH 5.0 aqueous buffer[7]. Therefore,various techniques have been used to increase its water solubility and/or water dispersibility and improve its oral bioavailability[8–10].
Amphiphilic block copolymer micelles have been used as vehicles for the solubilization and stabilization of Cur[11,12]. Cur has also been incorporated into micellar aggregates of crosslinked and random copolymers ofN-isopropylacrylamide withN-vinyl-2-pyrrolidone,and poly(ethyleneglycol)monoacrylate. The resulting nanoparticles(about 50 nm in diameter)were dispersed in aqueous media[13].Complex formation between Cur and cyclodextrins(CDs)also increases the water solubility of Cur[7,13].The hydrolytic stability of Cur improved aftercomplexation with CDs,but its photodecomposition rate increased compared with a Cur solution in an organic solvent. Formation of a 2:1 complex between β-CD and Cur has also been reported[14].Water-soluble polymers are also used to increase the water solubility of Cur[15],and binding with polyvinylpyrrolidone[16]and hyaluronic acid[17]has also been studied.Incorporation into an emulsion is also an effective way to enhance the oral administration of Cur[18–20].
Casein micelles have also been developed as nanocarriers for Cur[21–25].Complex formation of Cur with a casein micelle and its use for delivery to cancer cells was investigated[21]. The complex was analyzed using fuorescence spectroscopy [21,26,27].Encapsulation of Cur in a camel β-casein micelle has also been studied[28],as well as the binding of curcumin to αS1-casein,which was analyzed by fuorescence spectroscopy and circular dichroism[29,30].Recently,encapsulation of Cur in casein by spray-drying has been developed[31].Soy protein isolate can also be used as a complexation agent for Cur to improve its water solubility[32].
In the present study,a peptide mixture(Pep)was prepared by enzymatic hydrolysis of casein,and was used as a complexation agent for Cur.Recently,researchers have found that peptide mixtures obtained as protein hydrolysate were effective in enhancing the water solubility and/or water dispersibility of poorly water-soluble materials.The water solubility of a poorly water-soluble drug,indomethacin,was enhanced by complexation with Pep[33,34].The resulting complex between indomethacin and Pep is quite small and can pass through ultraflter membranes.Additionally,complexation with albumin hydrolysate as a peptide mixture enhanced the water dispersibility of extremely poorly water-soluble coenzyme Q10[35].The particle size of the complex between coenzyme Q10and albumin hydrolysate was 170–280 nm,suggesting that the complex is present as a hydrocolloidal material in aqueous media.Furthermore,the water dispersibility of paclitaxel was also enhanced by the complexation with Pep [36].
Peptide mixtures prepared from protein hydrolysate will contain diverse peptides with different molecular weights and amino acid sequences.Some of these exhibit affnity to poorly water-soluble materials on the basis of various interactions, such as hydrophobic and/or electrostatic interactions.For example,heme-iron-enriched polypeptide(digested hemoglobin,HIP),which is obtained by hydrolysis of hemoglobin using protease and subsequent enrichment by ultrafltration,is known to be complex between poorly water-soluble heme iron and peptide fragments[37–40].As the peptidic fraction of hemoglobin hydrolysate confers hydrophilicity to heme iron,HIP is apparently soluble in aqueous media and is more readily absorbed by the human body than heme iron alone[41].These examples demonstrate that complexation with Pep could increase the water solubility,and consequently the oral bioavailability,of Cur.In this study,casein was used as the resource to prepare Pep as it is an edible protein.Additionally, casein is a family of four phosphoproteins αS1-casein,αS2-casein,β-casein,and κ-casein,which show different hydrophilic/ hydrophobic balances,resulting in diverse peptides[42,43]. Therefore,Pep was fractionated using ammonium sulfate precipitation followed by ultrafltration in order to specify and use the peptides that are effective for enhancing the water dispersibility of Cur[36].
Pep was prepared by enzymatic hydrolysis of casein and fractionated by precipitation using ammonium sulfate solution and ulfrafltration[36].The complex between Cur and Pep was prepared by mixing an acetone solution of Cur and an aqueous solution of Pep,followed by removal of acetonein vacuoand lyophilization[33,35].The water dispersibility of Cur-Pep was compared with Cur alone and with other excipients.To estimate the structure of Cur-Pep in aqueous media, an aqueous suspension of Cur-Pep was fractionated by centrifugation followed by successive membrane fltrations.The particle size of Cur-Pep and the distribution of Cur in the fractions were analyzed.The zeta potential distribution of Cur-Pep and Pep was also measured to evaluate the surface charge of the materials.Cur-Pep was characterized using scanning electron microscopy(SEM),differential scanning calorimetry(DSC),and powder X-ray diffraction(XRD).The results were used to determine how Cur is incorporated into Cur-Pep.

Fig.1–Molecular structure of Cur.
2.Materials and methods
2.1.Materials
Cur,milk casein(Hammarsten grade),polyethylene glycol 4000 (PEG 4000,with average molecular weight 4000 g/mol), α-cyclodextrin,methyl-β-cyclodextrin,γ-cyclodextrin,glucose, and sucrose were purchased from Wako Pure Chemical Industries,Ltd.(Osaka,Japan).α-Chymotrypsin from bovine pancreas, pepsin from porcine gastric mucosa,and pancreatin from porcine pancreas were purchased from Sigma-Aldrich Japan K.K.(Tokyo,Japan).All other reagents were analytical grade. Disposable membrane flters(DISMIC?,pore size:0.80 μm (25CS080AN),0.45 μm(25CS045AN),or 0.20 μm(25CS020AN)) made of cellulose acetate were purchased from AdvantecToyo (Tokyo,Japan).The following ultrafltration membranes made of regenerated cellulose were purchased from Merck Milipore (Billerica,MA,USA)and used for fractionation of the peptides:Ultracel?5 kDa,molecular weight cut-off of 5000;Ultracel?3 kDa,molecular weight cut-off of 3000;and Ultracel?1 kDa, molecular weight cut-off of 1000.
2.2.Preparation and fractionation of Pep
Pep was prepared by enzymatic hydrolysis of casein according to similar procedures described previously[31].Casein(25 g) was hydrated in distilled water(500 ml)and the pH wasadjusted to 7.8 with 5 M NaOH using a pH controller(TOKOTDP-51,Tokyo,Japan).Casein was enzymatically hydrolyzed by adding α-chymotrypsin(125 mg)and calcium chloride dehydrate(1.5 g)with stirring(200 rpm)at 44±1°C.During the enzymatic hydrolysis,the pH of the mixture was kept at 7.8 using the pH controller.After 6 h,the mixture was heated at 81±2°C for 5 min to deactivate the enzyme.After cooling,the mixture was lyophilized to obtain casein hydrolysate(Pep)as a white powder.The molecular weight distribution of Pep was determined using gel fltration chromatography/highperformance liquid chromatography(GFC–HPLC;Prominence Isocratic System,Shimadzu,Kyoto,Japan).Isocratic HPLC was performed using a Shimadzu LC-10ADvppump unit(fow rate: 0.5 ml/min)together with Shimadzu Inertsil WP300 Diol(5 μm, 250 mm×7.6 mm I.D.)and Inertsil Diol(5 μm,250 mm×7.6 mm I.D.)columns,and detection of ultraviolet(UV)absorption with a Shimadzu SPD-10Avp UV–Vis detector at 230 nm.
Subsequently,Pep was fractionated by combining ammonium sulfate precipitation and ultrafltration as follows(Fig.2) [36].Pep(15 g)was dissolved in distilled water(300 ml)in an ice-water bath.The mixture was stirred for 30 min and then centrifuged at 10,000×g for 10 min at 4°C.The supernatant was treated with 34.2 g(19 wt%)ammonium sulfate and the precipitate was recovered.The resultant supernatant was treated with 28 wt%ammonium sulfate and the precipitate was recovered.In a similar manner,precipitates were obtained by adding 34 wt%and 40 wt%ammonium sulfate repeatedly.Each precipitate obtained by ammonium sulfate precipitation was dissolved again by adding distilled water,and the solution was ultrafltered using an ultrafltration unit(600 cm3,UHP-90K, Advantec Toyo)using an ultrafltration membrane with a molecular weight cut-off of 5000 g/mol at 0.3 MPa until 90 vol% of the fltrate had passed through the membrane.The retentate was lyophilized to obtain a fraction of Pep,and the permeate was successively ultrafltered again using ultrafltration membranes with molecular weight cutoffs of 3000 g/mol and 1000 g/ mol.During the ultrafltration,the pH of the mixture was kept at 7.0 by adding small amounts of 5 M NaOH and 6 M HCl.The retentate was also lyophilized to obtain a fraction of Pep.Finally, fractions A–L containing different peptides were prepared.
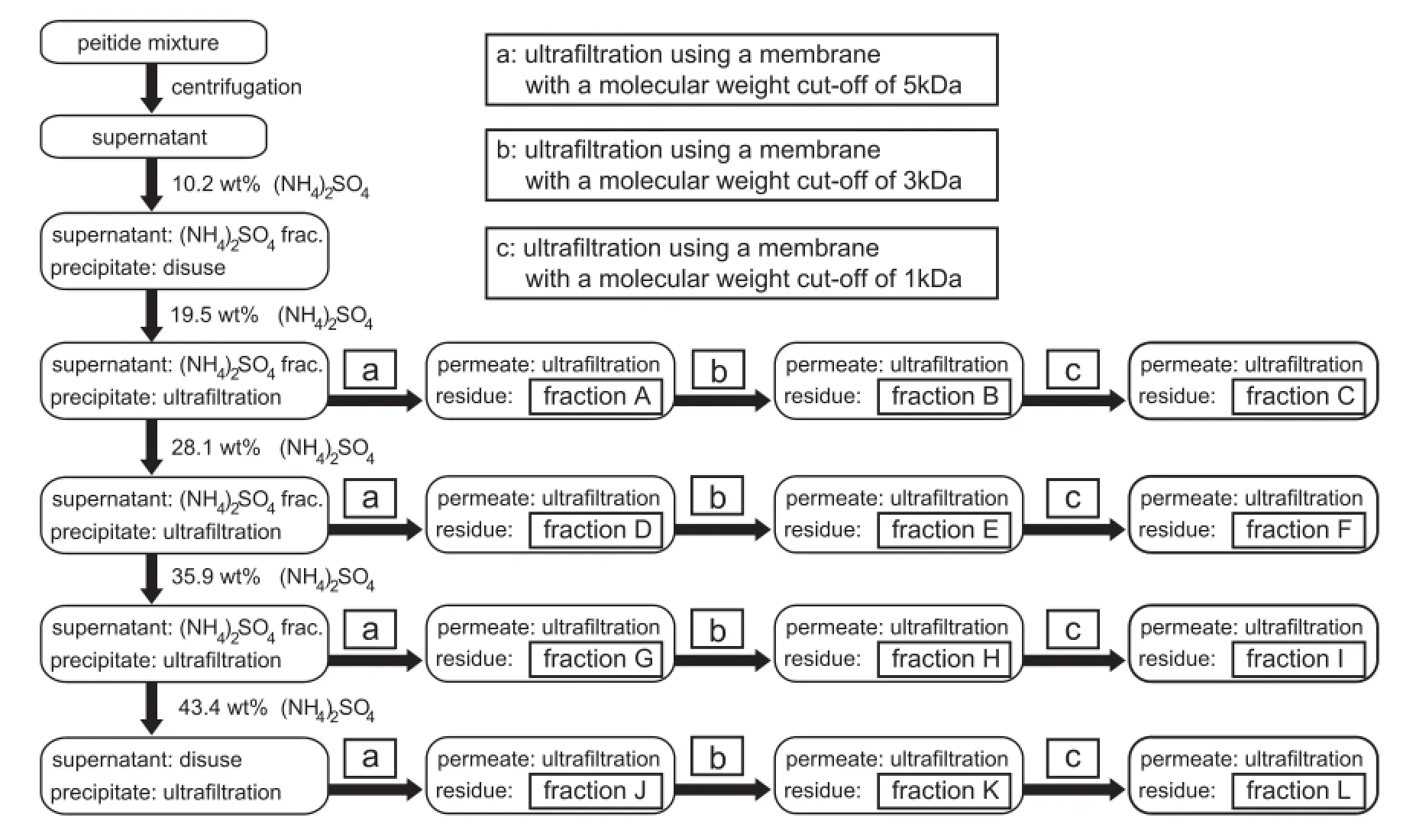
Fig.2–Flowchart for the fractionation of Pep by combining ammonium sulfate precipitation and ultrafltration.
2.3.Preparation of Cur-Pep and water dispersibility test
An acetone solution of Cur was prepared by dissolving Cur in acetone at a concentration of 0.1 g/l.An aqueous solution of Pep was prepared by dissolving 10 g/l of fractions A–L containing different peptides in water.Equal volumes(200 μl)of the aqueous and organic solutions were mixed in a stoppered polypropylene centrifuge tube and shaken using a thermoshaker(Hangzhou Allsheng Instruments MSC-100,Zhejiang,China)at 30°C for 1 h.After removing the acetonein vacuousing a centrifugal evaporator(Thermo Scientifc Savant SpeedVac SPD1010,MA,USA),the residue was lyophilized to obtain Cur-Pep as a white-yellow powder[33–36].
To evaluate the water dispersibility of Cur-Pep,1.0 ml of 10 mM sodium phosphate buffer at pH 6.8 was added to Cur-Pep.The aqueous mixture was shaken at 1500 rpm at 30°C for 1 h.After fltration of the aqueous mixture using a 0.80 μm membrane flter,the apparent solubility of Cur was determined using a UV–Vis spectrophotometer(JASCOV-660,Tokyo, Japan).As pure Cur is not soluble in aqueous media,the molar extinction coeffcient of Cur at 420 nm in acetone(ε, 5.84×104μM?1cm?1)was used to determine the concentration.
The effect of the quantity of excipient on the water dispersibility of Cur was studied as follows.An acetone solution(200 μL)containing 0.1 g/l Cur and an aqueous solution(200 μl)containing different concentrations of Pep(fraction A, 0–20 g/l)were mixed and shaken at 1500 rpm at 30°C for 1 h. After removing the acetonein vacuo,the residue was lyophilized to obtain Cur-Pep.In a similar manner,Cur solution (0.1 g/l)and an aqueous solution of excipients(PEG4000, α-cyclodextrin,methyl-β-cyclodextrin,γ-cyclodextrin,glucose and sucrose,0–20 g/l)were mixed to obtain the complexes between Cur and excipients(Cur-PEG4000,Cur-αCD,CurmβCD,Cur-γCD,Cur-Glu,and Cur-Suc),respectively.To determine the water dispersibility of the Cur complexes,1.0 ml of 10 mM sodium phosphate buffer at pH 6.8 was added to the Cur complex.After shaking at 30°C for 1 h,the aqueous mixture was fltered using 0.80 μm membrane flter.The apparent solubility of Cur was determined using HPLC(Shimadzu Prominence High Pressure Gradient System).The HPLC analysis system was based on gradient reverse-phase HPLC using a Shimadzu LC-20AB unit as a pump(fow rate:0.4 ml/min)together with a C18column(TSKgel-ODS-100Z,3.0×150 mm,3 μm)(TOSOH, Tokyo,Japan),and detection of absorption with a Shimadzu SPD-20A UV–Vis detector at 420 nm.The mobile phase consisted of two components:(A)water with 2%acetic acid,and (B)acetonitrile.
The effect of pH on the water dispersibility of Cur was studied as follows.Cur-Pep was prepared in a similar manner to that shown above by mixing an acetone solution(200 μl)containing 0.1 g/l Cur and an aqueous solution(200 μl)containing Pep(fraction A,5.0 g/l).Additionally,a blank sample(Cur-Blank)was prepared by mixing an acetone solution(200 μl) containing 0.1 g/l Cur and an aqueous solution(200 μl)without Pep,followed by lyophilization.Each sample was added to 1.0 ml of 10 mM sodium phosphate buffer solution at pH 2–8 and shaken at 30°C for 1 h.The aqueous mixture was fltered using a 0.80-μm membrane flter and the apparent solubility of Cur was determined using HPLC.The pH of the fltrate was measured using a pH meter(DKK-TOA Co.HM-25R,Tokyo,Japan).
The effect of treatment of simulated digestive fuid using pepsin and pancreatin on the water dispersibility of Cur was also studied as follows[44,45].Cur-Pep was prepared in a similar manner to that shown above.Each sample was added to 0.50 ml of hydrochloric acid(pH 2.0)containing 20 mg/l pepsin and shaken at 37°C for 1 h.Successively,to the mixture was added 0.50 ml of 50 mM sodium phosphate buffer solution at pH 7.6 containing 20 mg/l pancreatin and shaken at 37°C for 1 h.The aqueous mixture was fltered using a 0.45-μm membrane flter, and the apparent solubility of Cur was determined using a UV–Vis spectrophotometer.Same procedure was conducted without pepsin or pancreatin as a control sample.
2.4.Zeta potential of Cur-Pep
Cur-Pep was prepared in a similar manner to that shown above by mixing an acetone solution(200 μl)containing 0.1 g/l Cur and an aqueous solution(200 μl)containing Pep(fractionA,5.0 g/ l).Cur-Pep or Pep was added to 1.2 ml of 10 mM sodium phosphate buffer solution with pH of 2–8.The aqueous mixture was shaken and fltered using a 0.80-μm membrane flter.The pH of the fltrate was measured using a pH meter.The zeta potential of the fltrate was measured using a dynamic light scattering(DLS)particle size/zeta potential analyzer(Horiba SZ-100,Kyoto,Japan).
2.5.Material distribution and particle size analysis for Cur-Pep
Cur-Pep was prepared in a similar manner shown above by mixing an acetone solution(200 μl)containing 0.1 g/l Cur and an aqueous solution(200 μl)containing Pep(fraction A, 5.0 g/l).Cur-Pep(10 mg)was added to 10 ml of 10 mM phosphate buffer(pH 6.8)and the aqueous suspension was fractionated by centrifugation and membrane fltration as follows[31,33].The Cur-Pep suspension was centrifuged at 5000 rpm for 5 min and the supernatant was analyzed as a centrifuged sample,F1.The supernatant was fltered using a 0.80-μm membrane flter to obtain the fltrate,F2.Filtration using a 0.45-μm membrane flter followed by a 0.20-μm membrane flter was performed to obtain fractions F3 and F4,respectively.The concentration of Cur in fractions F1–F4 was determined using HPLC.The particle size of Cur-Pep in fractions F1–F4 was measured using a dynamic light scattering(DLS) particle size/zeta potential analyzer.The average particle size from DLS for each sample was determined as an average of more than four measurements.
2.6.Characterization of Cur-Pep
A reference mixture(abbreviated as Cur-Pep-R)was prepared by mixing Cur(6.0 mg)and Pep(30 mg)in the dry state to study the effect of the mixing method.Differential scanning calorimetry(DSC)analysis for Cur,Cur-Pep-R,Cur-Pep,and Pep (fraction A)was performed using a Shimadzu DTG-60/60H thermal analyzer from 25 to 300°C at a heating rate of 10°C/ min under nitrogen.The crystal structures of powder samples were also examined using XRD with Cu Kα radiation (MAXima_X,XRD-7000,Shimadzu).Measurements were performed at 40 kV and 40 mA.The 2θ angle was set from 1°to 70°,and the scanning rate was 2°/min.Cur,Pep,and Cur-Pep were also examined using scanning electron microscopy(SEM) (VE-8800,Keyence Co.,Osaka,Japan).
3.Results and discussion
3.1.Water dispersibility of Cur-Pep
Pep was fractionated using ammonium sulfate precipitation followed by ultrafltration in order to identify peptides which are effective for the complexation with Cur.Relatively hydrophobic peptides should precipitate under lower ammonium sulfate concentration.Therefore,peptides can be fractionated using different concentrations of ammonium sulfate on the basis of their hydrophilic/hydrophobic balance.Additionally,peptides were fractionated by molecular weight,using different ultraflters.From the GFC–HPLC analysis results,the molecular weights of the major components were 1100,2600, 7100,and 10,500 g/mol[33].Twelve Pep fractions A–L were obtained by the combination of ammonium sulfate precipitation followed by ultrafltration(Table 1):They should contain different peptides with different hydrophilic/hydrophobic balance and molecular weight.Fig.3 shows the apparent solubility of Cur-Pep prepared using different fractions at pH 6.8.Theapparent solubility of Cur was enhanced by complexation with Pep,and the solubilities of Cur-Pep prepared using fractions A,D,and G were higher than those prepared using other fractions.In particular,the solubility of Cur-Pep prepared using fraction A was the highest of all the complexes.Its apparent solubility was 5.19 mg/l,which is at least 3.8 times higher than those prepared using other fractions.
Recently,the molecular weight and sequence of major peptides in each fraction were identifed using matrix-assisted laser desorption/ionization time-of-fight/time-of-fight mass spectrometer with“LIFT”technique(MALDI LIFT-TOF/TOF MS) (Table 2)[36].As shown in the grand average of hydropathicity (GRAVY)of the identifed peptides[46],relatively hydrophobic peptides are contained in fractions A,B,and D,which are obtained as precipitate under lower ammonium sulfate concentration.In contrast,relatively hydrophilic peptides were present in fractions G,H,I,J,K,and L.Dominant peaks of 1718.0 and 1881.0 m/z detected in relatively hydrophobic fractions A, B,C,D,E,and F were assigned to be β-casein fragment 194–209 (β-CN f194–209)with the sequence QEPVLGPVRGPFPIIV and β-CN f193–209 with the sequence YQEPVLGPVRGPFPIIV,respectively.The result suggests that relatively hydrophobic peptides with high molecular weights are effective in enhancing the dispersibility of Cur.This is similar to the case of HIP,in which hydrophobic interactions are thought to be mainly involved in the complexation between heme iron and peptides[38,40].In contrast,relatively hydrophilic peptides with lower molecular weights are less effective for complexation with Cur.Based on this result,fraction A was used as Pep in the subsequent experiments.
The photograph in Fig.4 shows the aqueous suspension of Cur alone and Cur-Pep in 10 mM phosphate buffer(pH 6.8) before and after fltration using a 0.80-μm membrane flter.As the water solubility of Cur is quite low,Cur does not disperse in the aqueous media at all and precipitates at the bottom of sample tube.After fltration using a 0.80-μm membrane flter, the fltrate of Cur was colorless.In contrast,Cur-Pep disperses in aqueous media giving a yellow-white solution.After fltration,the solution of Cur-Pep became clear but remained yellow.Fig.5 shows the effect of the quantity of excipients(Pep (fraction A),PEG4000,α-cyclodextrin,methyl-β-cyclodextrin, γ-cyclodextrin,glucose,and sucrose)on the apparent solubility of Cur at pH 6.8.As shown in the profle for Cur-Pep,the apparent solubility of Cur increased as the quantity of Pep was increased.Saccharides are effective excipients for thepreparation of amorphous pharmaceutical solids[47];however, the solubility of Cur-Glu and Cur-Suc did not increase.The solubility of Cur-PEG4000 is also much lower than that of Cur-Pep.Macrocyclic cyclodextrins are well-known complexing agents for Cur,as Cur fts into their relatively hydrophobic cavity [7,13–15].However,the solubilities of Cur-mβCD and Cur-γCD were lower than that of Cur-Pep.Only Cur-αCD showed higher solubility than Cur-Pep at high concentrations.The procedure for the preparation of the Cur complex may not be optimal for cyclodextrins;however,Pep was more effective in enhancing the dispersibility of Cur than many of the cyclodextrins.
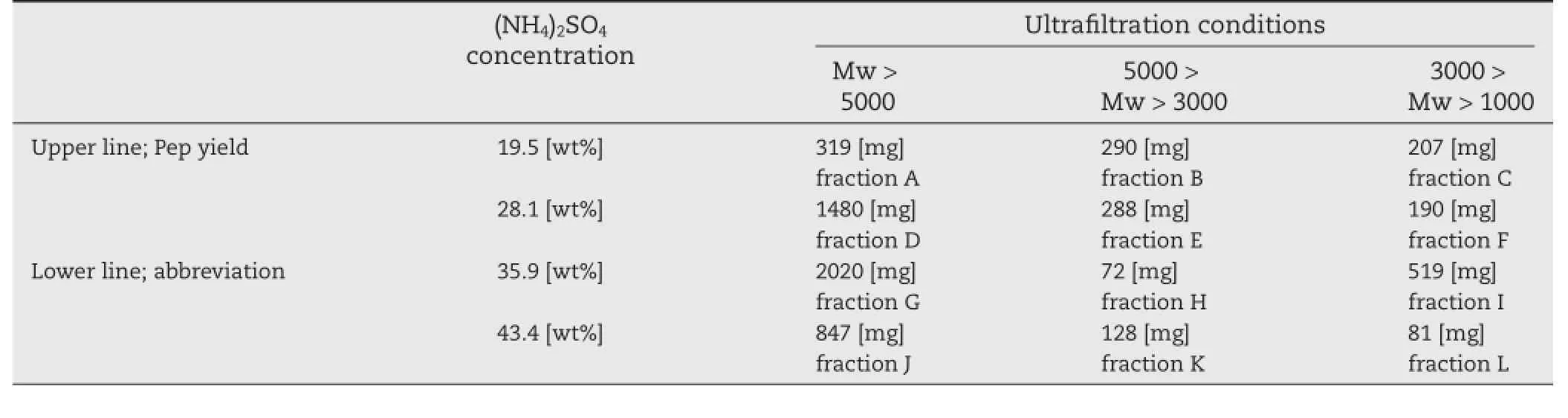
Table 1–Yields and abbreviations of Pep fractions obtained by the combination of ammonium sulfate precipitation and ultrafltration.
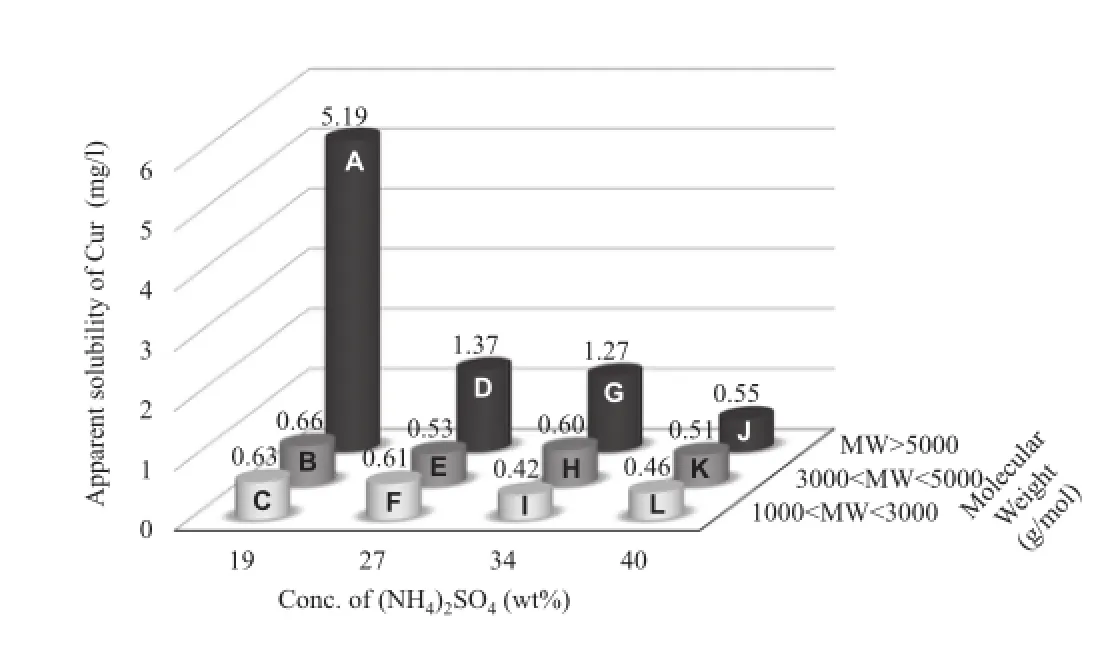
Fig.3–Apparent solubility of Cur-Pep prepared using different fractions.
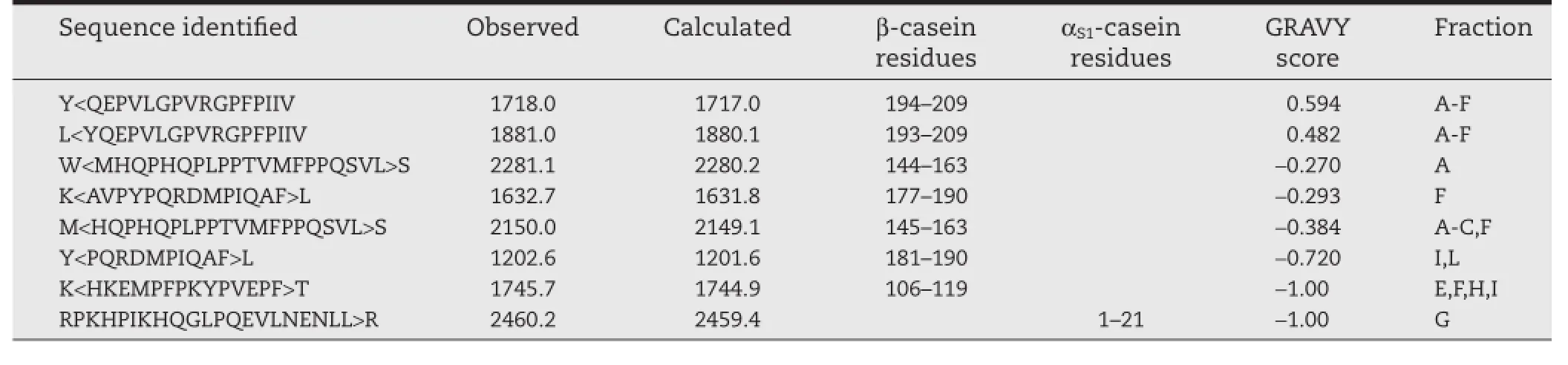
Table 2–Sequences of peptides in fractions A–L and the GRAVY scores[36].
The effect of pH on the apparent solubility of Cur-Pep and Cur-Blank is shown in Fig.6.Cur is present as the stable bisketo form under acidic or neutral conditions.Under basic conditions,Cur is deprotonated(pKavalues=7.75–7.80, 8.55,and 9.05)and degrades to ferulic acid(4-hydroxy-3-methoxycinnamic acid)and feruloylmethane(4-hydroxy-3-methoxycinnamoyl-methane)[48].Therefore,solubility tests were conducted at pH 2–8.Cur-Blank,which was prepared without Pep,was insoluble under all pH conditions investigated.The apparent solubilities of Cur-Pep are much higher than those of Cur-Blank over the pH range tested.However,the apparent solubility of Cur-Pep decreased around pH 4.The pH dependency of the Cur-Pep solubility is similar to that of the complex between peptides and coenzyme Q10,which is insoluble under weakly acidic conditions[35].The decrease in the apparent solubility of Cur-Pep could be due to the aggregation of Cur-Pep at a pH around the isoelectric point.The water solubility of HIP,which is a complex between heme iron and peptide fragments,also shows a similar pH dependency.HIP is soluble under strongly acidic or neutral conditions and insoluble under weakly acidic conditions because of the isoelectric point[38,40].
The effect of treatment of simulated digestive fuid using pepsin and pancreatin on the water dispersibility of Cur was also studied.As the Pep should be hydrolyzed using proteases,the apparent solubility of Cur-Pep after enzymatic treatment was 36%compared to that of a control sample without pepsin or pancreatin.This result suggests that Pep can be hydrolyzed and is safe when it is orally administered,while the dispersibility of Cur-Pep decreases.However,a peptide complex HIP is found to be more readily absorbed by the human body[41].Clinical study for oral administration of Cur-Pep should be conducted in future work.

Fig.4–Image of aqueous suspensions of Cur(left)and Cur-Pep(right)before and after fltration using a 0.80-μm membrane flter.
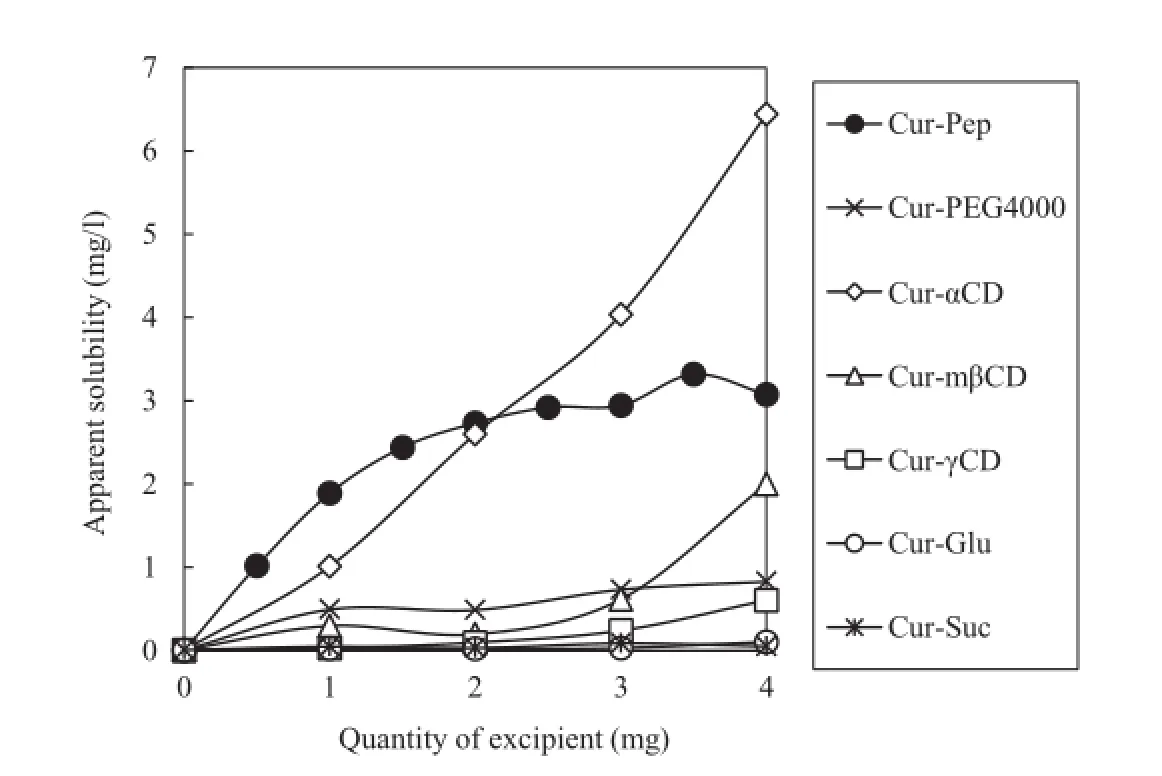
Fig.5–Effect of the quantity of excipient on the apparent solubility of Cur at pH 6.8(volume,1.0 ml).
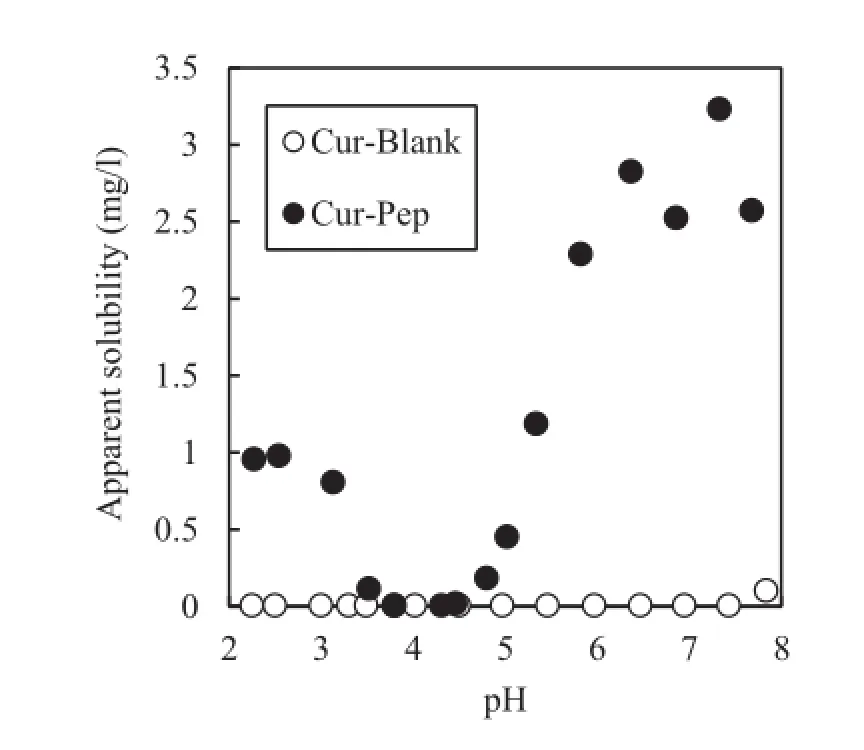
Fig.6–Effect of the equilibrium pH on the apparent solubility of Cur-Pep and Cur-Blank.
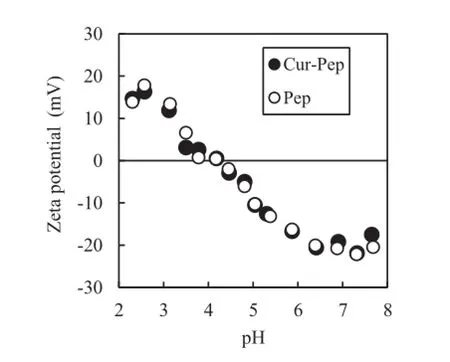
Fig.7–Zeta potential of aqueous suspensions of Cur-Pep and Pep as a function of pH.
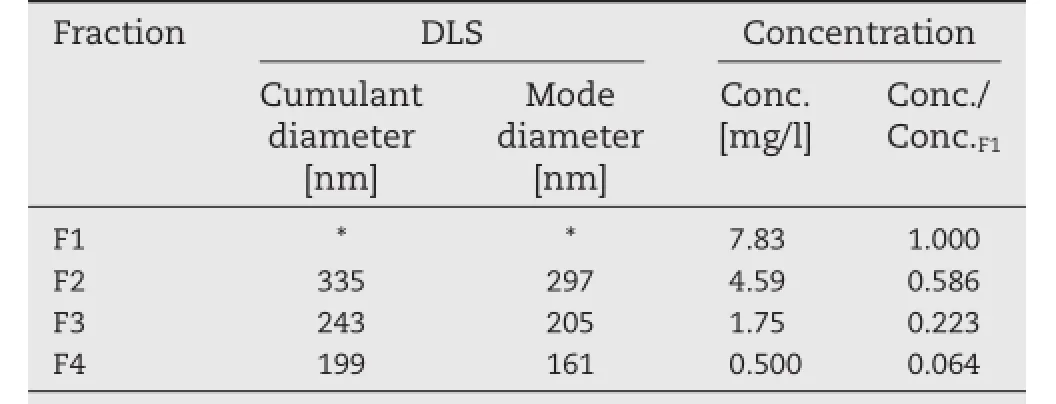
Table 3–Average particle size from dynamic light scattering(DLS)and concentration of Cur for the fractions of Cur-Pep.
3.2.Zeta potential of Cur-Pep
Fig.7 shows the zeta potential of aqueous suspensions of Cur-Pep and Pep as a function of pH.The zeta potentials of Cur-Pep and Pep decreased as the pH increased and the profles closely match each other.The isoelectric points of Cur-Pep and Pep are 4.18 and 4.17,respectively,demonstrating that Pep dominates the surface charge of Cur-Pep.As Pep consists of a mixture of peptides,it behaves as a mixture of amphoteric polyelectrolytes.The zeta potential of Cur-Pep supports the results regarding pH dependency of the apparent solubility of Cur-Pep shown in Fig.6.As the zeta potential of Cur-Pep at pH 4.2 approaches zero,the dispersibility of Cur-Pep decreases because of the aggregation of Cur-Pep.Thus,Cur-Pep disperses under strongly acidic and neutral conditions,but precipitates under weakly acidic conditions.
3.3.Material distribution and particle size analysis of Cur-Pep
An aqueous suspension of Cur-Pep was fractionated by centrifugation and fltration using membrane flters(Φ 0.80 μm, 0.45 μm,and 0.20 μm).Fig.8 shows typical particle size distributions of fractions F2–F4 of the aqueous suspensions of Cur-Pep determined using DLS.The particle size of F1 could not be recorded because the fraction contained larger particles. The particle sizes for F2–F4 of Cur-Pep were polydisperse and 160–330 nm.Therefore,Cur-Pep is present as a colloidal material with wide particle size distributions.
Table 3 summarizes the average particle size from DLS and the concentration of Cur for the fractions of Cur-Pep.The distribution of Cur in each fraction was consistent with the average particle size:The relative concentration of Cur in each fraction compared with F1 decreased from 0.586 to 0.064,indicating that the particles of Cur-Pep were gradually captured by fltration using 0.80-μm,0.45-μm,and 0.20-μm membrane flters. These results show that Cur-Pep exists as a hydrocolloidal material in aqueous media with polydisperse particle size,and the particle size is around 160–330 nm.The hydrocolloidal behavior of Cur-Pep is similar to that of the peptide complexes containing coenzyme Q10[35]and paclitaxel[36].On the other hand,the complex between Pep and indomethacin is not a hydrocolloidal material and is permeable through ultraflter membranes[33].
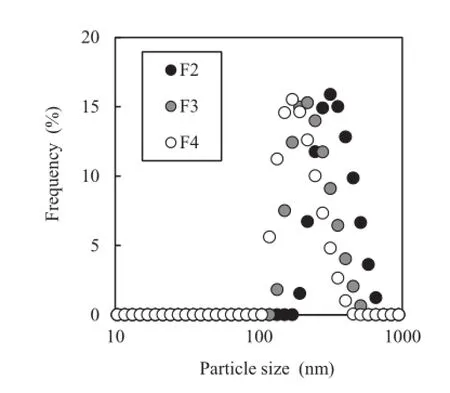
Fig.8–Particle size distribution of Cur-Pep in aqueous media determined using dynamic light scattering(DLS).
3.4.Characterization of Cur-Pep
Fig.9 shows SEM micrographs of Cur,Pep,and Cur-Pep.The morphology of Cur-Pep was similar to that of Pep.As the crystalline structure of Cur was not observed in Cur-Pep,Cur-Pep was found to be a homogeneous material with its main body based on that of Pep.
DSC and XRD of Cur-Pep were examined to study the crystallinity of Cur in the complex[9,15,33].Fig.10 shows the DSC thermograms of Cur,Cur-Pep-R,Cur-Pep,and Pep.The DSC thermogram of Cur showed an endothermal peak at 184°C,which can be attributed to the melting point of Cur(183°C).Cur-Pep-R,which is a mixture of solid Pep and Cur(5:1,w/w),showed a slight endothermic peak at 184°C,indicating that a small quantity of crystallized Cur is present.However,no endothermic peak between 170 and 190°C was observed for Cur-Pep. The XRD patterns of Cur,Cur-Pep-R,Cur-Pep,and Pep are shown in Fig.11.The XRD data for Cur show the presence of crystalline Cur[9,49].As the XRD pattern of Cur-Pep-R can be superimposed on that of Cur,this shows that Cur-Pep-R also contains crystalline Cur.In contrast,no diffraction peak of Cur was observed in the XRD pattern of Cur-Pep,which indicates that the Cur in Cur-Pep is amorphous.The results suggest thatCur in Cur-Pep is incorporated into the peptide matrix and is not present as a crystalline structure.
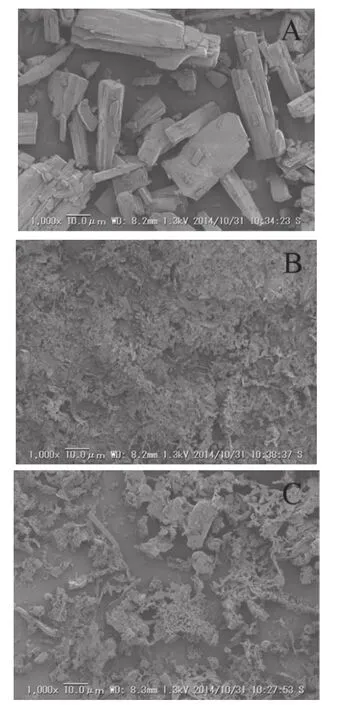
Fig.9–Scanning electron micrographs of Cur(A),Pep(B), and Cur-Pep(C)at 1000×magnifcation.
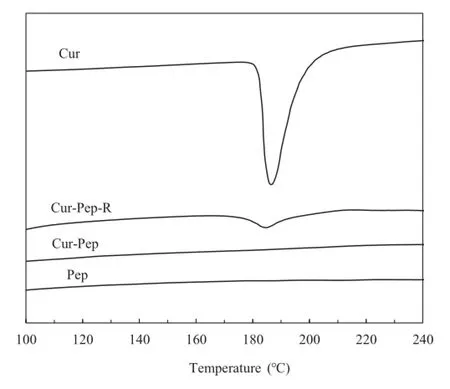
Fig.10–Differential scanning calorimetry(DSC)curves for Cur,Cur-Pep-R,Cur-Pep,and Pep.
4.Conclusion
A peptide mixture from casein hydrolysate(Pep)enhances the water-dispersibility of curcumin by complexation.In particular,relatively hydrophobic peptides with high molecular weights act as an effective excipient in for Cur.The water dispersibility of curcumin in weakly acidic and neutral conditions increased after complexation with Pep.Mass distribution of curcumin and particle analysis by DLS showed the complex was present as a hydrocolloidal material.DSC thermograms and XRD data showed curcumin was present in an amorphous state in the complex.Increased water dispersibility of the complex would increase the oral bioavailability of poorly water-soluble curcumin,and a clinical study on the absorption of curcumin in the human body should be conducted in future.Complexation with Pep would enhance the photochemical stability of curcumin,which should be studied in future work.
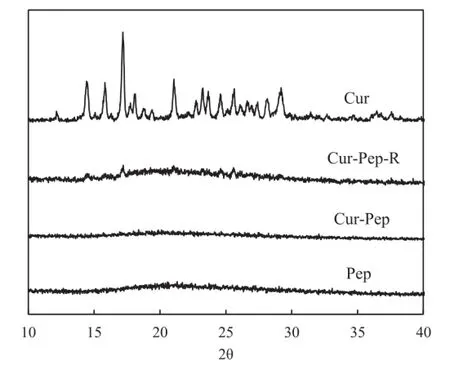
Fig.11–X-ray diffraction patterns for Cur,Cur-Pep-R,Cur-Pep,and Pep.
Acknowledgments
This research has been funded by the Japan Society for the Promotion of Science(JSPS)(No.LR029)through the“Funding Program for Next GenerationWorld-Leading Researchers(NEXT Program),”initiated by the Council for Science and Technology Policy(CSTP).
R E F E R E N C E S
[1]Aggarwal BB,Kumar A,Bharti AC.Anticancer potential of curcumin:preclinical and clinical studies.Anticancer Res 2003;23:363–398.
[2]Aggarwal BB,Harikumar KB.Potential therapeutic effects of curcumin,the anti-infammatory agent,against neurodegenerative,cardiovascular,pulmonary,metabolic, autoimmune and neoplastic diseases.Int J Biochem Cell Biol 2009;41:40–59.
[3]Kunchandy E,Rao MNA.Oxygen radical scavenging activity of curcumin.Int J Pharm 1990;58:237–240.
[4]Sharma OP.Antioxidant activity of curcumin and related compounds.Biochem Pharmacol 1976;25:1811–1812.
[5]Sharma RA,Gescher AJ,Steward WP.Curcumin:the story so far.Eur J Cancer 2005;41:1955–1968.
[6]Anand P,Kunnumakkara AB,Newman RA,et al. Bioavailability of curcumin:problems and promises.Mol Pharm 2007;4:807–818.
[7]Tonnesen HH,Masson M,Loftsson T.Studies of curcumin and curcuminoids.XXVII.Cyclodextrin complexation: solubility,chemical and photochemical stability.Int J Pharm 2002;244:127–135.
[8]Huang Q,Yu H,Ru Q.Bioavailability and delivery of nutraceuticals using nanotechnology.J Food Sci 2010;75:R50–R57.
[9]Pawar YB,Shete G,Popat D,et al.Phase behavior and oral bioavailability of amorphous curcumin.Eur J Pharm Sci 2012;47:56–64.
[10]Khadka P,Ro J,Kim J,et al.Pharmaceutical particle technologies:an approach to improve drug solubility, dissolution and bioavailability.Asian J Pharm Sci 2014;9:304–316.
[11]Ma Z,Haddadi A,Molavi O,et al.Micelles of poly(ethylene oxide)-b-poly(ε-caprolactone)as vehicles for the solubilization,stabilization,and controlled delivery of curcumin.J Biomed Mater Res A 2008;86:300–310.
[12]Letchford K,Liggins R,Burt H.Solubilization of hydrophobic drugs by methoxy poly(ethylene glycol)-blockpolycaprolactone diblock copolymer micelles:theoretical and experimental data and correlations.J Pharm Sci 2008;97:1179–1190.
[13]Bisht S,Feldmann G,Soni S,et al.Polymeric nanoparticleencapsulated curcumin(“nanocurcumin”):a novel strategy for human cancer therapy.J Nanobiotechnology 2007;5:art. no.3.
[14]Li H,Sun J,Wang Y,et al.Structure-based in silico model profles the binding constant of poorly soluble drugs with β-cyclodextrin.Eur J Pharm Sci 2011;42:55–64.
[15]Mulik R,Mahadik K,Paradkar A.Development of curcuminoids loaded poly(butyl)cyanoacrylate nanoparticles:physicochemical characterization and stability study.Eur J Pharm Sci 2009;37:395–404.
[16]Manju S,Sreenivasan K.Synthesis and characterization of a cytotoxic cationic polyvinylpyrrolidone-curcumin conjugate. J Pharm Sci 2011;100:504–511.
[17]Manju S,Sreenivasan K.Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability.J Colloid Interface Sci 2011;359:318–325.
[18]Cui J,Yu B,Zhao Y,et al.Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm 2009;371:148–155.
[19]Wang X,Jiang Y,Wang YW,et al.Enhancing antiinfammation activity of curcumin through O/W nanoemulsions.Food Chem 2009;108:419–424.
[20]Shaikh J,Ankola DD,Beniwal V,et al.Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer.Eur J Pharm Sci 2009;37:223–230.
[21]Sahu A,Kasoju N,Bora U.Fluorescence study of the curcumin-casein micelle complexation and its application as a drug nanocarrier to cancer cells.Biomacromolecules 2008;9:2905–2912.
[22]El-Salam MHA,El-Shibiny S.Formation and potential uses of milk proteins as nano delivery vehicles for nutraceuticals:a review.Int J Dairy Technol 2012;65:13–21.
[23]Elzoghby AO,El-Fotoh WSA,Elgindy NA.Casein-based formulations as promising controlled release drug delivery systems.J Control Release 2011;153:206–216.
[24]Pan K,Luo Y,Gan SJ,et al.pH-Driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity.Soft Matter 2014;10:6820–6830.
[25]Oshima T,Yamashita R,Baba Y.Preparation of the complex between curcumin and casein by kneading and its water dispersibility.Kagaku Kogaku Ronbun 2015;41:381–386.
[26]Rahimi Yazdi S,Corredig M.Heating of milk alters the binding of curcumin to casein micelles.A fuorescence spectroscopy study.Food Chem 2012;132:1143–1149.
[27]Mehranfar F,Bordbar AK,Keyhanfar M,et al. Spectrofuoremetric and molecular docking study on the interaction of bisdemethoxycurcumin with bovine β-casein nanoparticles.J Lumin 2013;143:687–692.
[28]Esmaili M,Ghaffari SM,Moosavi-Movahedi Z,et al.Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin:food industry application.LWT Food Sci Technol 2011;44:2166–2172.
[29]Sneharani AH,Singh SA,Rao AGA.Interaction of αS1-casein with curcumin and its biological Implications.J Agric Food Chem 2009;57:10386–10391.
[30]Bourassa P,Bariyanga J,Tajmir-Riahi HA.Binding sites of resveratrol,genistein,and curcumin with milk α-and β-caseins.J Phys Chem B 2013;117:1287–1295.
[31]Pan K,Zhong Q,Baek SJ.Enhanced dispersibility and bioactivity of curcumin by encapsulation in casein nanocapsules.J Agric Food Chem 2013;61:6036–6043.
[32]Tapal A,Tiku PK.Complexation of curcumin with soy protein isolate and its implications on solubility and stability of curcumin.Food Chem 2012;130:960–965.
[33]Inada A,Oshima T,Takahashi H,et al.Enhancement of water solubility of indomethacin by complexation with protein hydrolysate.Int J Pharm 2013;453:587–593.
[34]Oshima T,Inada A,Baba Y.Evaluation of the hydrophilic and hydrophobic balance for the complex between indomethacin and casein hydrolysate using an aqueous two-phase system.Solvent Extr Res Dev Jpn 2013;20:71–77.
[35]Matsushita N,Oshima T,Takahashi H,et al.Enhanced water dispersibility of coenzyme Q10 by complexation with albumin hydrolysate.J Agric Food Chem 2013;61:5972–5978.
[36]Inada A,Oshima T,Baba Y.Enhancing the water dispersibility of paclitaxel by complexation with hydrophobic peptides.Colloids Surf B Biointerfaces 2015;135:408–415.
[37]Lebrun F,Bazus A,Dhulster P,et al.Infuence of molecular interactions on ultrafltration of a bovine hemoglobin hydrolysate with an organic membrane.J Memb Sci 1998;146:113–124.
[38]Lebrun F,Bazus A,Dhulster P,et al.Solubility of heme in heme-iron enriched bovine hemoglobin hydrolysates.J Agric Food Chem 1998;46:5017–5025.
[39]Oshima T,Niide T,Shiraki H,et al.Preparation and separation of heme iron preparation from fsh blood of cultured yellowtail.Kagaku Kogaku Ronbun 2011;37:51–56.
[40]Todaka M,Oshima T,Baba Y.Production and characterization of hydrophilic heme iron preparation from fsh blood.J Chem Eng Jpn 2014;47:893–899.
[41]Seligman PA,Moore GM,Schleicher RB.Clinical studies of HIP:an oral heme-iron product.Nutr Res 2000;20:1279–1286. [42]Holt C.Structure and stability of bovine casein micelles.Adv Protein Chem 1992;43:63–153.
[43]De Kruif CG,Huppertz T,Urban VS,et al.Casein micelles and their internal structure.Adv Colloid Interface Sci 2012;171–172:36–52.
[44]Jinsmaa Y,Yoshikawa M.Enzymatic release of neocasomorphin and β-casomorphin from bovine β-casein. Peptides 1999;20:957–962.
[45]Hernández-Ledesma B,Quirós A,Amigo L,et al. Identifcation of bioactive peptides after digestion of human milk and infant formula with pepsin and pancreatin.J Mol Biol 1982;157:105–132.
[46]Kyte J,Doolittle RF.A simple method for displaying the hydropathic character of a protein.J Mol Biol 1982;157:105–132.
[47]Yu L.Amorphous pharmaceutical solids:preparation, characterization and stabilization.Adv Drug Deliv Rev 2001;48:27–42.
[48]Wang YJ,Pan MH,Cheng AL,et al.Stability of curcumin in buffer solutions and characterization of its degradation products.J Pharm Biomed Anal 1997;15:1867–1876.
[49]Wang YJ,Song L,Shen Y,et al.Polymeric micelles for parenteral delivery of curcumin:preparation, characterization and in vitro evaluation.Colloids Surf A Physicochem Eng Asp 2011;390:25–32.
*< class="emphasis_italic">Corresponding author.
.University of Miyazaki,1-1 Gakuen Kibanadai Nishi,Miyazaki 889-2192,Japan.Tel.:+81 985 58 7321;fax:+81 985 58 7323.
E-mail address:oshimat@cc.miyazaki-u.ac.jp(T.Oshima).
http://dx.doi.org/10.1016/j.ajps.2016.05.001
1818-0876/?2016 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年5期
Asian Journal of Pharmacentical Sciences2016年5期
- Asian Journal of Pharmacentical Sciences的其它文章
- Quantitative determination of metaxalone in human plasma by LC-MS and its application in a pharmacokinetic study
- Effects of duration of phenytoin administration on mRNA expression of cytochrome P450 and P-glycoprotein in the liver and small intestine of rats
- Effect of surface ligand density on cytotoxicity and pharmacokinetic profle of docetaxel loaded liposomes
- Stability of freeze-dried pH-responsive dextrin nanogels containing doxorubicin
- Evaluation of pharmacokinetics underlies the collaborated usage of lamivudine and oxymatrine in beagle dogs
- An intravenous clarithromycin lipid emulsion with a high drug loading,H-bonding and a hydrogen-bonded ion pair complex exhibiting excellent antibacterial activity
