Huangqi Guizhi Wuwu Decoction for treating diabetic peripheral neuropathy: a meta-analysis of 16 randomized controlled trials
Bing Pang, Tian-yu Zhao, Lin-hua Zhao Fang Wan Ru Ye Qiang Zhou, Feng Tian, Xiao-lin Tong Guang’anmen Hospital of China Academy of Chinese Medical Sciences, Beijing, China2 Department of Endocrinology, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China Digestive Disease Diagnosis and Treatment Center, Beijing Chinese Medicine Hospital, Capital Medical University, Beijing, China Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing, China
Huangqi Guizhi Wuwu Decoction for treating diabetic peripheral neuropathy: a meta-analysis of 16 randomized controlled trials
Bing Pang1,#, Tian-yu Zhao2,#, Lin-hua Zhao1, Fang Wan1, Ru Ye1, Qiang Zhou3, Feng Tian4, Xiao-lin Tong1,*
1 Guang’anmen Hospital of China Academy of Chinese Medical Sciences, Beijing, China
2 Department of Endocrinology, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
3 Digestive Disease Diagnosis and Treatment Center, Beijing Chinese Medicine Hospital, Capital Medical University, Beijing, China
4 Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing, China
How to cite this article: Pang B, Zhao TY, Zhao LH, Wan F, Ye R, Zhou Q, Tian F, Tong XL (2016) Huangqi Guizhi Wuwu Decoction for treating diabetic peripheral neuropathy∶ a meta-analysis of 16 randomized controlled trials. Neural Regen Res 11(8)∶1347-1358.
Funding: This work was supported by a grant from the National Basic Research Program of China (973 Program), No. 2010CB530600; and Institutes Project from Guang'anmen Hospital of China Academy of Chinese Medical Sciences, No. 2011261.
Xiao-lin Tong, M.D.,
xiaolintong66@sina.com.
#These authors contributed
equally to this study.
orcid:
0000-0002-0069-5055
(Bing Pang)
Accepted: 2016-07-03
Graphical Abstract
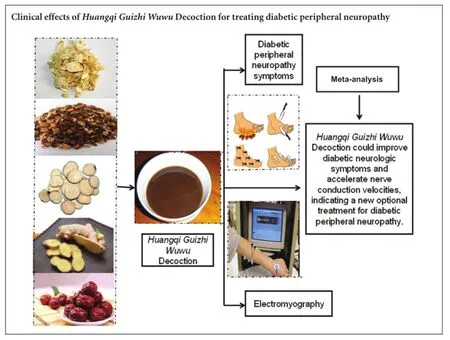
OBJECTIVE: This meta-analysis was performed to systematically assess the efficacy and safety of the Chinese herbal medicine Huangqi Guizhi Wuwu Decoction (HGWWD) for treating diabetic peripheral neuropathy.
DATA SOURCES: Six electronic databases, including the Cochrane Library, MEDLINE database, Chinese Biomedical Database, Chinese National Knowledge Infrastructure Database, Chinese Science and Technique Journals Database, and the Wanfang Database, were search ed on the internet for randomized controlled trials published up until 1 December 2015. The search terms included “Chinese herbal medicine”, “diabetic peripheral neuropathy” and “randomized controlled trials” in Chinese and in English.
DATA SELECTION: We included randomized controlled trials using HGWWD/modified HGWWD for the treatment group, without restriction for the control group. We assessed literature quality in accordance with the Cochrane Review Handbook. A random or a fixed effects model was used to analyze outcomes using RevMan 5.2 software.
OUTCOME MEASURES: The primary outcomes were changes in symptoms and nerve conduction velocities. The secondary outcomeswere fasting blood glucose and hemorheological indexes.
RESULTS: Sixteen randomized controlled trials, with a total of 1,173 patients, were included. Meta-analysis revealed that the efficacy of HGWWD for diabetic peripheral neuropathy was significantly superior compared with the control treatment (i.e., control group) (risk ratio = 0.36, 95% confidence interval (CI): 0.29-0.46, Z =8.33, P < 0.00001) Compared with the control group, there was an increase in median motor nerve conduction velocity (mean difference (MD) = 3.46, 95%CI: 1.88-5.04, Z = 4.30, P < 0.01) and median sensory nerve conduction velocity (MD = 3.30, 95%CI: 2.04-4.56, Z = 5.14, P < 0.01). There was also an increase in peroneal motor nerve conduction velocity (MD = 3.22, 95%CI: 2.45-3.98, Z = 8.21, P < 0.01) and peroneal sensory nerve conduction velocity (MD = 3.05, 95%CI: 2.01-4.09, Z = 5.75, P < 0.01) in the treatment groups. No significant difference in fasting blood glucose was found between the treatment groups and the control groups (MD = -0.12, 95%CI: -0.42-0.19, Z = 0.76, P = 0.45). Plasma viscosity was significantly decreased after treatment (MD = -0.11, 95%CI: -0.21 to -0.02, Z = 2.30, P = 0.02). No significant difference in fibrinogen was detectable (MD = -0.53, 95%CI: -1.28-0.22, Z = 1.38, P = 0.17). Four trials reported that treatment groups experienced no adverse reactions. Adverse events were not mentioned in the other 12 trials. No trial reported the incidence of complications, quality of life outcomes, or health economics.
CONCLUSION: HGWWD treatment improves diabetic neurologic symptoms and ameliorates nerve conduction velocities. Our study suggests that HGWWD may have significant therapeutic efficacy for the treatment of diabetic peripheral neuropathy. However, the methodological quality of the randomized controlled trials was generally low. Larger and better-designed randomized controlled trials are required to more reliably assess the clinical effectiveness of HGWWD.
nerve regeneration; meta-analysis; diabetic peripheral neuropathy; randomized controlled trials; Huangqi Guizhi Wuwu Decoction; traditional Chinese medicine; mecobalamin; efficacy; nerve conduction velocities; fasting blood glucose; hemorheology; neural regeneration
Background
Diabetic peripheral neuropathy (DPN) is a common and severe complication of diabetes mellitus (Dong et al., 2016). DPN can cause progressive nerve fiber degeneration as well as sensory and motor nerve dysfunction, which is characterized by unremitting pain (hyperalgesia), sensory loss and limb impairment that can progress to muscular dystrophy in late DPN stages (Boulton et al., 2005). Recent reports suggest that ~50% of those with diabetes mellitus develop nerve damage within 10-20 years after diagnosis (Spruce et al., 2003; Tesfaye et al., 2012). Tesfaye’s group estimated that DPN may affect as many as 236 million people worldwide (Tesfaye et al., 2012). DPN symptoms worsen at night, and result in sleep and mood disturbance, and poor treatment causes further serious complications (diabetic foot ulcers, infections, and gangrene) (American Diabetes Association, 2013). Thus, DPN represents a major problem to the health care system (Davies et al., 2015). At present, treatment for DPN chiefly includes diabetic management via tight glucose control and pain relief for neuropathy (Hemmingsen et al., 2013; Davies et al., 2015). Therapeutic strategies include aldose reductase inhibitors, antioxidant therapy, neurotrophic drugs and analgesics, which all have potential toxicity and side-effects, as well as poor tolerability. Moreover, many of these therapies may be ineffective for many diabetic patients (Gao et al., 2012). Thus, traditional Chinese medicine may hold some promise for specific DPN patients.
DPN may fall under the traditional Chinese medicine categories of “blood impediment” (China Association of Traditional Chinese Medicine, 2007). Clinical studies indicate that qi asthenia-associated blood stasis, and vessel and collateral obstruction are common in DPN (Wang et al., 2011; Zhou et al., 2013). Huangqi Guizhi Wuwu Decoction (HGWWD) is a classical prescription described in Jingui Yaolue, written by Zhang Zhong-jing during the Han dynasty. HGWWD is composed of Radix Astragali seu Hedysari, Ramulus Cinnamomi, Radix Paeoniae Alba, Rhizoma Zingiberis Recens and Fructus Jujubae, all of which are reported to improve qi and promote the flow of yang, invigorating the blood to promote coronary circulation (Bai et al., 2015). HGWWD is used to treat “blood impediment” characterized by a feeling of numbness and pain, and rough pulse, consistent with the features of DPN. Recent studies (Hua et al., 2008; Bian, 2010; Wang, et al., 2012) suggest that the mechanisms of action of HGWWD in the treatment of DPN in rats include an amelioration of morphological changes and an improvement of nerve function, probably through improving expression of multiple neurotrophic factors, enhancing blood flow and reducing free radical production. HGWWD may improve subjective symptoms associated with this progressive disabling disorder as well (Tong et al., 2006; Zhang et al., 2014). HGWWD exerts multiple therapeutic effects by acting on multiple targets. Although previous studies (Li, 2002; Liu, 2005; Chen et al., 2006; Ji et al., 2007; Bian et al., 2010; Wang and Luan, 2011; Hu et al., 2013) suggest that HGWWD is therapeutically effective for DPN, a systematic evaluation of these studies is required to more accurately assess the efficacy and safety of the medicine.
Systematic evaluation of randomized controlled trials (RCTs) is a reliable method for verifying the validity and safety of certain therapies. A few systematic reviews and meta-analyses (Chen et al., 2013) have examined the efficacy of Chinese herbal medicines for DPN, but these studies did not focus on a specific formulation. In the present systematic study, we evaluate the efficacy of a classical prescription-HGWWD-by evaluating clinical trials. Our findings should serve as a reference for clinicians seeking effective treatments for DPN.
Data and Methods
Database and search strategy
Trials were identified from the following six electronic databases: Cochrane Library, MEDLINE database, Chinese Biomedical Database, Chinese National Knowledge Infrastructure Database, Chinese Science and Technique Journals Database, and the Wanfang Database. We searched all trials published before 1 December 2015. Search terms (free words search) were as follows: “diabetes” OR “diabetic” OR “diabetes mellitus”; (“diabetic peripheral neuropathy” OR “peripheral nervous system disease” OR “diabetic neuropathies”) AND(“Huang Qi Gui Zhi Wu Wu” OR “Huangqi Guizhi Wuwu”O(jiān)R “Huangqi Guizhi Wuwu Tang/Decoction”) AND (“randomized controlled trial” OR “controlled clinical trial” OR“random” OR “randomly” OR “randomized” OR “control”). Different search strategies were used for Chinese and foreign language databases. Conference abstracts were searched manually. No language, publication or date limitations were used.
Inclusion and exclusion criteria
The study was restricted to RCTs that compared HGWWD/modified HGWWD with a control group. We assessed HGWWD/modified HGWWD as the treatment group, without restriction for the control group, whether mecobalamin, no treatment or placebo. RCTs that used HGWWD/modified HGWWD plus conventional Western treatment (mecobalamin) compared with conventional Western treatment (mecobalamin) alone were included as well. Baseline data, where available, were included.
Inclusion criteria: (1) This analysis included DPN patients irrespective of gender, age or ethnicity, but all the patients were diagnosed with diabetes mellitus by clearly defined or internationally recognized criteria; (2) patients with paresthesia or hypesthesia in the four limbs or in the lower extremities, including pain, tingling, numbness, weakness or burning sensation; (3) patients with neurologic abnormalities, including an abnormal tendon reflex, or the absence of a sense of vibration in the lower extremities; (4) reduced motor nerve conduction velocity (MNCV) and sensory nerve conduction velocity (SNCV), as demonstrated by a nerve conduction test (China Association of Traditional Chinese Medicine, 2007; Chen 2013).
Exclusion criteria: (1) Treatments that combined other traditional Chinese medicine therapies, such as acupuncture, acupoint injection or the use of other Chinese herbs were excluded, in order to more specifically assess the effects of HGWWD alone; (2) non-randomized trials were excluded; (3) patients with sensorimotor polyneuropathy associated with other diseases were excluded.
Data extraction
Two authors (Lin-hua Zhao and Fang Wan) extracted data independently. The data included general trial characteristics (title, authors, year and source), baseline patient and disease data (sample size, age, gender and disease course), interventions (component and dosage of Chinese herbs, and details of the control interventions), and outcomes (clinical efficacy and other clinically relevant outcomes, adverse events and length of follow-up). Discrepancies were settled by consensus or by a third party (Xiao-lin Tong).
Quality assessment
Two authors (Ru Ye and Qiang Zhou) assessed the risk of bias in trials according to the Cochrane Handbook for meta-analysis of the treatments (Higgins and Green, 2013), based on the following six items: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias. We categorized each item into one of three levels-“high risk”, “l(fā)ow risk” or“unclear-as follows: low risk of bias (all the items had a low risk of bias), high risk of bias (at least one item had a high risk of bias), and unclear risk of bias (at least one item had an unclear risk of bias). Discrepancies in interpretation were resolved by consensus or by a third party (Xiao-lin Tong).
Outcome measures
Clinical efficacy was the main outcome, and was based on changes in symptoms and nerve conduction velocities (NCVs) (Ministry of Health, China, 2002). The secondary outcomes were fasting blood glucose and hemorheological indexes at the end of treatment.
Improvements were categorized as significantly effective (disappearance of subjective symptoms, recovered tendon reflex, and NCV increased significantly), effective (alleviated subjective symptoms, improved tendon reflex, and NCV increased) or ineffective (no improvement in symptoms, tendon reflex or NCV). NCV was assessed by median MNCV, median SNCV, peroneal MNCV and peroneal SNCV.
Statistical analysis
RevMan 5.2 software (Cochrane Collaboration, Oxford, UK) was used for data analysis. Publication bias was examined by funnel plots. In the outcomes, the data for efficacy were dichotomous, and others were continuous. Dichotomous data were expressed as the risk ratio (RR), and continuous outcomes between groups as mean difference (MD), both with a 95% confidence interval (95%CI), using a fixed-effects or random-effects model. A significant difference for the heterogeneity test was considered when P < 0.05, and substantial heterogeneity was indicated by an I2of 50% or more. In such cases, a random effects model was used; otherwise a fixed effects model was used (i.e., when P > 0.05 or I2< 50%).
Results
Description of trials
Our primary searches identified 372 references from the databases. Of these, 209 references were repeated literature and were excluded. After reading the titles and summaries, another 117 references were excluded because they were repeated literature, case reports, experimental studies, retrospective studies or literature reviews. A total of 46 references were retrieved for further assessment. After a detailed evaluation of the full text, an additional 30 references were excluded. Among these, seven trials were excluded because they used another traditional Chinese medicine therapy in the treatment group. Four trials were excluded because of incomplete or erroneous baseline or treatment data. Seven studies claimed that the trials were RCTs, but they lacked a controlgroup. The therapies in six trials were not in accordance with the inclusion criteria. The remaining six excluded trials were theoretical or non-clinical experiments. The search results are displayed in Figure 1.
Included trials
We included 16 trials (Li, 2002; Liu, 2005; Chen and Liu, 2006; Ji, 2007; Lian et al., 2007; Shu, 2007; Bian, 2010; Lu and You, 2010; Ye, 2010; Zhu, 2010; Wang and Luan, 2011; Hu and Wang, 2012; Jiang, 2012; Han, 2013; Yang, 2013; Zhang and Fu, 2013). All were RCTs with two parallel arms. All trials were conducted and published in China before 1 December 2015. The characteristics of the included trials are summarized in Table 1.
HGWWD decoction for treating DPN
Participants
In this meta-analysis, a total of 1,173 participants with DPN were included (619 patients and 554 controls). All trials were hospital-based and included inpatients and/or outpatients. Trial samples ranged from 36 to 225 participants, and a total of 622 males and 551 females were included. The age of participants ranged from 25 to 78 years. In six trials (Li, 2002; Liu, 2005; Lian et al., 2007; Lu and You, 2010; Zhu, 2010; Yang, 2013), enrolled patients suffered from type 2 diabetes, and in one trial (Shu, 2007), enrolled patients with either type 1 or type 2 diabetes were included. In nine trials, the type of diabetes was not reported. Diagnostic criteria for diabetes were necessary. Thirteen trials (Li, 2002; Liu, 2005; Chen and Liu, 2006; Lian et al., 2007; Shu, 2007; Bian, 2010; Ye, 2010; Zhu, 2010; Wang and Luan, 2011; Hu and Wang, 2012; Han, 2013; Yang, 2013; Zhang and Fu, 2013) mentioned World Health Organization diabetes mellitus diagnostic criteria (1999) or the American Diabetes Association criteria. In these 13 trials, acquired nerve symptoms, including pain, tingling, numbness, weakness, paralysis, abnormal tendon reflex and delayed nerve conduction were reported, and other neuropathic diseases were excluded. In three trials (Ji, 2007; Lu and You, 2010; Jiang, 2012), internal diabetes mellitus diagnostic criteria were used, and acquired nerve symptoms, including pain, tingling, numbness, weakness, paralysis, abnormal tendon reflex and delayed nerve conduction were reported, and other neuropathic diseases were excluded. Six trials (Liu, 2005; Bian, 2010; Lu and You, 2010; Zhu, 2010; Hu et al., 2012; Han, 2013) mentioned that the pattern of the syndrome was consistent with qi asthenia-caused blood stasis, while the remaining 10 trials did not classify the syndrome according to traditional Chinese medical theory.
Treatments
Seven trials (Li, 2002; Liu, 2005; Shu, 2007; Bian, 2010; Lu and You, 2010; Zhu, 2010; Hu and Wang, 2012) used modified HGWWD, and compared this treatment group with a group given mecobalamin. In two trials (Wang, 2011; Jiang, 2012), modified HGWWD was compared with patients given no treatment. In seven trials (Chen and Liu, 2006; Ji, 2007; Lian et al., 2007; Ye, 2010; Han, 2013; Yang, 2013; Zhang and Fu, 2013), modified HGWWD plus mecobalamin was compared with mecobalamin alone. Hypoglycemic therapy was concomitantly given in both groups to control glycemia. Trial durations varied from 2 weeks to 12 weeks.
Outcomes
All trials used clinical efficacy based on changes in symptoms and NCV reported at the end of treatment, which was used as the main outcome index. We used FBG and hemorheological indexes as secondary outcome measures in the present meta-analysis. Adverse events were also recorded. None of the trials reported complications, reduced quality of life, or economic effects.
Risk of bias in the included trials
The quality assessment of the included trials is shown in Table 2. No trial reported details of sample size calculations, and none of the 16 trials were double-blind, placebo-controlled trials. Eight trials (Liu, 2005; Chen, et al., 2006; Shu, 2007; Ji, 2007; Hu and Wang, 2012; Jiang, 2012; Han, 2013; Zhang and Fu, 2013) described methods of randomization using a random number table. The remaining eight trials indicated “randomly allocating”, but the method of randomization was not provided. No trial stated how allocation concealment or blinding was performed. All the 16 trials provided complete baseline information, and described similarities between comparison groups. No trial reported participant losses, so attrition bias was uncertain. Selective reporting was difficult to assess, and trial protocols were unavailable. All trials were generally assessed low in quality and contained risk of bias, suggesting that future studies might influence the confidence intervals in this meta-analysis and require revising the conclusion.
Meta-analysis results
Efficacy
The results of the 16 trials were included in our meta-analysis and demonstrated a significant difference in clinical efficacy between the treatment groups and the control groups. These trials showed insignificant heterogeneity of the trial results (χ2= 8.49, P = 0.83, I2= 0%). Thus, a fixed effects model was used for statistical analysis. Treatment groups were superior to control groups in terms of efficacy (n = 1,173, RR = 0.36, 95%CI: 0.29-0.46, Z = 8.33, P < 0.00001) (n = 1,173, RR = 1.33, 95%CI: 1.24-1.42, Z = 8.39, P < 0.01). To compare the clinical efficacy of modified HGWWD with the control group, subgroup analysis was performed. There was a significant difference between HGWWD alone and the control groups (n = 566, RR = 0.31, 95%CI: 0.22-0.45, Z = 6.42, P < 0.00001), without heterogeneity (chi-square=1.70, P = 0.99, I2= 0%). A significant difference in clinical efficacy was also found between HGWWD plus conventional Western drug and control groups (n = 607, RR = 0.41, 95%CI: 0.30-0.57, Z = 5.34, P < 0.00001; n = 607, RR = 1.28, 95%CI: 1.17-1.40, Z = 5.39, P < 0.01), without heterogeneity (n = 607, RR = 0.41, 95%CI: 0.30-0.57, Z = 5.34, P < 0.00001) (χ2= 6.50, P = 0.37, I2= 8%) (Figure 2).

Figure 1 Flow chart of the literature screen.
NCVs
The pooled analysis of NCVs taken as a continuous measurement was not different between treatment groups in any of the trials. After 2 to 12 weeks, NCVs differed significantly between treatment groups and control groups. To compare NCVs in the modified HGWWD groups with those in the control groups, subgroup analysis was performed.
Eight trials, involving a total of 711 patients, reported median MNCV as an outcome (MD = 3.46, 95%CI: 1.88-5.04, Z = 4.30, P < 0.01) (Figure 3). Significant heterogeneity between trials was observed (χ2= 50.75, P < 0.01, I2= 86%), and accordingly, a random effects model was used for statistical analysis. Four trials compared the median MNCV between HGWWD alone and a control group (n = 286, MD = 3.56, 95%CI = 0.76-6.36, Z = 2.49, P = 0.01), with heterogeneity (χ2= 32.71, P < 0.01, I2= 91%), and four trials compared the median MNCV between HGWWD plus conventional Western drug and a control group (n = 425, MD = 3.31, 95%CI: 1.46-5.15, Z = 3.51, P < 0.01), with heterogeneity (χ2= 14.87, I2= 80%, P < 0.01).
Nine trials, involving a total of 765 patients, reported median SNCV as an outcome (MD = 3.30, 95%CI: 2.04-4.56, Z = 5.14, P < 0.01) (Figure 4). Significant heterogeneity between trials was observed (χ2= 38.39, P < 0.01, I2= 79%), and therefore, a random effects model was used for statistical analysis. Five trials compared the median SNCV between HGWWD alone and a control group (n = 340, MD = 3.47, 95%CI: 1.61-5.32, Z = 3.66, P < 0.01), with heterogeneity (χ2= 16.18, I2= 75%, P < 0.01), and four trials compared the median SNCV between HGWWD plus conventional Western drug and a control group (n = 425, MD = 3.14, 95%CI: 1.17-5.12, Z = 3.11, P < 0.01), with heterogeneity (χ2= 22.21, I2= 86%, P < 0.01).
Nine trials, involving a total of 765 patients, reported peroneal MNCV as an outcome (MD = 3.22, 95%CI: 2.45-3.98, Z = 8.21, P < 0.01) (Figure 5). Heterogeneity between trials was significant (χ2= 16.02, P = 0.04, I2= 50%), and accordingly, a random effects model was used for statistical analysis. Five trials compared the peroneal MNCV between HGWWD alone and a control group (n = 340, MD = 3.87, 95%CI: 3.07-4.66, Z = 9.54, P < 0.01), without heterogeneity (chi-square = 2.77, P = 0.60, I2= 0%), and four trials compared the peroneal MNCV between HGWWD plus conventional Western drug and a control group (n = 425, MD = 2.52, 95%CI: 1.56-3.48, Z = 5.15, P < 0.01), with heterogeneity (χ2= 5.82, P = 0.12, I2= 48%).
Eight trials, involving a total of 711 patients, reported peroneal SNCV as an outcome (MD = 3.05, 95%CI: 2.01-4.09, Z = 5.75, P < 0.01) (Figure 6). Significant heterogeneity between trials was observed (χ2= 26.65, I2= 74%, P < 0.01), and therefore, a random effects model was used for statistical analysis. Four trials compared peroneal SNCV between HGWWD alone and a control group (n = 286, MD = 4.27, 95%CI: 2.73-5.82, Z = 5.43, P < 0.01), with heterogeneity (χ2= 9.83, P = 0.02, I2= 69%), and four trials compared the peroneal SNCV between HGWWD plus conventional Western drug and a control group (n = 425, MD = 1.94, 95%CI: 1.27-2.62, Z = 5.68, P < 0.01), without heterogeneity (χ2= 0.48, P = 0.92, I2= 0%).
Glycemic control
In four trials (Li, 2002; Liu, 2005; Bian, 2010; Lu and You, 2010), changes in fasting blood glucose levels were assessed, and they did not show heterogeneity (χ2= 1.78, I2= 0%, P = 0.62). Thus, a fixed effects model was used for statistical analysis. A meta-analysis of four trials revealed no significant difference in changes in fasting blood glucose levels between the treatment groups and the control groups (n = 204, MD = -0.12, 95%CI: -0.42-0.19, Z = 0.76, P = 0.45) (Figure 7).

Figure 2 Clinical efficacy of HGWWD in the treatment of DPN.

Figure 3 Effects of HGWWD on median MNCV in patients with DPN.

Figure 4 Effects of HGWWD on median SNCV in patients with DPN.

Figure 5 Effects of HGWWD on peroneal MNCV in patients with DPN.
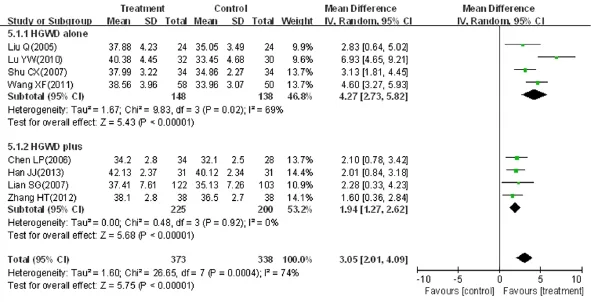
Figure 6 Effects of HGWWD on peroneal SNCV in patients with DPN.
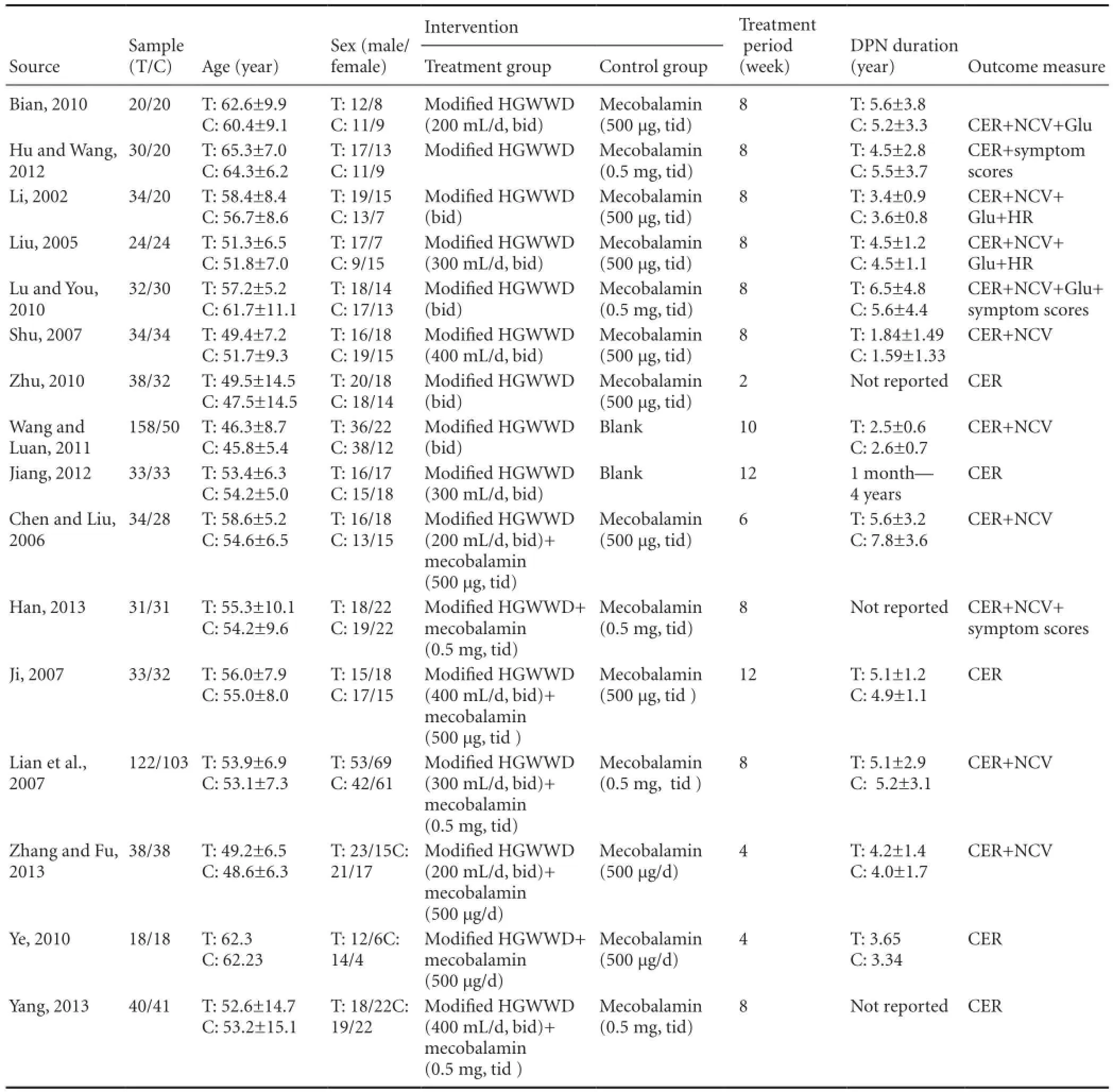
Table 1 Characteristics of the trials included in the meta-analysis
Hemorheology
Two trials (Li, 2002; Liu, 2005) evaluated changes in hemorheological indexes. In this review, we mainly assessed plasma viscosity and fibrinogen variations. Two trials did not show evidence of heterogeneity in plasma viscosity (χ2= 0.48, P = 0.49, I2= 0%). Thus, a fixed effects model was used for statistical analysis. There was a statistically significant difference between the two groups in plasma viscosity (n = 102, MD = -0.11, 95%CI: -0.21 to -0.02, Z = 2.30, P = 0.02) (Figure 8). Heterogeneity in fibrinogen between trials was significant (χ2= 3.93, P = 0.05, I2= 75%), and therefore, a random effects model was used for statistical analysis. The meta-analysis of the two trials showed that the treatment groups were not statistically different from the control groups in the reduction in fibrinogen (n = 102, MD = -0.53, 95%CI: -1.28-0.22, Z = 1.38, P = 0.17) (Figure 9).
Adverse and other effects
In four trials (Liu, 2005; Bian, 2010; Wang and Luan, 2011; Jiang, 2012), the treatment groups were reported to have experienced no adverse reactions. Adverse events were not mentioned in the other 12 trials, and therefore, the safety of the Chinese herbal medicine therapy could not be assessed. Only one trial (Liu, 2005) reported follow-up.
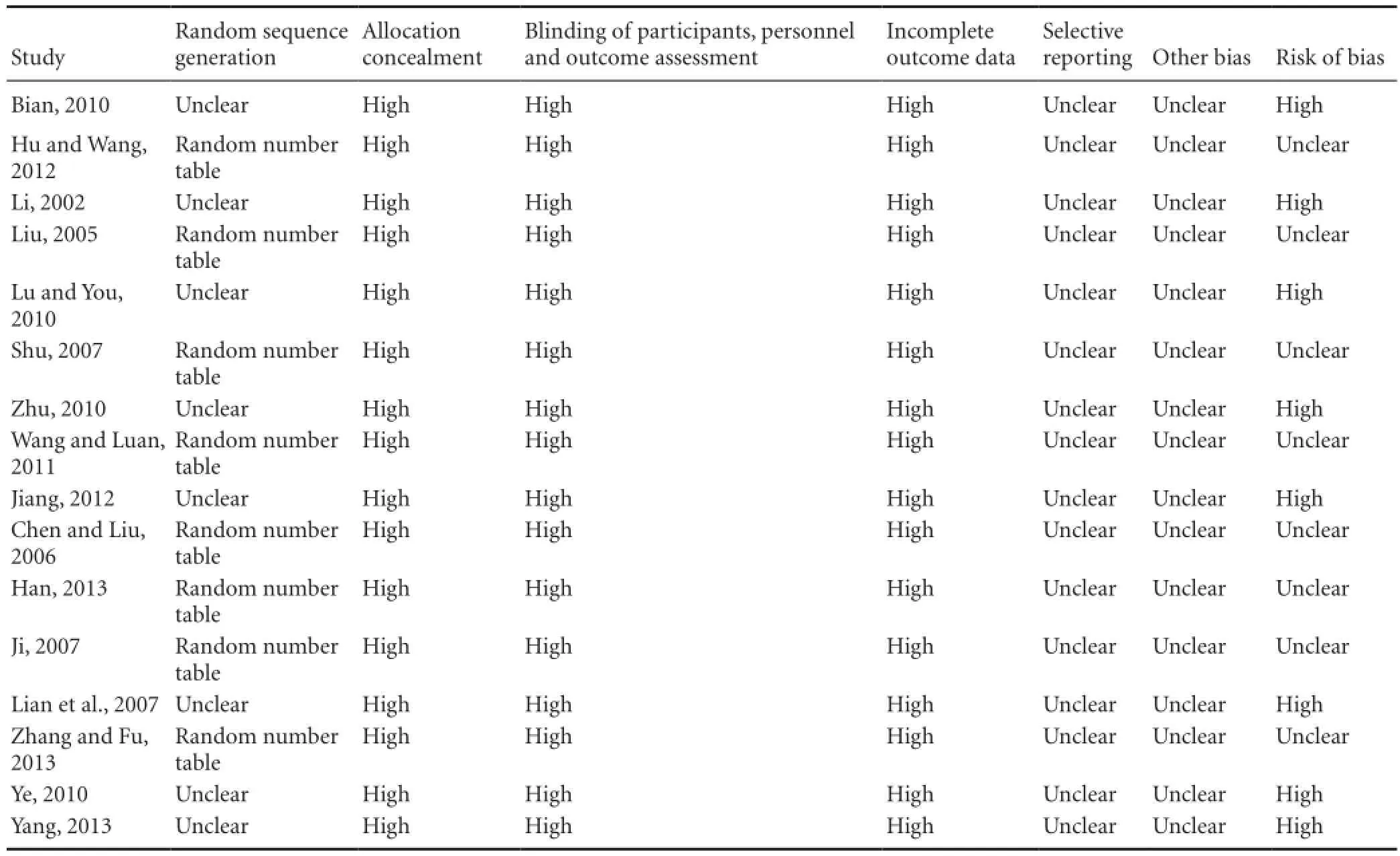
Table 2 Quality assessment of the trials included in the meta-analysis
The funnel shape of the plot was not completely symmetrical, indicating a potential publication bias. Although we conducted comprehensive searches and attempted to avoid bias, all trials were published in China, and accordingly, we could not exclude potential publication bias (Figure 10).
Discussion
DPN is a chronic, progressive disease. Treatment for DPN is aimed at improving the quality of life and preventing further nerve damage and complications (diabetic foot ulcers, infection, and gangrene) (American Diabetes Association, 2013). Because treatment by conventional Western medicine is of limited clinical efficacy, safer and more effective therapies are urgently needed. Clinical practice has shown that the combination of traditional Chinese medicine and Western medicine has substantial therapeutic potential for treating diabetes and its complications (Tong, 2012). Clinical trials and research on traditional Chinese medicine for treating DPN, including herbs and acupuncture, have been reported (Zhang et al., 2014; Cui et al., 2015). HGWWD is widely used to treat discomfort of the limbs in clinical practice in China. Gao et al. (2012) reported positive results with this decoction for treating DPN. Therefore, we used meta-analysis of currently published trials to assess the effectiveness of the herbal medicine for treating DPN.
Analysis of efficacy
In this meta-analysis, 16 trials involving 1,173 participants were included. The data revealed that the efficacy of HGWWD for DPN was significantly superior to that of the treatments for the control group. This finding suggests that HGWWD has therapeutic efficacy for DPN. However, the methodological quality of the trials was generally low, and there is the possibility of bias. Therefore, a more definitive statement of the clinical efficacy of HGWWD requires larger and better-designed RCTs.
There are a number of limitations of this meta-analysis. Although the decocting of HGWWD in the included trials was generally similar, and administration was twice per day, the herb ingredients, dosage and treatment periods varied among the trials. The period of treatment ranged from 2 weeks to 12 weeks. No trial reported sample size calculations, so assessment of clinical efficacy was made difficult, which might have negatively impacted the reliability of the outcomes.
We assessed clinical efficacy based on changes in symptoms and NCV as the main outcome indexes. Only half of the clinical trials provided NCV examination results, and those that did, examined different nerves. Consequently, clinical trials should standardize DPN NCV examinations to enhance the reliability of analysis. Limb discomfort is an important clinical characteristic, and clinical efficacy assessment should include evaluation of symptomatic variation. The Toronto Clinical Scoring System is widely used to evaluate DPN, and the scores correlate with NCV examinations (Perkins et al., 2001; Boulton et al., 2005). However, no trial used this scoring system, and three trials (Lu et al., 2010; Hu et al., 2012; Han, 2013) adopted a questionnaire based on theGuiding principles for clinical research of new Chinese patent medicine (Ministry of Health, China, 2002). Thus, it is urgent to standardize the assessment of symptoms. The clinical trials mainly assessed outcomes using substitutive indexes (NCV, and response rate of clinical symptoms); however, no trials assessed quality of life in patients with endpoint criteria (incidence of diabetic foot ulcers, amputation rates, and disability rates).

Figure 7 Effects of HGWWD on fasting blood glucose in patients with DPN.

Figure 8 Effects of HGWWD on plasma viscosity in patients in DPN.
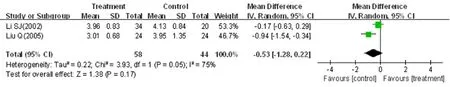
Figure 9 Effects of HGWWD on fibrinogen in patients with DPN.

Figure 10 Funnel plot for assessing publication bias.
In the included trials, only four trials (Li, 2002; Liu, 2005; Bian, 2010; Lu and You, 2010) examined fasting blood glucose changes. Although our meta-analysis revealed no significant difference in this variable between the treatment and control groups, we could not fully evaluate the influence of blood glucose. Only a few trials reported hypoglycemic effects, but some reports suggest that Huangqi (Radix Astragali seu Hedysari) and Baishao(Radix Paeoniae Alba) have hypoglycemic effects, and these herbs are components of HGWWD. Therefore, it is unclear whether the HGWWD formulation affects blood glucose and whether the amelioration of NCV is related to improvement of blood glucose. Although one trial (Zhou et al., 2013) proposed that the HGWWD formulation may improve blood glucose, they did not provide support for this statement.
In the present meta-analysis, we found a statistically significant difference in the reduction in plasma viscosity, although there was no statistically significant difference in the reduction in fibrinogen. Previous studies (Hua et al., 2008; Wang et al., 2009, 2010, 2012) suggest that HGWWD inhibits coagulation and platelet aggregation. One trial (Wang, et al., 2009) showed that Huangqi (Radix Astragali seu Hedysari) decreases blood viscosity and inhibits coagulation and platelet aggregation. Guizhi (Ramulus Cinnamomi) dilates blood vessels and regulates the circulation. Nevertheless, clinical data are presently lacking, and additional clinical trials and experiments are needed to clarify the effect of HGWWD on the circulatory system.
Quality of the evidence
All of the RCTs were of very low quality in terms of design, reporting and methodology. Eight trials (Liu, 2005; Chen and Liu, 2006; Shu, 2007; Ji, 2007; Jiang, 2012, Hu and Wang, 2012; Han, 2013; Zhang and Fu, 2013) mentioned the use of a random number table, while another eight trials only indicated “randomly allocating”, with no detailed information. No trial mentioned allocation concealment, suggesting selection bias. No trials mentioned blinding methods that might rule out performance and detection biases.
In this meta-anlysis, NCV was an objective index that should not be affected by blinding, but patients and researchers were aware of the treatments, which might affect subjective symptoms. Herbs were also not standardized, and the traditional Chinese medicine treatment was not compared to an approved medication for DPN. Instead, it was compared to an alternative or complementary method for treating DPN. No trials reported participant losses, so attrition bias was unclear. As such, the improvements in qualitative outcomes should be regarded with caution. All trials were published in China.
Potential biases in the review process
The asymmetrical funnel plot demonstrates the potential publication bias. Funnel plots are a visual aid to identify publication bias or systematic heterogeneity. All of the 16 trials were included in these funnel plots, recognizing the substantial heterogeneity of the treatment, trial size and design. However, none of the trials found a negative effect, indicating publication bias. Although we undertook extensive searches for unpublished literature, we found no negative trials. However, trials with large positive results are often much easier to publish than trials with negative results. Therefore, it is likely that publication bias is present, affecting the reliability of the meta-analysis.
Safety and follow-up
Inadequate reporting on adverse events in the included trials was a problem. Four trials reported that the treatment groups experienced no adverse reactions. Adverse events were not mentioned in other 12 trials. Only one trial (Liu, 2005) reported follow-up. Therefore, long-term effectiveness, side effects, and health outcomes could not be evaluated.
Future clinical trials should put emphasis on the following aspects: (1) Enhancing the quality of the methodology, including assessing sample size, increasing the power of the test, and improving randomization, allocation concealment, the blinding method, as well as safety reporting and detailed follow-up. (2) Side effects and long-term effects of HGWWD should be studied along with an objective endpoint (diabetic foot ulcers, amputation and disability rates) to evaluate the quality of life. (3) Future trials should standardize the composition of the herbal mixture, and examine the dose-effect relationships. This should help optimize the efficacy of the Chinese herbal medicine, either when used alone or in combination with a Western medical treatment.
Conclusion
Our meta-analysis suggests that the Chinese herbal medicine HGWWD is better for improving NCVs and alleviating subjective DPN symptoms compared with control treatment. However, the long-term effectiveness and safety of HGWWD for DPN are uncertain, and most trials included in this review were of poor quality. Thus, the limitations of this meta-analysis should be taken into consideration. Well-designed, large-scale, high-quality multicenter RCTs are required to provide better outcome assessments. Furthermore, long-term outcome observations, including mortality, quality of life and adverse events, are also necessary to more reliably assess the clinical efficacy of HGWWD for DPN.
Author contributions: BP and TYZ conceived the study and wrote the paper. LHZ and FW did data collection. RY and QZ accessed the risk of bias of references. FT performed statistical analysis. XLT participated in the design of the study and coordination. All authors approved the final version of the paper. Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
American Diabetes Association (2013) Standards of medical care in diabetes-2013. Diabetes Care 36:S11-66.
Bai Q (2015) The influence of Huangqi Guizhi Wuwu Decoction on diabetic peripheral neuropathy and nerve conduction velocity. Zhong Cheng Yao 37:962-964.
Bian XJ (2010) Theoretical, clinical and experimental studies in the treating diabetic peripheral neuropathy with modified Huangqi Guizhi Wuwu Decoction (Dissertation). Nanjing: Nanjing Zhongyiyao Daxue.
Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D (2005) Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 28:956-962.
Chen LP, Liu M (2006) Observation on the effect of modified Huangqi Guizhi Wuwu Decoction combined with mecobalamin in treating thirty-four patients with diabetic peripheral neuropathy. Xin Zhong Yi 38:56-57.
Chen W, Zhang Y, Li X, Yang G, Liu JP (2013) Chinese herbal medicine for diabetic peripheral neuropathy. Cochrane Database Syst Rev 10:CD007796.
China Association of Traditional Chinese Medicine (2007) Guideline for TCM Diabetes Prevention and Treatment, China. Beijing: Traditional Chinese Medicine Press of China.
Coppini DV (2016) Enigma of painful diabetic neuropathy:can we use the basic science, research outcomes and real-world data to help improve patient care and outcomes? Diabet Med. Doi: 10.1111/ dme.13089.
Cui J, Kong DM, Hou YX, Xing M, Feng L, Xu HS (2015) Study on the clinical therapeutic evaluation of acupuncture in the treatment of diabetic peripheral neuropathy. Zhong Hua Zhong Yi Yao Za Zhi 30:626-628.
Davies B, Cramp F, Gauntlett-Gilbert J, Wynick D, McCabe CS (2015) The role of physical activity and psychological coping strategies in the management of painful diabetic neuropathy: a systematic review of the literature. Physiotherapy 101:319-326.
Dong HY, Jiang XM, Niu CB, Du L, Feng JY, Jia FY (2016) Cerebrolysin improves sciatic nerve dysfunction in a mouse model of diabetic peripheral neuropathy. Neural Regen Res 11:156-162.
Gallagher HC, Gallagher RM, Butler M, Buggy DJ, Henman MC (2015) Venlafaxine for neuropathic pain in adults. Cochrane Database Syst Rev 8:CD011091.
Gao C, Song JS, Xue XH, Xiong J, Shang TG (2012) Effect comparison of huangqi guizhi wuwu decoction and western medicine for treating diabetic peripheral neuropathy: a systematic review. Liaoning Zhongyi Zazhi 6:993-1000.
Han JJ (2013) Effectiveness observation on sixty-two cases of diabetic peripheral neuropathy with combination of modified Huangqi Guizhi Wuwu Decoction and methylcobalamin. Anhui Yiyao 17:849-850.
Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal TP, Wetterslev J (2013) Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 11:CD008143.
Higgins JPT, Green S (2013) Corchrane Reviewers’ Handbook 5.2 [updated March 2013], ReviewManager (RevMan) [Computer program]. Version 5.2.
Hu DY, Wang HY (2012) Clinical study of modified Huangqi Guizhi Wuwu Decoction in treating diabetic peripheral neuropathy. Zhongguo Zhongyiyao Yuancheng Xiandai Jiaoyu 10:74-75.
Hua WJ, Bu P (2008) 68 cases by combination of Chinese and western therapies on diabetic peripheral neuropathy. Jiangsu Zhong Yiyao 40:65-66.
Ji HM (2007) Observation on the effect of modified Huangqi Guizhi Wuwu Decoction in treating thirty-three patients with diabetic peripheral neuropathy. Zhongguo Xiandai Yisheng 45:62.
Jiang BS (2012) Effectiveness analysis on forty cases of diabetic peripheral neuropathy with modified Huangqi Guizhi Wuwu Decoction. Zhongwai Yiliao 1:108-109.
Li SJ (2002) Clinical observation of thirty-four cases with modified Huangqi Guizhi Wuwu Decoction in treating diabetic peripheral neuropathy. Zhongguo Yiyao Xuebao 17:540-542.
Lian SG, Liu HL, Yang YW, Li XX (2007) Clinical observation on Chinese and Western Medicine treatment on 122 diabetic peripheral neuropathy patients. Henan Zhongyi 27:56-57.
Liu Q (2005) Clinical research on the treatment of diabetic peripheral neuropathy with modified Huangqi Guizhi Wuwu Decoction (Dissertation). Chengdu, China: Chengdu University of TCM.
Lu YW, You L (2010) Clinical observation on the treatment of diabetic peripheral neuropathy with modified Huangqi Guizhi Wuwu Decoction. Zhongguo Zhongyi Jizheng 19:1297-1298.
Ministry of Health, PR. China (2002) Guiding principles for clinical research of new Chinese patent medicine (Pilot). Beijing: China Medical Science Press.
Negi G, Nakkina V, Kamble P, Sharma SS (2015) Heme oxygenase-1, a novel target for the treatment of diabetic complications: focus on diabetic peripheral neuropathy. Pharmacol Res 102:158-167.
Perkins BA, Olaleye D, Zinman B, Bril V (2001) Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care 24:250-256.
Qin BF, Weng WL, Zhang W, Ye Q, Zhu XY, Pan WD (2015) Effect of Jiawei Huangqi Guizhi Wuwu Decoction on vascular endothelial growth factor in patients with diabetic peripheral neuropathy. Hebei Zhongyi 37:73-75.
Shu CX (2007) Clinical application on the treatment of diabetic peripheral neuropathy with modified Huangqi Guizhi Wuwu Decoction. Guangxi Zhongyiyao 30:12-13.
Spruce MC, Potter J, Coppini DV (2003) The pathogenesis and management of painful diabetic neuropathy: a review. Diabet Med 20:88-98.
Tesfaye S, Selvarajah D (2012) Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev 28:8-14.
Tong XL, Dong L, Chen L, Zhen Z (2012) Treatment of diabetes using traditional Chinese medicine: past, present and future. Am J Chin Med 40:877-886.
Tong Y, Hou H (2006) Effects of Huangqi Guizhi Wuwu Decoction on diabetic peripheral neuropathy. J Altern Complement Med 12:506-509.
Wang BD, Shen XH (2012) Clinical observation on the treatment of diabetic peripheral neuropathy with modified Huangqi Guizhi Wuwu Decoction. Zhongguo Yiyao Zhinan 10:262-264.
Wang XF, Luan ZM (2011) Clinical observation of 108 cases with modified Huangqi Guizhi Wuwu Decoction in treating diabetic peripheral neuropathy. HeilongJiang Yiyao Zazhi 24:795-796.
Wang XR, Luo K, Li YY, Tao SY, Liu J (2009) Effectiveness observation on the treatment of diabetic peripheral neuropathy with modified Huangqi Guizhi Wuwu Decoction. Yixue Zongshu 15:3353-3354.
Wang ZF, Wang JY (2010) Clinical study of modified Huangqi Guizhi Wuwu Decoction in treating diabetic peripheral neuropathy. Qingdao Yiyao Weisheng 42:275-276.
Wang ZQ, Pang GM, Yan Y, Yao PY (2011) Distributive study of TCM syndrome on diabetic peripheral neuropathy. Zhongyi Xuebao 26:487-489.
Yang LL (2013) Observation on forty cases of diabetic peripheral neuropathy with modified Huangqi Guizhi Wuwu Decoction. Henan Zhongyi 33:853-854.
Ye WP (2010) Combination of Chinese and western therapies in treating thirty-five patients with diabetic peripheral neuropathy. Guangming Zhongyi 25:2281.
Zhang HT, Fu CH (2013) Modified Huangqi Guizhi Wuwu Decoction on thirty-eight diabetic peripheral neuropathy cases. Zhongguo Minzu Minjian Yiyao 22:80-82.
Zhang HY, Ji SX, Chen Q (2015) Treating chronic diabetic complications with Huangqi Guizhi Wuwu Decoction. Liaoning Zhongyi Zazhi 42:1116-1117.
Zhang JY, Zhang W, Tian SH, Shi GB (2014) Research progress of Huangqi Guizhi Wuwu Decoction in the treatment of diabetic peripheral neuropathy. Liaoning Zhongyiyao Daxue Xuebao 16:122-124.
Zhang YL, Zhang XK (2014) Discussion of Huangqi Guizhi Wuwu Decoction in the treatment of diabetic peripheral neuropathy. Heilongjiang Zhongyiyao 2:41-42.
Zhou Q, Peng ZP, Zhao XY, Jin MS, Pang B (2013) Clinical Experience of Huangqi Guizhi Wuwu Decoction in Treatment of DPN by Professor Tong Xiao Lin through theory of “collateral disease”. Anhui Zhongyi Xueyuan Xuebao 2:44-46.
Zhu XM (2010) Clinical observation of seventy cases on the treatment of diabetic peripheral neuropathy with modified Huangqi Guizhi Wuwu Decoction. Liaoning Zhongyi Zazhi 37:1067-1068.
Copyedited by Patel B, Norman C, Wang J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.189202
*Correspondence to:
- 中國神經(jīng)再生研究(英文版)的其它文章
- Secondary parkinsonism induced by hydrocephalus after subarachnoid and intraventricular hemorrhage
- No synergism between bis(propyl)-cognitin and rasagiline on protecting dopaminergic neurons in Parkinson's disease mice
- Association between chromosomal aberration of COX8C and tethered spinal cord syndrome: arraybased comparative genomic hybridization analysis
- Rebuilding motor function of the spinal cord based on functional electrical stimulation
- Acellular allogeneic nerve grafting combined with bone marrow mesenchymal stem cell transplantation for the repair of long-segment sciatic nerve defects: biomechanics and validation of mathematical models
- Antioxidative mechanism of Lycium barbarum polysaccharides promotes repair and regeneration following cavernous nerve injury

