Rebuilding motor function of the spinal cord based on functional electrical stimulation
Xiao-yan Shen, Wei Du Wei Huang Yi Chen Electronic Information School, Nantong University, Nantong, Jiangsu Province, China2 Co-innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China Medical School, Nantong University, Nantong, Jiangsu Province, China
Rebuilding motor function of the spinal cord based on functional electrical stimulation
Xiao-yan Shen1,2,*, Wei Du1, Wei Huang1, Yi Chen3
1 Electronic Information School, Nantong University, Nantong, Jiangsu Province, China
2 Co-innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China
3 Medical School, Nantong University, Nantong, Jiangsu Province, China
How to cite this article: Shen XY, Du W, Huang W, Chen Y (2016) Rebuilding motor function of the spinal cord based on functional electrical stimulation. Neural Regen Res 11(8)∶1327-1332.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81371663, 61534003; and the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions of China, No. PPZY2015B135.
Xiao-yan Shen, Ph.D.,
xiaoyansho@ntu.edu.cn.
orcid:
0000-0003-4551-186X (Xiao-yan Shen)
Accepted: 2016-07-20
Graphical Abstract
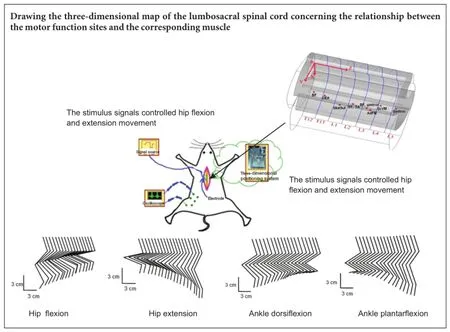
Rebuilding the damaged motor function caused by spinal cord injury is one of the most serious challenges in clinical neuroscience. The function of the neural pathway under the damaged sites can be rebuilt using functional electrical stimulation technology. In this study, the locations of motor function sites in the lumbosacral spinal cord were determined with functional electrical stimulation technology. A three-dimensional map of the lumbosacral spinal cord comprising the relationship between the motor function sites and the corresponding muscle was drawn. Based on the individual experimental parameters and normalized coordinates of the motor function sites, the motor function sites that control a certain muscle were calculated. Phasing pulse sequences were delivered to the determined motor function sites in the spinal cord and hip extension, hip flexion, ankle plantarflexion, and ankle dorsiflexion movements were successfully achieved. The results show that the map of the spinal cord motor function sites was valid. This map can provide guidance for the selection of electrical stimulation sites during the rebuilding of motor function after spinal cord injury.
nerve regeneration; spinal cord injury; functional electrical stimulation; rebuilding motor function; movement control; spinal cord; lumbosacral spinal cord; motor function sites; hip extension movement; hip flexion movement; ankle plantarflexion; ankle dorsiflexion; neural regeneration
Introduction
Spinal cord injury (SCI) can impair the motor function of patients to different degrees, and directly impacts the patients’ lifestyle and quality of life. Thus, there is an urgent need to explore effective ways to restore motor function of patients with SCI (Griffin et al., 2009; Musienko et al., 2009; Borton et al., 2014). Functional electrical stimulation (FES) has been widely used as a method to artificially activate the motor system after central nervous system injury (Dejan, 2014). So far, FES has been used in clinical trials successfully (Hart et al., 1998; Burridge et al., 2007). It has been used to control the arm and hand or to promote standing, balance, posture and gait training in the lower limbs (Pfurtscheller et al., 2003; Kim et al., 2008; Braz et al., 2009). Also, FES induces limb movements and promotes the motor function recovery of animals with SCI (Mushahwar et al., 2002, 2004; Bamford et al., 2005; Mondello et al., 2014). Because limb movements cannot occur with only a single muscle contraction, a natural motor behavior is formed by the combination of muscle synergies (d’Avella et al., 2003; Drew et al., 2008; Yu et al., 2009). Electrical microstimulation research has provided the most direct and powerful evidence that the nervous system encodes motor primitives, whether the corresponding relationship exists in the spinal cord or cortex (Tresch et al., 1999; Graziano et al., 2002; Haiss et al., 2005; Zimmermann et al., 2011). There is no suitable spinal cord motor function map that is used for FES, so the positions of many implanted microelectrodes have been determined by trial and error of a specific movement (Lemay et al., 2009). Therefore, FES technology has been used to determine the corresponding relationships between the motor function sites in the spinal cord and the muscles that control the hindlimb movements. On this basis, rebuilding hindlimb motor function with FES was studied.
Materials and Methods
Animals
Seven adult specific-pathogen-free male Sprague-Dawley rats (~250 g body weight) were provided by the Experimental Animal Center of Nantong University in China (animal license No. SCXK (Su) 2014-0001). After intraperitoneal anesthesia with chloral hydrate (4 mL/kg), the rats’ backs and left hindlimbs were shaved and laminectomies were performed at the T12-L5vertebral segments (the distance of the spinous process of the thoracic vertebra is shortest between the ninth, the tenth and the eleventh). The spinous process trended to caudal-ward above the ninth thoracic vertebra, the tenth was in the neutral position, and the spinous process trended to head-ward below the eleventh (Shang et al., 2013). The reference electrode was implanted in the adjacent paraspinal muscle. Bipolar needle electrodes were implanted in the eight muscles of the left hindlimb: biceps femoris, obliquus externus abdominis, glutaeus superficialis, semitendinosus, tibialis anterior, adductor femoris magnus, sacrococcygeus ventralis medialis, and gastrocnemius, which were
used to confirm whether the relevant muscle contraction was induced by the electrical stimulation. Rats were placed on a heating pad to maintain body temperature at approximately 37°C. All surgical procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the local Animal Studies Committee.
Stimulation electrode and stimulation parameters
Monopolar stimulation with a tungsten needle microelectrode (WE30030.5A3; MicroProbes for Life Science, Gaithersburg, MD, USA) with a diameter of 2-3 μm and 30-70 μm exposed at the tip was used to stimulate the spinal cord. Monophasic, cathodic, voltage-controlled (400-800 mV) pulse trains generated by a stimulator (master 9, A.M.P.I, Jerusalem, Israel) were applied in the experiment. Each train consisted of 40 negative pulses, and each pulse lasted 200 μs with a frequency of 33 Hz. The pulse trains were repeated every 4 seconds.
Experimental procedure
The experimental procedure of positioning the spinal cord motor function sites of the rat via FES is shown in Figure 1. Each rat was laid down on the Stereotaxic System (51750; Stereotaxic Instrument, Stoelting, IL, USA) and the spinal cord was fixed with the rat spinal cord adaptor (51695, Stereotaxic Instrument). FES was used in the exposed spinal cord, and the spinal cord locations that could induce a certain muscle contraction were determined. The locations of the stimulation sites were described as the positions in the corresponding vertebral segment, denoted as (X, Y, Z). X is the distance that the stimulation site deviated from the posterior median sulcus of the spinal cord (left ‘-’, right ‘+’). Y is the depth of the stimulation site (distance from the highest location of the spinal cord surface). Z is the distance from the stimulation site to the rostral margin of the corresponding vertebral segment. Electrical stimulation was delivered to the stimulation electrode, which was moved in the cross-sectional and the rostrocaudal directions of the spinal cord at certain intervals. Stimulation intensity was regulated and the different locations that could induce hindlimb muscle contraction were determined. The region that induced a certain muscle contraction was determined. The thresholds of the different stimulation sites in the region were compared, and the stimulation site with the lowest threshold was determined as the optimal spinal cord stimulation site (the rats were anesthetized deeply). The optimal stimulation sites that control different muscle contraction were normalized. The stimulation sites of other rats were calculated according to the processed data. Phasing pulse sequences were delivered to the different motor function sites in the spinal cord to induce hindlimb coordinated movements. Electromyography (EMG) signals were recorded (2 kHz), amplified and filtered (30-1,000 Hz).
Normalized coordinate description of the stimulation sites
To describe the motor function locations in the spinal cord accurately, the coordinates that control different muscle con-traction were normalized. Through analysis and verification of the two normalization methods relative to the vertebral segment or the total length of spinal cord (Huang et al., 2012), the normalization method relative to the vertebral segment was adopted to describe the motor function sites. Coordinate X was normalized by the half transverse diameter of the spinal cord D/2 (D was the transverse diameter of the spinal cord). Coordinate Y was normalized by the transverse diameter (D). Z was normalized by the length L of the vertebral segment in the rostrocaudal direction.
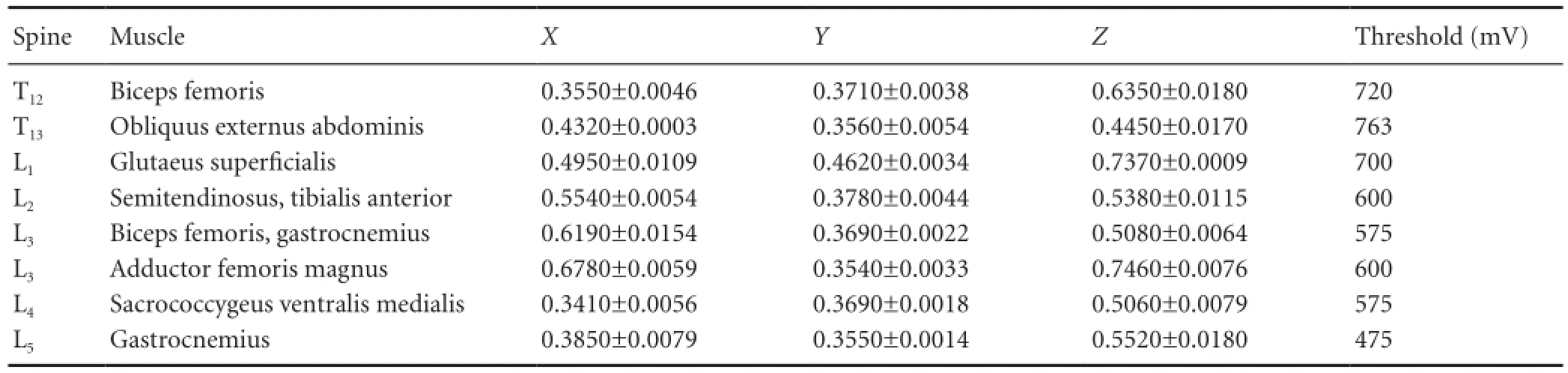
Table 1 Normalized coordinates of motor function sites in the spinal cord
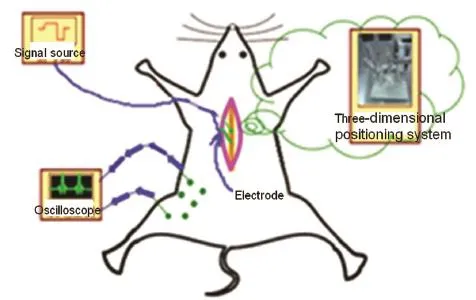
Figure 1 Experimental schematic indicating the positioning of the motor function sites in the spinal cord of the rat via functional electrical stimulation.
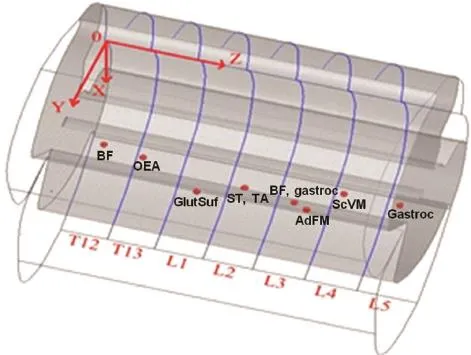
Figure 2 The three-dimensional map depicts the spinal cord motor function sites.
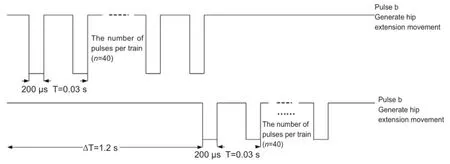
Figure 3 Stimulus signals controlling hip flexion and extension movement.
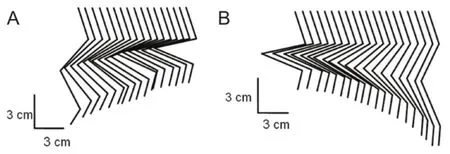
Figure 4 Hip extension and flexion movements based on functional electrical stimulation.
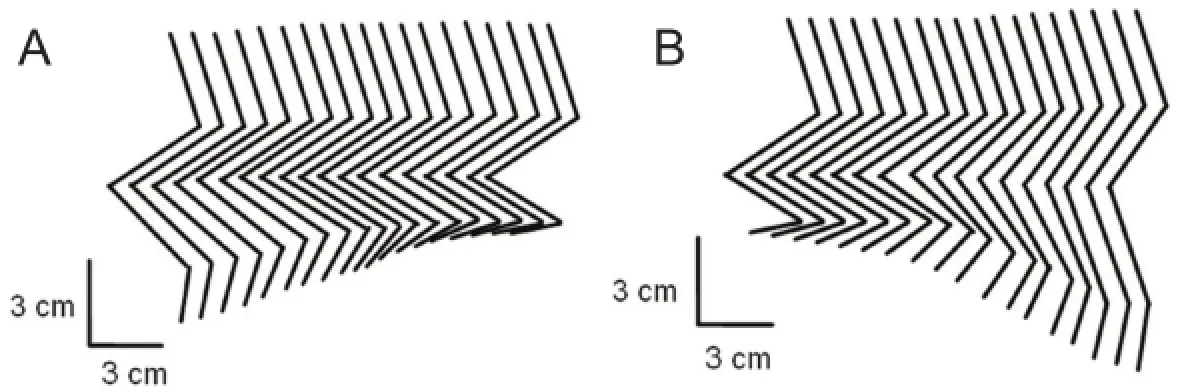
Figure 5 Ankle plantarflexion and dorsiflexion movements based on functional electrical stimulation.
Results
The map about motor function sites
Following the above experimental procedure, the experiment of positioning the motor function sites with a frequency of 1 Hz was conducted on the six rats. The coordinates of the spinal cord motor function sites that induce muscle contraction were normalized and recorded as (X, Y, Z). The data on the normalized coordinates are shown in Table 1.
According to the experimental data in Table 1, based on the average diameter of the spinal cord and the average length of each rat’s spine, a three-dimensional map of the spinal cord stimulation sites that can induce hindlimb muscle contraction is shown in Figure 2. The spinal segments corresponding to the T12-L5vertebral segments are depicted in Figure 2. The vertebral segments were separated by blue lines. The locations of the optimal spinal cord motor function sites that can induce biceps femoris, obliquus externus abdominis, glutaeus superficialis, semitendinosus, tibialis anterior, adductor femoris magnus, sacrococcygeus ventralis medialis, and gastrocnemius to produce muscle contraction are shown with red dots on the map. Determining the corresponding relationship between the motor function sites and the muscles can provide guidance for the next few experiments to achieve hip flexion, hip extension, ankle plantarflexion, and dorsiflexion movements.
Movement control with FES
Lumbosacral spinal cord can induce simple muscle contraction with low-frequency electrical stimulation, but coordinated movements can only be induced at a higher frequency (Lavrov et al., 2015). According to the data in Table 1, the transverse diameter and the length of the vertebral segments of the 7thrat, the positions that control the hip flexion, hip extension, ankle plantarflexion and dorsiflexion could be calculated.
Hip movement control with FES
The location calculated with related data that control the hip flexion of the 7thrat is located in the spinal cord corresponding to the first lumbar vertebra, near coordinates (X, Y, Z) = 0.8, 1.5, 3.6 mm. The location that controls the hip flexion is located in the third lumbar vertebra, near coordinates (X, Y, Z) = 1.0, 1.3, 3.1 mm.
The extension and flexion movements of the hip joint are controlled by two phasing pulse sequences as shown in Figure 3. Hip flexion movement was generated with pulse train a, and hip extension movement was generated with pulse train b. Stimulation signals a and b were delivered to two tungsten electrodes that were located in the correspondingspinal cord motor function sites. The threshold voltage of the hip flexion movement was 800 mV and the threshold voltage of the hip extension movement was 250 mV.
The kinematics of the hip movements of the left hindlimb are displayed in Figure 4. As can be seen in the figure, accompanying the hip flexion and extension movements, slight movements of other joints can be evoked within these locations.
Ankle movement control with FES
The experimental procedure of ankle movement control was consistent with the experimental procedure of hip movement control. The stimulus signals shown in Figure 3 were still used, but the difference was that ankle plantarflexion movement was generated with pulse train a and ankle dorsiflexion movement was generated with pulse train b. The location that controls the ankle dorsiflexion of the 7thrat is located in the spinal cord corresponding to the second lumbar vertebra, near coordinates (X, Y, Z) = 0.9, 1.3, 3.3 mm. The location that controls the ankle plantarflexion is located in the third lumbar vertebra, near coordinates (X, Y, Z) = 1.1, 1.2, 4.6 mm. The kinematics of the ankle movements of the left hindlimb is shown in Figure 5. Accompanying the ankle dorsiflexion and plantarflexion movements, slight movements of the knee joint can also be seen in Figure 5.
Discussion
In this study, FES technology was used to position the motor function sites in the spinal cord that can induce hindlimb muscle contraction. The normalized locations of motor function sites were summarized and a three-dimensional map of the relationship between the motor function sites and the corresponding muscles was drawn. All this can be the basis of further study using functional reconstruction experiments in SCI rats via electrical means. Phasing pulse sequences were delivered to the corresponding motor function sites in the spinal cord, and hip extension, hip flexion, ankle plantarflexion, and dorsiflexion movements were achieved successfully. Thus, fluid movements of the hindlimb can be induced using phasing pulse sequences that are delivered to a small amount of electrodes implanted in the corresponding functional sites of the lumbosacral spinal cord.
Some scholars have drawn maps to describe the output response of electrical stimulation at the ventral horn of the spinal cord or the motoneuron distribution in the lumbosacral spinal cord (Mushahwar and Horch et al., 1997, 1998). However, so far, there is no description of the corresponding relationship between the optimal motor function sites and the corresponding muscles. Independent control of all the joints and muscles can be achieved with the above corresponding relationship and the parameters of the animal model. In the experiment, a simple muscle contraction response could only be seen with a stimulus frequency of 1 Hz, while a variety of hindlimb movements could be induced with frequency ~30 Hz. This is because of the varieties of motoneurons that are compactly distributed in the lumbosacral spinal cord (Vanderhorst et al., 1997; Yakovenko et al., 2002). When electrical stimulation was delivered to the tip of the microelectrode implanted in the lumbosacral spinal cord, the motoneurons located at the tip of and around the microelectrode were activated simultaneously, and the motor output was expressed as the co-effect of multiple muscle contractions. In addition, because the number of interneurons is seven times that of the motoneurons within these stimulated regions (Henneman, 1980), the flexion and extension movements of the joints and other coordinated movements might arise from the activation of neuronal circuitry spanning the lumbosacral, and indirectly activated motoneurons. It is reported that rhythmic activities can be evoked with tonic intraspinal microstimulation (Guevremont et al., 2006; Lavrov et al., 2015). However, alternating, locomotor-like stepping was not found in our experiments, which may be because of suppression from the higher central nervous system. More experiments need to be conducted on spinal rats to investigate the mechanism of the locomotion generated by FES in the spinal cord.
As a form of FES, intraspinal microstimulation shows promise in clinical applications, and indices better selective activation of locomotor-related networks in the spinal cord than does epidural electrical stimulation (Musbahwar et al., 1998, 2000). More importantly, intraspinal microstimulation can activate propriospinal pathways located superficially, making it more feasible in clinical applications (Lavrov et al., 2015). Further, intraspinal microstimulation can evoke natural movements of the hindlimb by recruiting muscles in a synergistic way, and preferentially recruits fatigue-resistant muscle fibers, so the issue of fatigue is reduced significantly during electrical stimulation (Mushahwar and Horch et al., 2000; Bamford et al., 2011; Verhaagen et al., 2012). Because of the complexity of the neural networks in the lumbosacral spinal cord, further investigations will be needed into the distribution of motoneurons, interneurons and axons in the lumbosacral spinal cord, which requires more electrophysiological studies (Mushahwar et al., 2002). Other methods can be combined to promote the recovery of hindlimb motor function in follow-up experiments, such as pharmacological therapy and treadmill training. Research on this topic will provide guidance on the subsequent experiment addressing the recovery of motor function.
Author contributions: The overall design of the experiment was agreed by all authors after extensive discussions. XYS designed the study. WH performed the study. YC drew the diagrams. WH and WD analyzed the experimental data and wrote the paper. All authors approved the final version of the paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
References
Bamford JA, Mushahwar VK (2011) Intraspinal microstimulation for the recovery of function following spinal cord injury. Progress Brain Res 194:227.
Bamford JA, Putman CT, Mushahwar VK (2005) Intraspinal microstimulation preferentially recruits fatigue resistant muscle fibres and generates gradual force in rat. J Physiol 569:873-884.
Borton D, Bonizzato M, Beauparlant J, Digiovanna, J., Moraud, EM, Wenger N (2014) Corticospinal neuroprostheses to restore locomotion after spinal cord injury. Neurosci Res 78:21-29.
Braz GP, Russold M, Davis GM (2009) Functional electrical stimulation control of standing and stepping after spinal cord injury: a review of technical characteristics. Neuromodulation 12:180-190.
Burridge J, Haugland M, Larsen B (2007) Phase II trial to evaluate the ActiGait implanted drop-foot stimulator in established hemiplegia. J Rehabil Med 39:212-218.
d’Avella A, Saltiel P, Bizzi E (2003) Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci 6:300-308.
Dejan BP (2014) Advance in functional electrical stimulation. J Electromyogr Kinesiol 24:795-802.
Drew T, Kalaska J, Krouchev N (2008) Muscle synergies during locomotion in the cat: a model for motor cortex control. J Physiol 586:1239-1245.
Graziano MSA, Taylor CSR, Moore T (2002) Complex movements evoked by microstimulation of precentral cortex. Neuron 34:841-851.
Griffin L, Decker MJ, Hwang JY (2009) Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol 19:614-622.
Guevremont L (2006) Locomotor-related networks in the lumbosacral enlargement of the adult spinal cat: activation through intraspinal microstimulation. IEEE Trans Neural Syst Rehabil Eng 14:266-272.
Haiss F, Schwarz C (2005) Spatial segregation of different modes of movement control in the whisker representation of rat primary motor cortex. J Neurosci 25:1579-1587.
Hart RL, Kilgore KL, Peckham PH (1998) A comparison between control methods for implanted FES hand-grasp systems. IEEE Trans Rehabil Eng 6:208-218.
Henneman E (1980) Organization of the spinal cord and its reflexes. Med Physiol 762-786.
Huang W, Shen XY, Huang TP (2012) Experimental research on the Reference coordinates for functional localization of spinal cord. The 6thInternational Conference on Bioinformatics and Biomedical Engineering (iCBBE) 245-248.
Kim CS, Eom GM, Hase K (2008) Stimulation pattern-free control of fes cycling: simulation study. IEEE Trans Syst Man Cybern 38:125-134.
Lavrov I, Musienko PE, Selionov VA, Zdunowski S, Roy RR, Edgerton VR (2015) Activation of spinal locomotor circuits in the decerebrated cat by spinal epidural and/or intraspinal electrical stimulation. Brain Res 1600:84-92.
Lemay MA, Grasse D, Grill WM (2009) Hindlimb endpoint forces predict movement direction evoked by intraspinal microstimulation in cats. IEEE Trans Neural Syst Rehabil Eng 17:379-389.
Mondello SE, Kasten MR, Horner PJ (2014) Therapeutic intraspinal stimulation to generate activity and promote long-term recovery. Front Neurosci 8:21.
Mushahwar VK, Aoyagi Y, Stein RB (2004) Movements generated by intraspinal microstimulation in the intermediate gray matter of the anesthetized, decerebrate, and spinal cat. Can J Physiol Pharmacol 82:702-714.
Mushahwar VK, Gillard DM, Gauthier MJ (2002) Intraspinal microstimulation generates locomotor-like and feedback-controlled movements. IEEE Trans Neural Syst Rehabil Eng 10:68-81.
Mushahwar VK, Horch KW (1997) Proposed specifications for a lumbar spinal cord electrode array for control of lower extremities in paraplegia. IEEE Trans Rehabil Eng 5:237-243.
Mushahwar VK, Horch KW (1998) Selective activation and graded recruitment of functional muscle groups through spinal cord stimulation. Ann N Y Acad Sci 860:531-535.
Mushahwar VK, Horch KW (2000) Selective activation of muscle groups in the feline hindlimb through electrical microstimulation of the ventral lumbo-sacral spinal cord. IEEE Trans Neural Syst Rehabil Eng 8:11-21.
Mushahwar VK, Saigal R (2002) Spinal cord stimulation for restoration of locomotion. Engineering in Medicine and Biology 24thAnnual Conference and the Annual Fall Meeting of the Biomedical Engineering Society EMBS/BMES Conference, 2002. Proceedings of the Second Joint. IEEE 3:2041-2042.
Musienko P, van den Brand R, Maerzendorfer O, Larmagnac, A (2009) Combinatory electrical and pharmacological neuroprosthetic interfaces to regain motor function after spinal cord injury. IEEE Trans Biomed Eng 56:2707-2711.
Pfurtscheller G, Müller GR, Pfurtscheller J (2003) ‘Thought’-control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci Lett 351:33-36.
Shang YL, Li YF, Ning YF (2013) Anatomy reference location for model of SCI rats. Anat Res 35:412-414.
Tresch MC, Bizzi E (1999) Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low threshold cutaneous stimulation. Exp Brain Res 129:401-416.
Vanderhorst VG, Holstege G (1997) Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol 382:46-76.
Verhaagen J, McDonald III JW (2012) Spinal cord stimulation: therapeutic benefits and movement generation after spinal cord injury. Handb Clin Neurol 109:283.
Yakovenko S (2002) Spatiotemporal activation of lumbosacral motoneurons in the locomotor step cycle. J Neurophysiol 87:1542-1553.
Yu XN, Qian JG (2009) The function of functional electrical stimulation to lower extremity of hemipleg. Nanjing Tiyu Nxueyuan Xuebao (Ziran Kexueban) 8:26-29.
Zimmermann JB, Seki K, Jackson A (2011) Reanimating the arm and hand with intraspinal microstimulation. J Neural Eng 8:054001.
Copyedited by Jackson C, Norman C, Wang J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.189199
*Correspondence to:
 中國(guó)神經(jīng)再生研究(英文版)2016年8期
中國(guó)神經(jīng)再生研究(英文版)2016年8期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Secondary parkinsonism induced by hydrocephalus after subarachnoid and intraventricular hemorrhage
- Huangqi Guizhi Wuwu Decoction for treating diabetic peripheral neuropathy: a meta-analysis of 16 randomized controlled trials
- No synergism between bis(propyl)-cognitin and rasagiline on protecting dopaminergic neurons in Parkinson's disease mice
- Association between chromosomal aberration of COX8C and tethered spinal cord syndrome: arraybased comparative genomic hybridization analysis
- Acellular allogeneic nerve grafting combined with bone marrow mesenchymal stem cell transplantation for the repair of long-segment sciatic nerve defects: biomechanics and validation of mathematical models
- Antioxidative mechanism of Lycium barbarum polysaccharides promotes repair and regeneration following cavernous nerve injury
