Correlation of the mean activity coefficient of aqueous electrolyte solutions using an equation of state
Seyed Hossein Mazloumi*
Chemical Engineering Department,Faculty of Engineering,Ferdowsi University of Mashhad,Mashhad,Iran
1.Introduction
Electrolyte solutions appear in many important chemical systems such as extraction,distillation,biological processes.To correctly design and optimize such processes,precise knowledge of thermo-physical properties of electrolyte solutions is necessary.Due to the presence of charged particles and their interactions between ions and molecular species,modeling of electrolyte solution is a difficult and challenging task.Thermodynamic modeling of electrolyte solutions can be carried out using activity coefficient models[1–10]or by equation of state approach[11–26].The main advantage of the latter method is that a residual Helmholtz energy model has implemented so many useful thermo-physical properties can be derived by proper thermodynamic relations.
However it should be noted that the application of the EOS method for electrolyte solutions is almost a new approach and more complicated rather than the activity coefficient models.The species interactions in electrolytes that result into a more non-ideal solutions than non-electrolytes even at very low concentration,are different from the repulsive and dispersive(attractive)forces taken into account by most non-electrolyte EOS[23].In most electrolyte equations of state(eEOS),a non-electrolyte equation of sate is used as a base,and due to the presence of ions,some extra expressions such as a version of Mean Spherical Approximation(MSA)theory and the Born equation are added.The eEOSs of Planche and Renon[13],Jin and Donohue[14],Fürst and Renon[15],Wu and Prausnitz[16],Galindo et al.[17]Myers et al.[18],Clarke and Boishni[19],Cameretti et al.[20],Tan et al.[21],Liu et al.[22],Lin et al.[23],Kim and Lee[24],Inchekel et al.[25],and Haghtalab and Mazloumi[26]are some examples of published electrolyte equations of state.
Haghtalab and Mazloumi[27]have developed two non-electrolyte equations of state based on a new coordination number model for square well fluids.The non-electrolyte non-cubic SW proposed in our previous work[27]has the advantage of usinga very accurate repulsive term of the Carnahan–Starling equation that is in very good agreement with simulation data.Also,the attractive part(dispersion)contribution of EOSs is usually empirical orpure correlation of simulation data,while the attractive part of the present EOS is expressed by an equation that has a theoretical backbone obtained from a square well potential function.The aim of this study is to extend the SWEOS to aqueous electrolyte solutions and to verify its capability in representation of the thermodynamic properties of electrolyte solutions.
2.Thermodynamic Model
To establish a formulation for a residual Helmholtz energy model,some authors[16,18,25]have used a thermodynamic cycle in which an electrolyte solution is created from an ideal gas mixture.The Born equation is used to uncharge the ions at temperature T and volume V at an ideal gas state resulting in a mixture including ungraded ions and solvent molecules.The repulsive and attractive interactions of various uncharged species are expressed by the non-cubic SWEOS in the step of isothermal compression of ideal gas mixture to liquid state at
V.In the next step,the Born equation with a negative sign is again applied to charge the neutral ions.Finally the long range interactions of ions are expressed by the MSA equation.The total residual Helmholtz energy can be expressed as

Table 1 The fitted parameters and the results of correlation of mean activity coefficients and prediction of osmotic coefficients of various aqueous electrolyte solutions by the new eEOS and comparisons with MSW EOS[18],the data were taken from Ref.[32]
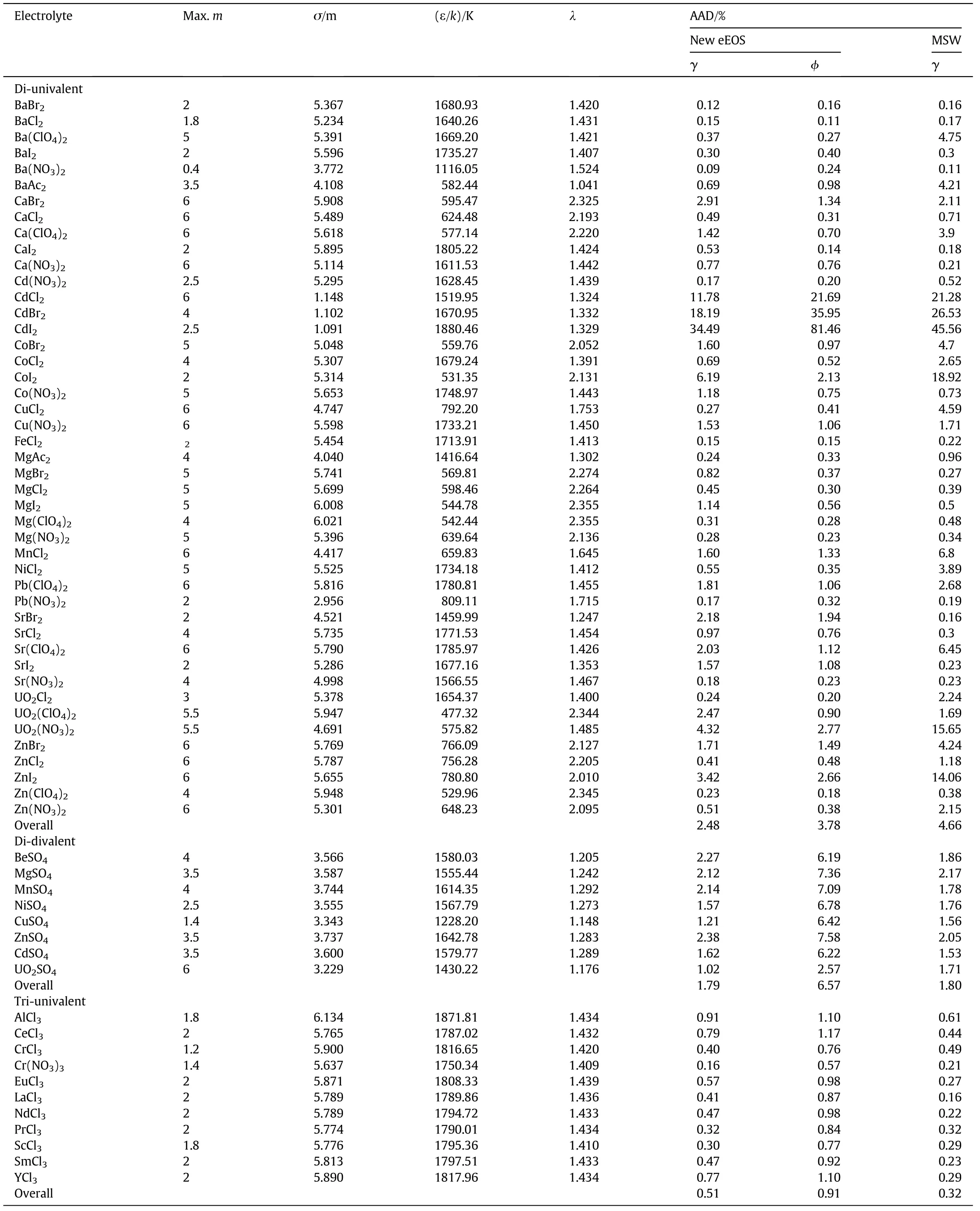
Table 1(continued)

where the superscript id denotes the ideal gas mixture and the subscript Born,SW and MSA stand for the Born equation,square well equation of sate and mean spherical approximation,respectively.Having the residual Helmholtz energy,the equation of state in terms of compressibility factor can be derived by differentiating the total Helmholtz energy equation with respect to density.Also,the chemical potential equation can be obtained by the derivative of the total residual Helmholtz energy with respect to the mole number.
The Born equation[28]for discharging and charging processes of ions in the thermodynamic cycle is expressed as
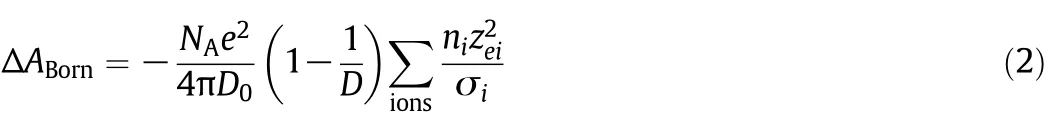
where NAis the number,e is the unit of elementary charge,D0is the per-mittivityof the free space,D is the dielectric constant, n is the number of moles,zeiis the charge number of ion and σ is the diameter parameter.Depending on which expression is used for dielectric constant,the Born equation may or may not be contributed to the computation of the properties of electrolyte solutions.Using a dielectric constant dependent only on temperature,the Born equation has no effect on the calculation of compressibility factor.In addition,because of linear dependency of the Born equation to the mole number,the Born equation contribution to the chemical potential equation vanishes.
The square-well equation of state that is composed of the Carnahan–Starling repulsive term and a square-well potential based attractive term [27] is expressed as

where ntis the total number of moles,R is the gas constant,T is the absolute temperature,z is the maximum attainable coordination number,m is the orientational parameter,y=τV0/V,V0is the closed packed volume,V is the total volume andThe following mixing rules are used in this work,


where ε is the square well potential depth,σ is the diameter of the particle,and λσ is the potential range.It should be noted that the summation is over all ionic and molecular species in Eqs.(4)–(7).
In electrolyte EOSs,the long range contribution of ions to the total Helmholtz energy is often expressed by a version of MSA theory.In this work a simple and explicit version of primitive MSA is adopted for the electrostatic interaction of ions in the electrolyte solution[29],

where κ is the Debye screening length,Γ is the MSA screening parameterandwhere summation is over ions.For consistency,the MSA that is derived in the McMillan–Mayer framework should be converted to the Lewis–Randall framework,however it has been shown that this incompatibility can be ignored[30],particularly the present model have a number of adjustable parameters which compensate the error[18].It should be noted that the MSA model has no effect on chemical potential of solvent molecule because the summations in the MSA term are over ions.So the total Helmholtz energy equation used in this work is expressed as,

whereis the mean activity coefficient based on mole fraction and υ+and υ-are the number of cations and anions,respectively.The osmotic coefficient can be calculated as

where Msis molecular weight of solvent and Cmis molality.
3.Results and Discussion
The new electrolyte EOS is applied for representation of the mean activity coefficient and osmotic coefficient of various strong aqueous electrolyte solutions at 25°C and 0.1 MPa.To do this, first,the parameters of the EOS for water should be determined.The experimental data of saturated vapor pressure and liquid density of water[31]at the reduced temperature of 0.44–0.94 are used to fi t the parameters of the EOS.So the values of 0.2803 nm,909.048 K and 1.286 are obtained for σ,ε/k and λ of water,respectively.The percentage of absolute average deviations(AADs)of the EOS for calculating saturated vapor pressure and liquid density of water are 0.71 and 1.13,respectively.
The new eEOS has three adjustable parameters(σ,ε/k and λ)for each ion,so the number of fitted parameters for each strong aqueous electrolyte is six which is high.To reduce the number of parameters,the assumption of equality of parameters of cation and anion is used.Thus the adjustable parameters of the new eEOS per each electrolyte are σ,ε/k and λ that are obtained by correlating experimental data of the mean activity coefficient[32]of more than 130 strong aqueous electrolytes.To calculate the activity and osmotic coefficients of aqueous electrolytes at a given temperature and pressure, first the new pressure explicit equation of state is solved numerically to obtain the liquid volume of the mixture,then the chemical potential and fugacity coefficients of all species are calculated.After that the activity and osmotic coefficients are obtained using Eqs.(17)–(20).The fitted parameters and the AADs(=100/NP∑|γCalculated? γExperiment|/γExperiment)have been reported in Table 1.The overall AADs%of the new eEOS in correlating the mean activity coefficient of 59 uni-univalent electrolytes,9 uni-divalent electrolytes,45 di-univalent electrolytes,8 didivalent electrolytes and 11 tri-univalent electrolytes are 0.30,0.25,2.48,1.79 and 0.50,respectively.Also Table 1 gives a comparison of the results of the new eEOS with that of Myers et al.eEOS(MSW)[18].The overall AADs%of the MSW eEOS in correlating the mean activity coefficient of uni-univalent electrolytes,uni-divalent electrolytes,diunivalent electrolytes, di-divalent electrolytes and tri-univalent electrolytes are 0.42,0.24,4.66,1.80 and 0.32,respectively.As one can see,in two types of electrolytes,i.e.1:1 and 2:1,the new eEOS is better and in 3:1 electrolytes,the MSW is more accurate.In 1:2 and 2:2 electrolytes,the results are the same.However,the overall AADs for all 132 electrolytes are 1.13 for the new eEOS and 1.93 for MSW.Generally the new eEOS is more accurate than MWS eEOS[18],although both eEOSs represent the experimental data successfully.Also included in Table 1 are the results of the new eEOS in predicting the osmotic coefficient of various electrolytes.The overall AADs of the new eEOS in predicting the osmotic coefficients of uni-univalent,uni-divalent,diunivalent,di-divalent and tri-univalent electrolytes are 0.29,0.32,3.78,6.27 and 0.91,respectively.As one can see,the results of the new eEOS are quite satisfactory.
Fig.1 shows the comparison of results of the new eEOS with experimental data in correlating the mean activity coefficient of four electrolytes.Shown in Fig.2 are the results of the new eEOS in predicting the osmotic coefficient of some electrolytes.As it can be observed,the agreement of the results of the present model and the experiments is very good.Plotted in Fig.3 is the application of the new eEOS to correlate the mean activity coefficient of a number of strong aqueous electrolyte solutions up to saturation molalities.As it can be seen,then ewe EOS is capable to represent the mean activity coefficient at high molality as well as low molality.It should be noted that the adjusted parameters for the electrolytes shown in Fig.3 are different from those listed in Table 1 because the range of molalities is different.

Fig.1.Correlation of activity coefficients of some aqueous electrolytes.Solid lines represent the results of the new eEOS and the symbols are the experimental data[32].

Fig.2.Prediction of osmotic coefficients of some aqueous electrolytes. Solid lines represent the results of the new eEOS and the symbols are the experimental data[32].

Fig.3.Correlation of activity coefficient of some uni-univalent aqueous electrolytes up to saturation molalities. Solid lines represent the results of the new eEOS and the symbols arethe experimental data[33].
To examine the capability of new eEOS for the other temperatures and pressures,four electrolytes,i.e.NaCl,NaBr,CaCl2and Na2SO4at temperature range of 0–100 °C have been chosen and listed in Table 2.The following temperature dependency is adopted for the eEOS parameters,

The adjusted coefficients of Eq.(21)and the results of the eEOS in calculating activity coefficient,osmotic coefficient and solution density have been listed in Table 3 for four aqueous electrolytes.The parameters of eEOS for NaCl and NaBr solutions have been obtained during correlating experimental data of both mean activity coefficient and solution density. Thus the AADs% of the eEOS in correlating mean activity coefficients of these electrolytes are higher than those reported in Table 1. It should be noted that the results are still satisfactory and show the good qualification of the new eEOS for representation of the experimental data at high temperatures. Fig. 4 shows the results of the eEOS in correlating density of aqueous NaCl at temperatures of 303, 353 and 373 K and pressure 1 bar. Plotted in Figs. 5 and 6 are the results of the new eEOS in predicting the density and the mean activity coefficient, respectively,of aqueous NaCl at temperatures of 303, 353 and 373 K and pressure 200 bar. Fig. 7 shows the correlation of the mean activity coefficients of aqueous NaBr,CaCl2and Na2SO4at273K.The predictions of the osmotic coefficients of four aqueous electrolytes at temperature 273 K are represented by Fig.8.As one can see,the new eEOS has been successfully applied for representation of activity coefficient,osmotic coefficient and density of the electrolytes at a temperature range of 0–100 °C.
4.Conclusions
A new electrolyte equation of sate was introduced for electrolyte solution that is applicable over wide ranges of composition,temperature and pressure.It consists of four terms:the Carnahan–Starling repulsive term,a square-well potential based attractive term,an explicit Mean Spherical Approximation theory(MSA)and the Born term.However,in this work,the Born term vanishes in pressure and chemical potential equations because of the dependency of the dielectric constant on temperature only.Correlation of mean activity coefficient and prediction of osmotic coefficient of 132 strong aqueous electrolyte solutions at 25°C and 0.1 MPa were carried out successfully by the new eEOS.It is alsocapable to correlate the activity coefficient of some electrolytes up to saturation molalities.Moreover the extension of the new eEOS for correlation and prediction of mean activity coefficient,osmotic coefficient and solution density of some aqueous electrolytes at a temperature range of 0–100°Cand pressures up to20 MPa was done with satisfactory results.The results demonstrate that the new eEOS is capable for good representation of the studied thermodynamic properties of aqueous electrolyte solutions at a wide range of temperatures,pressure and composition.

Table 2 The properties,temperature and molality ranges of various electrolytes used in this study
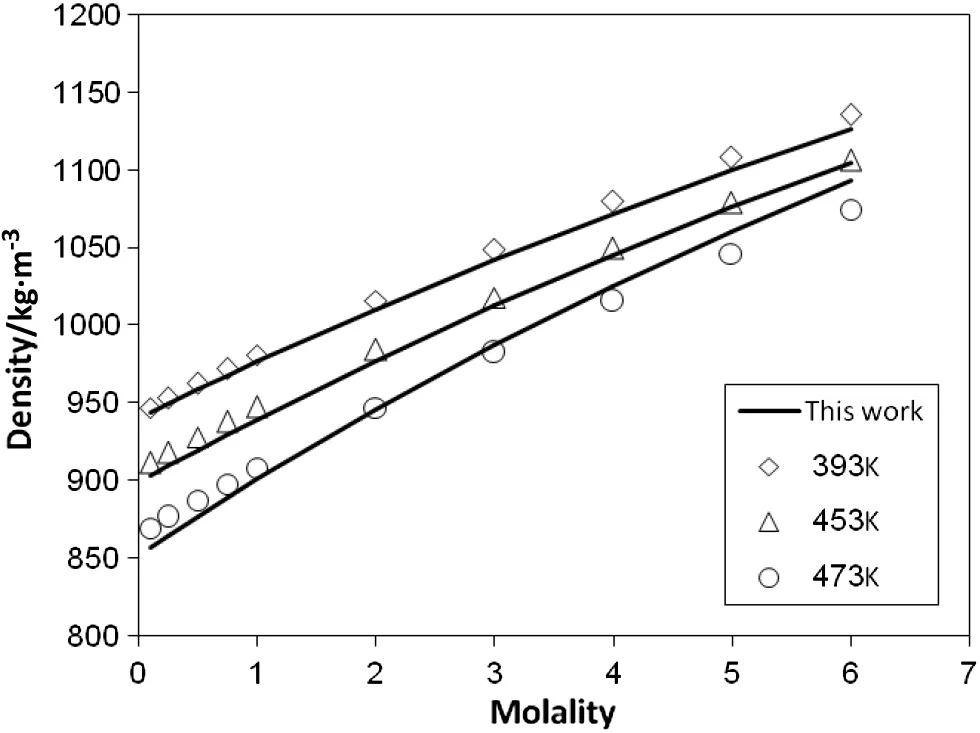
Fig.4.Correlation of densities of aqueous NaCl at different temperatures and pressure 0.1 MPa.Solid lines represent the results of the new eEOS and the symbols are the experimental data[34].

Fig.5.Prediction of densities of aqueous NaCl at different temperatures and pressure 20 MPa.Solid lines represent the results of the new eEOS and the symbols are the experimental data[34].

Table 3 The coefficients of temperature dependent parameters of the present model and the percent of absolute average deviations from experimental data for different aqueous electrolytes

Fig.6.Prediction of activity coefficients of aqueous NaCl at different temperatures and pressure 20 MPa.Solid lines represent the results of the new eEOS and the symbols are the experimental data[34].

Fig.7.Correlation of activity coefficients of some aqueous electrolytes at temperature 273 K.Solid lines represent the results of the new eEOS and the symbols are the experimental data[35–37].


Fig.8.Predictions of osmotic coefficients of some aqueous electrolytes at temperature 273 K.Solid lines represent the results of the new eEOS and the symbols are the experimental data[34–37].


[1]K.S.Pitzer,Activity coefficients in Electrolyte Solutions,second ed.CRC Press,Boca Raton,FL,1991.
[2]C.C.Chen,L.B.Evans,A local composition model for the excess Gibbs energy of aqueous electrolyte systems,AIChE J.32(1986)444–454.
[3]A.Haghtalab,J.H.Vera,A nonrandom factor model for the excess Gibbs energy of electrolyte solutions,AIChE J.34(1988)803–813.
[4]A.Haghtalab,S.H.Mazloumi,A nonelectrolyte local composition model and its application in the correlation of the mean activity coefficient of aqueous electrolyte solutions,Fluid Phase Equilib.275(2009)70–77.
[5]A.Haghtalab,M.Dehghani tafti,Electrolyte UNIQUAC-NRF model to study the solubility of acid gases in alkanolamines,Ind.Eng.Chem.Res.46(2007)6053–6060.
[6]A.Haghtalab,A.Shojaeian,Modeling solubility of acid gases in alkanolamines using the nonelectrolyte Wilson-nonrandom factor model,Fluid Phase Equilib.289(2010)6–14.
[7]A.Haghtalab,K.Peyvandi,Generalized Electrolyte-UNIQUAC-NRF model for calculation of solubility and vapor pressure of multicomponent electrolytes solutions,J.Mol.Liq.165(2012)101–112.
[8]M.R.Dehghani,H.Modarress,M.Monirfar,Measurement and modelling of mean activity coefficients of aqueous mixed electrolyte solution containing glycine,J.Chem.Thermodyn.38(2006)1049–1053.
[9]A.Haghtalab,A.Shojaeian,S.H.Mazloumi,Nonelectrolyte NRTL-NRF model to study thermodynamics of strong and weak electrolyte solutions,J.Chem.Thermodyn.43(2011)354–363.
[10]S.Kumar Dash,A.N.Samanta,S.S.Bandyopadhyay,(Vapour+liquid)equilibria(VLE)of CO2 in aqueous solutions of 2-amino-2-methyl-1-propanol:New data and modelling using eNRTL-equation,J.Chem.Thermodyn.4(2011)1278–1285.
[11]P.W.J.Derks,J.A.Hogendoorn,G.F.Versteeg,Experimental and theoretical study of the solubility of carbon dioxide in aqueous blends of piperazine and N-methyldiethanolamine,J.Chem.Thermodyn.42(2010)151–163.
[12]A.T.Zoghi,F.Feyzi,M.R.Dehghani,Modeling CO2solubility in aqueous N-methyldiethanolamine solution by electrolyte modified Peng–Robinson plus association equation of state,Ind.Eng.Chem.Res.51(2012)9875–9885.
[13]H.Planche,H.Renon,Mean spherical approximation applied to a simple but nonprimitive model interaction for electrolyte solutions and polar substances,J.Phys.Chem.85(1981)3924–3929.
[14]G.Jin,M.D.Donohue,An equation of state for electrolyte solutions.1.Aqueous systems containing strong electrolytes,Ind.Eng.Chem.Res.27(1988)1073–1084.
[15]W.Fürst,H.Renon,Representation of excess properties of electrolyte solutions using a new equation of state,AIChE J.39(1993)335–343.
[16]J.Z.Wu,J.M.Prausnitz,Phase equilibria for systems containing hydrocarbons,water,and salt:An extended Peng-Robinson equation of state,Ind.Eng.Chem.Res.37(1998)1634–1643.
[17]A.Galindo,A.Gil-Villegas,G.Jackson,A.N.Burgess,SAFT-VRE:Phase behavior of electrolyte solutions with the statistical associating fluid theory for potentials of variable range,J.Phys.Chem.B 103(1999)10272–10281.
[18]J.A.Myers,S.I.Sandler,R.H.Wood,An equation of state for electrolyte solutions covering wide range of temperature,pressure and composition,Ind.Eng.Chem.Res.41(2002)3282–3297.
[19]M.A.Clarke,P.R.Bishnoi,Development of a new equation of state for mixed salt and mixed solvent systems,and application to vapour–liquid and solid(hydrate)–vapour–liquid equilibrium calculations,Fluid Phase Equilib.220(2004)21–35.
[20]L.F.Cameretti,G.Sadowski,J.M.Mollerup,Modeling of aqueous electrolyte solutions with perturbed-chain statistical associated fluid theory,Ind.Eng.Chem.Res.44(2005)3355–3362.
[21]S.P.Tan,H.Adidharma,M.Radosz,Statistical associating fluid theory coupled with restricted primitive model to represent aqueous strong electrolytes,Ind.Eng.Chem.Res.44(2005)4442–4452.
[22]Z.Liu,W.Wang,Y.Li,An equation of state for electrolyte solutions by a combination of low-density expansion of nonprimitive mean spherical approximation and statistical associating fluid theory,Fluid Phase Equilib.227(2005)147–156.
[23]Y.Lin,K.Thomsen,J.C.Hemptinne,Multicomponent equations of state for electrolytes,AIChE J.53(2007)989–1005.
[24]Y.S.Kim,C.S.Lee,An electrolyte equation of state based on a hydrogen-bonding nonrandom lattice fluid model for concentrated electrolyte solutions,Ind.Eng.Chem.Res.47(2008)5102–5111.
[25]R.Inchekel,J.Hemptinne,W.Fürst,The simultaneous representation of dielectric constant,volume and activity coefficients using an electrolyte equation of state,Fluid Phase Equilib.271(2008)19–27.
[26]A.Haghtalab,S.H.Mazloumi,A square-well equation of state for aqueous strong electrolyte solutions,Fluid Phase Equilib.285(2009)96–104.
[27]A.Haghtalab,S.H.Mazloumi,A new coordination number model for development of a square-well equation of state,Fluid Phase Equilib.280(2009)1–8.
[28]M.Born,Volumenund hydratationswarme der ionen,Zeitschrift Zeitschrift für Physik 1(1920)45–49.
[29]A.Harvey,T.W.Copeman,J.M.Prausnitz,Explicit approximation of the mean spherical approximation for electrolyte systems with unequal ion sizes,J.Phys.Chem.92(1988)6432–6436.
[30]C.A.Haynes,J.Newman,On converting from the McMillan-Mayer framework I.Single-solvent system,Fluid Phase Equilib.145(1998)255–263.
[31]R.H.Perry,D.W.Green,Perry’s Chemical Engineers’Handbook,sixth ed.McGraw-Hill,Tokyo,Japan,1988.
[32]R.A.Robinson,R.H.Stokes,Electrolyte Solutions,seconded.Butter worths,London,1970.
[33]W.J.Hamer,Y.C.Wu,Osmotic coefficients and mean activity coefficients of uniunivalent electrolytes in water at 25°C,J.Phys.Chem.Ref.Data 1(1972)1074–1099.
[34]K.S.Pitzer,J.C.Peiper,R.H.Busey,Thermodynamic properties of aqueous sodium chloride solutions,J.Phys.Chem.Ref.Data 13(1984)1–102.
[35]D.G.Archer,Thermodynamic properties of the NaBr+H2Osystem,J.Phys.Chem.Ref.Data 20(1991)509–555.
[36]J.Ananthaswamy,G.Atklnson,Thermodynamics of concentrated electrolyte mixtures.5.A review of the thermodynamic properties of aqueous calcium chloride in the temperature range 273.15-373.15 K,J.Chem.Eng.Data 30(1985)120–128.
[37]H.F.Holmes,R.E.Mesmer,Thermodynamics of aqueous solutions of the alkali metal sulfates,J.Solut.Chem.15(1986)495–517.
 Chinese Journal of Chemical Engineering2016年10期
Chinese Journal of Chemical Engineering2016年10期
- Chinese Journal of Chemical Engineering的其它文章
- An investigation on dissolution kinetics of single sodium carbonate particle with image analysis method
- Rapid synthesis of CNTs@MIL-101(Cr)using multi-walled carbon nanotubes(MWCNTs)as crystal growth accelerator☆
- Hydrodynamic cavitation as an efficient method for the formation of sub-100 nm O/W emulsions with high stability
- Isobaric vapor–liquid equilibrium for binary system of aniline+methyl-N-phenyl carbamate☆
- Proposal and evaluation of a new norm index-based QSAR model to predict pEC50and pCC50activities of HEPT derivatives☆
- Removal of Reactive Red 198 from aqueous solution by combined method multi-walled carbon nanotubes and zero-valent iron:Equilibrium,kinetics,and thermodynamic
