Removal of Reactive Red 198 from aqueous solution by combined method multi-walled carbon nanotubes and zero-valent iron:Equilibrium,kinetics,and thermodynamic
Sudabeh Pourfadakari,Nader Youse fi,Amir Hossein Mahvi,3,4,*
1Department of Environmental Health Engineering,School of Health,Shiraz University of Medical Sciences,Shiraz,Iran
2Department of Environmental Health Engineering,School of Public Health,Tehran University of Medical Sciences,Tehran,Iran
3Center for Solid Waste Research,Institute for Environmental Research,Tehran University of Medical Sciences,Tehran,Iran
4National Institute of Health Research,Tehran University of Medical Sciences,Tehran,Iran
1.Introduction
When Marvene organic dye was synthesized by Perkins in 1875,dying industries were quickly developed[1].In general,both natural and synthetic dyes are widely utilized in textile,leather,printing,cosmetics,and other industries[2].Overall,dyes are classified into three categories of anionic(acidic,reactive,and direct),cationic(basic),and non-ionic(disperse)dyes.[3].Reactive dyes which are among the major synthetic dyes are widely used in textile industries.In addition,they are one of the most problematic dyes since they have high solubility in water and are found in high concentrations in wastewater[4,5].Also,it is estimated that 20%–30%of the overall dyes used worldwide are reactive dyes[6].The precipitation of colored wastewater can extinguish the water body and kill the aquatic animals.Still,some dyes contain toxic and carcinogenic compounds and are hazardous to human health.Therefore,it is necessary to remove the existing dyes from the sewage systems of textile industries[5,7,8].Because of their complex structure,synthetic dyes cannot be effectively degraded using biological methods[8].Asa result,dyes are treated through physical and chemical methods,such as coagulation, flocculation,oxidation,membrane filtration,ion exchange,ozone generation,precipitation,chemical oxidation,enzymes or a combination of these methods.At any rate,using such methods involves certain limitations,such as high costs for utilization,production of hazardous byproducts,and excessive energy consumption[9–12].The adsorption method is extensively used for the treatment of colored wastewater and relies on various natural and synthetic(non)-organic adsorbents,including activated carbon,raw coal,natural zeolite,fugitive dust,and clay.Among these adsorbents,activated carbon is commonly utilized for removing the organic compounds[13].Considering these shortcomings, researchers have focused on optimal adsorbents with high adsorption capacity. Carbon nanotubes, divided into two groups of single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs), are used as a new small sized adsorbent with a hollowed and stratified structure. This shows a higher capacity for adsorption compared to the activated carbon. Also,it can remove dyes and other pollutants from waste water [14–16]. In recent years, studies have concentrated on the decomposition of organic pollutants through new treatment methods using zero-valent iron (ZVI) powder [17]. ZVI is one of the most abundant types of metal on earth and is given priority because of its strong synthetic tendency, inexpensiveness,and high effectiveness in decomposing different insecticides(DDT, DDD), halogenated organic compounds, aromatics, surfactants,and azo dyes [18,19]. ZVI (as a powder) is a strong remediation, is oxidized in aqueous environments, and produces free electrons which act as remediating factors and help remediate organic compounds [17,20].Considering the advantages of ZVI and MWCNTs, the main purpose of the present study is to investigate the efficiency of the kinetic process of ZVI/MWCNTs to remove Reactive Red 198 from aqueous environments.
2.Material and Methods
2.1.Material
The Reactive Red198(above95%purity)was bought from Hamedan Alvan Sabet Company and used with no further treatment.This dye is widely used for the dyeing of cellulose and cotton and commonly used in several industries such as textile dyeing and printing.Characteristics and chemical structures of RR198 are presented in Table 1.In addition,iron powder with above 98%purity and effective particle size of150μ was bought from Merck Company,Germany and used in the experiments without any further treatment.Besides,MWCNTs with above 95%purity,1–30 nm diameter,specific surface area of 270 m2·g?1,and electrical conductivity of 1500 s·m?1were prepared by the ResearchInstitute of Petroleum Industry and used without any further treatment as the adsorbent.Transmission electron microscopy(TEM)and scanning electron microscopy(SEM)images of MWCNTs have been shown in Fig.1.

Table 1 The properties of the dye used in the study
Other chemicals used in the study also had the laboratory purity of Merck.To prepare the dye solution, first the stock solution of 500 mg·L?1concentration was prepared out of the RR198 using deionized water.Then,the experimental solutions were daily prepared from the stock solution in 20,50,100,and 200 mg·L?1concentrations.
2.2.Methods
The present applied research was carried out at a laboratory scale.To perform the experiments,laboratory jars(250 ml)were used.In addition,hydrochloric acid and caustic were used in order to organize pH.In order to remove the dye in this study,the effect of the following variables was investigated:pH in the range of(3–10),temperature(283–303 K),contact time(5–120 min),ZVI dose(200–5000 mg·L?1),and dose of MWCNTs(100–600 mg·L?1).After mixing was performed by Erlen shaker(model 430R)at 150 r·min?1,the samples were centrifuged at 4000 r·min?1for 10 min and passed through 0.2 μm filter papers.The maximum rate of dye adsorption at the wavelength of 518nm was measured by the UV/Vis spectrophotometer(German Hatch—DR5000)according to the standard method[21,22].Besides,the concentration of the remaining dye was determined through the calibration curve.
The adsorption capacity of the adsorbent and the removal efficiency was respectively calculated using the following equations:

where:Re,is the removal efficiency,qt,the amount of the adsorbed dye(mg·g?1),C0,the initial concentration of dye in the solution(mg·L?1),Ct,concentration of the dye remaining in the solution(mg·L?1),V,volume(L),and m,the dose of the adsorbent used(g).
3.Results and Discussion
3.1.The effect of pH
pH is an important parameter in the adsorption process.The effect of pH on dye removal was assessed at 5000 mg·L?1iron powder,100 mg·L?1initial dye concentration,600 mg·L?1adsorbent dose,and different contact times(5–120 min)at 303 K.The effect of the primary pH on the rate of dye adsorption is illustrated in Fig.2.According to Fig.2,as pH increased from 3 to10,during 120 min,the dye removal efficiency decreased from 99.78%to 89.7%.This might be due to the increase in the H+ions in the environment,decrease in the OH?,and increase in the positive ions on the surface of the adsorbent.This process will lead to the electrostatic force among the dye molecules(negatively charged)and the surface of the adsorbent(positively charged).In other words,the charge on the surface of the adsorbent is affected by the pH of the solution[23].In acidic solutions, the iron powder turns into Fe2+,while two free electrons are produced and sedimentation of Fe(OH)3does not take place.This shows that iron powder is solved faster in lower pH levels.On the other hand,in acidic environments,the surface of the iron powder is continuously cleaned and will accordingly produce more free electrons and enhance the efficiency of the dye removal process[24].Machado et al.[25]conducted a study on removing the reactive red M-2BE by MWCNTs and activated carbon and showed that the highest removal efficiency was achieved at pH=2,which is caused by the electrostatic reaction between the adsorbents and dye molecules in the acidic pH. Also, this result is confirmed by Yao et al.[26].
3.2.The effect of contact time
As far as time is concerned, the effect of the reaction between the adsorbent and adsorbate is among the most important parameters affecting the adsorption process.Fig.3 illustrates the effect of time on the efficiency of removing the RR198 at pH=3 for different dye concentrations by using 600 mg·L?1MWCNTs and 5000 mg·L?1zero-valent iron,at 303 K by increasing the contact time from 60 to 120 min,in dye concentration of 100 mg·L?1,RR198 dye removal efficiency reached 99.68%from 96.8%.The results showed that adsorption of the RR198dye followed a fast process at the preliminary stages and the longer the process,the higher the removal percentage.However,the adsorption reaches equilibrium after 100 min.This happens because at the primary stages,a great number of activated sites on the surface of the adsorbent are not yet occupied,but as time passes,the remaining empty sites are rigidly filled with dye molecules.Shahryari et al.[27]in a research on the adsorption of Methylene blue through CNTs,found that the rate of Methylene blue adsorption was primarily rapid,whereas it gradually dropped until it reached the equilibrium time of 120 min.

Fig.1.SEM(a)and TEM(b)images of the MWCNTs.

Fig.2.The effect of pH on the adsorption of RR198 using ZVI/MWCNTs at(dose of iron powder=5000 mg·L?1;MWCNTs=600 mg·L?1,dye concentration=100 mg·L?1).

Fig.3.The effect of the equilibrium time on the adsorption process of RR198 using mixed method at pH=3 in different dye concentrations(dose of iron powder=5000 mg·L?1;MWCNTs=600 mg·L?1).
3.3.The effect of adsorbent dose
One of the parameters affecting the adsorption process is the amount of the adsorbent.In order to determine the effect of the doses of iron powder and MWCNTs on the dye removal,different iron powder doses were added to different doses of MWCNTs at pH=3 and 100 mg·L?1initial dye concentration at the equilibrium time.The results are presented in Fig.4.According to this figure,higher doses can lead to an increase in the efficiency of the dye removal process;such a way that in200mg·L?1iron powder and100mg·L?1MWCNTs,the efficiency reached 37.48%and in 5000 mg·L?1iron powder and 600 mg·L?1MWCNTs,the removal efficiency reached 99.16%.To describe the process more clearly,the model of the multivariate linear regression was drawn using MATLAB software for the ZVI/MWCNT method and according to Fig.5,the best fitness was related to the linear regression coefficient with R2>0.9756.This research showed that an increase in the adsorbent dose could enhance the removal efficiency.Also,according to Fig.6 by increasing the dose of MWCNTs from 100 to 600 mg·L?1in 100 mg·L?1dye concentration and 5000 mg·L?1iron powder dose,the dye removal efficiency reached 99.12%from 95.65%.Due to the fact that by increasing the dose of MWCNTs from 100 to 600 mg·L?1in constant dose of iron powder,only 3.47%increase was observed in dye removal efficiency(from 95.65%to 99.12%),this process can be effective in dye removal.In other words,high efficiency can be obtained in low doses of carbon nanotubes in the presence of iron particles and higher doses of MWCNTs are not required.In order for better understanding of the effect of MWCNT dose on dye removal efficiency,multivariate linear regression model was drawn for various adsorbent doses using MATLAB software,the best fitness was related to(R2=0.98).As can be seen in Fig.7,by increasing the iron powder dose from 200 to 5000 mg·L?1at MWCNT dose of 600 mg·L?1and dye concentration of 100 mg·L?1,RR198 dye removal efficiency increased from 82.93%to 99.16%.Overall,these results indicated that by increasing the dose of iron powder,the efficiency of the process increased,as well.Therefore,an increase of iron powder dose has a considerable effect on dye removal.Furthermore,a multivariate linear regression model was drawn in order to determine the effect of iron powder dose on the efficiency of dye removal, the best fitness was related to(R2=0.95).The most important factor here is that increasing the ZVI dose will increase the primary matter producing free electrons.As a result,the rate of free electron production will increase which subsequently enhances the removal efficiency.In general,iron powder reaction depends on the surface area;the smaller the surface area and the larger the particles surface area,the higher the reactivity of the particles will be[28].With an increase in the adsorbent CNT dose,the adsorption sites and the surface are extended and more activated sites will be available.The results of the study carried out by Gong et al.[29]showed that by increasing the MMWCNT dose from 0.3 to 0.9 g·L?1,MB,NR,and BCB dyes removal efficiency reached from 30%to 99.1%,17.11%to 98.33%,and17.6%to98.8%,respectively.In the higher dose of the adsorbent(0.9g·L?1),on the other hand, the dye adsorption process reached its equilibrium and the rate of dye removal remained approximately constant. This is due to the fact that the high dose of the adsorbent increases the viscosity and stops the diffusion of the dye molecules on the surface of the MWCNTs.

Fig.4.The effect of different dosages of ZVI and MWCNTs on the efficiency of the removal process(pH=3;contact time=100 min;dye concentration=100 mg·L?1).

Fig.5.Modeling the multivariate linear regression of RR198 through the mixed method(contact time=100 min;pH=3)different doses of ZVI/MWCNTs in 100 mg·L?1dye concentration.
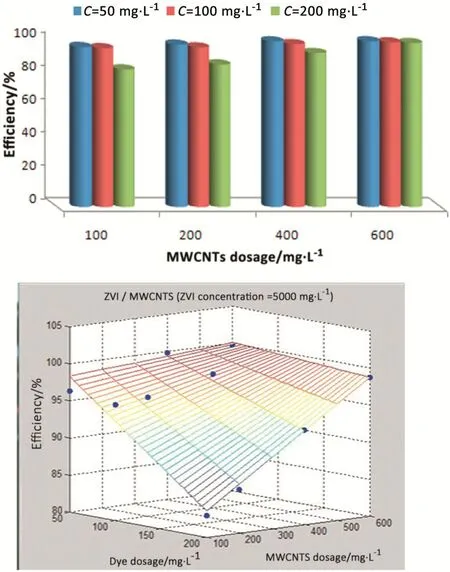
Fig.6.The effect of MWCNT dosage on the adsorption efficiency at pH=3,5000 mg·L?1 ZVI,different dye concentration,and equilibrium time,and multivariate linear regression model for RR198 dye.

Fig.7.The effect of zero-valent iron on the adsorption efficiency at pH=3,600 mg·L?1 MWCNTs,different dye concentrations,and equilibrium time and multivariate linear regression model for RR198 dye.
3.4.The effect of initial dye concentration
Initial dye concentration is one of the main parameters of dye removal.As Fig.8 depicts,by decreasing the initial dye concentration,dye removal efficiency increased.At 5000 mg·L?1dose of iron powder and 600 mg·L?1dose of MWCNTs in the combined method,by increasing the initial dye concentration from20 to200mg·L?1,the dye removal efficiency decreased from 100%to 98.63%,while the adsorption capacity increased from 3.57 to 35.22 mg·L?1.This is due to the fact that in lower concentrations,there are a larger number of unsaturated places on the adsorbent surface for adsorbing the dye.By increasing the dye concentration,however,these places are reduced and repulsive forces between the adsorbed dye molecules on the adsorbent surface increase[30].Natarajan et al.[31]performed a study on Rhoda mine Blue dye removal and showed that by increasing the initial dye concentration,the removal efficiency reduced from 96%to 51%.An increase in the adsorption capacity by the increase in the initial dye concentration is due to the increase in the force resulting from dye concentration gradient for overcoming the resistance toward mass transfer between liquid and solid phases.

Fig.8.The effect of initial dye concentration on the adsorption efficiency at 600 mg·L?1 MWCNTs,5000 mg·L?1zero-valent iron,pH=3,and equilibrium time.
3.5.The effect of temperature
To determine the effect of temperature on the rate of dye removal at different temperatures(283–303 K),constant doses of ZVI and MWCNTs were added to different concentrations(20–200 mg·L?1)at the acidic pH at the equilibrium time.According to Fig.9,by increasing the temperature from 283 to 303 K in 100 mg·L?1concentration,the removal efficiency increased from 89.44%to 99.11%.Also,the adsorption capacity increased from 15.97 mg·g?1to 17.69 mg·g?1.This occurs because in an endothermic process,as the temperature increases,the motion of the dye ions increases and the adsorption capacity of the adsorbent is enhanced[32].The results of the study by Dizge et al.[33]showed that as the temperature increased from 293 K to 323 K,the efficiency of the dye removal process was enhanced,as well.In the study by Fan et al.[34]on methylene orange removal,by increasing the temperature from 293 to 313 K,dye removal efficiency increased from 72.2%to 98.3%and higher temperatures decreased the required time for dye removal.(See Fig.10.)

Fig.9.The effect of the temperature on the equilibrium capacity of the adsorption process through the mixed method in different dye concentrations(pH=3;contact time=100 min;constant ZVI/MWCNT dosage).
3.6.The isotherm of adsorption
To describe the mutual behavior of the adsorbent and the adsorbate and predict the adsorption capacity of the adsorbent,the isotherm equitation plays a key role as it is one of the fundamental parameters of designing the system[35].The three models,Temkin,Langmuir,and Freundlich,are used to describe the behavior of RR198 adsorption in the mixed ZVI/MWCNTs method.In addition,the experiments were performed by changing the initial dye concentration from 20 to 200 mg·L?1at temperature of 293 K,and the equilibrium time of 100 min.The results and isothermal equations presented in Table 2 indicate that Freundlich isotherm(R2=0.996 at 293 K)is better correlated with the other isotherms under investigation.Besides,by increasing the dye equilibrium concentration,the adsorption equilibrium capacity increased and maximum adsorption capacity of ZVI was obtained 33.88 mg·g?1.

Table 2 Linear equations and the results of the isotherm calculations[36,37]
3.7.Kinetic studies
Kinetic studies are used for designing and modeling the processes and the reactions performed in the reactor.These studies also provide important information about the mechanism of RR198 adsorption onto ZVI/MWCNTs which is necessary to depict the adsorption rate of the adsorbate and control the residual time of the whole adsorption process.In this research,pseudo- first-order,pseudo-second-order,and intra-particle diffusion models were utilized.[38,39].
A,pseudo- first-order equation:

where qeand qtare the amounts of dye adsorbed on the adsorbent at equilibrium and at the time of t,respectively(mg·g?1),k1is the equilibrium rate constant of pseudo- first-order(min?1),and t is the contact time(min).The slope and intercept of the plot of lg(qe?qt)versus t were used to determine the pseudo- first-order rate constant,k1.
B,pseudo-second-order equation:

where C is the intercept and kdifis the intra-particle diffusion rate constant(mg·g·min?0.5)which can be determined by the slope of the linear plot of q versus t1/2.
According to Table 3,pseudo-second-order kinetics was the dominant synthetics for the mixed ZVI/MWCNT method with R2=0.999 in 50 and 200 mg·L?1concentrations and R2=1 in 100 mg·L?1concentration.Konicki et al.[40]conducted a study on Direct red 23 dye and the obtained results showed that Freundlich isotherm(R2=0.959)and Pseudo-second-order kinetic model(R2>0.99)were the best models for describing the reaction of adsorption.The results of the study conducted by Lin et al.[41]on removing Basic Green 5 dye by Titania nanotubes showed that Basic Green 5 adsorption followed a Pseudo-second-order kinetics model for dye concentration of 900 mg·L?1with R2> 0.999.
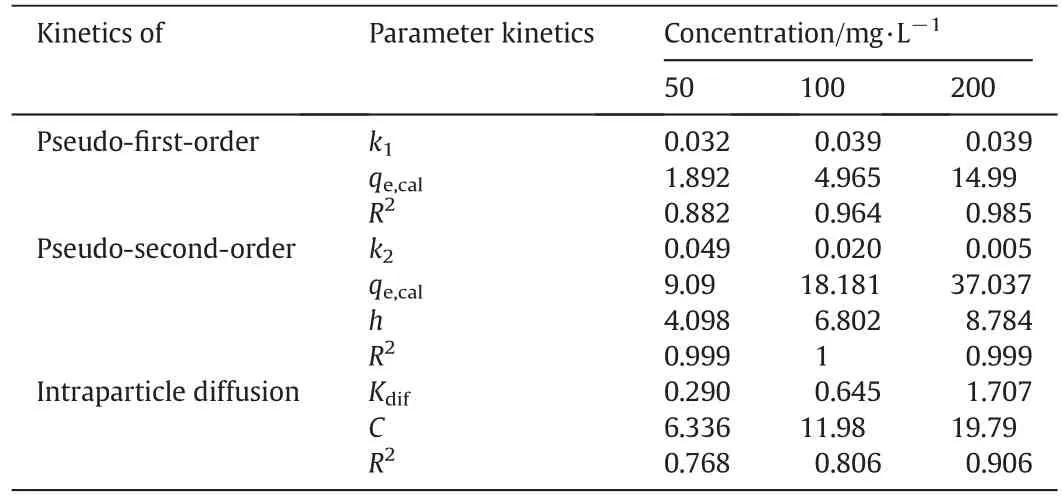
Table 3 The results of the kinetics under investigation
3.8.Estimation of thermodynamic parameters

Thermodynamic parameters provide information regarding the inherent energetic changes associated with adsorption.Therefore,(ΔG0,kJ·mol?1),entropy (ΔS0,J·mol?1·K?1) and enthalpy (ΔH0,kJ·mol?1)changes were determined by using Vant Hoff equation to elucidate the process of adsorption.where KLis the thermodynamic equilibrium constant(mol?1),R is the gas constant(8.314 J·mol?1·K?1),T is temperature(K),and ΔS0and ΔH0were determined from the slope of linear plotting between ln K and 1/T according to Eq.(8).Also,the ΔG0values were computed according to Eq.(9)[42].As Table4depicts,the values of Gibbs free energy(ΔG0)were negative in the temperature range of 283–303K con firming that the adsorption of RR198 onto ZVI was spontaneous and thermodynamically favorable.When the temperature increases from 283 to 303 K,ΔG0decreases from ?96.81 to ?103.65 kJ·mol?l,which indicates that adsorption,is more spontaneous at higher temperature.The value of enthalpy change ΔH0shows the exothermic or endothermic of the adsorption process.Kara et al.[43]suggested that the ΔH0of physisorption is smaller than 40 kJ·mol?l.On the other hand,the positive value of ΔH0(91.76kJ·mol?l)indicates that the adsorption process of RR-198 onto ZVI/MWCNTs is endothermic and chemisorption.The results of the study conducted by Lin et al.[44]on removing Acid Black 24 dye also showed that by increasing the temperature from 283 to 323 K reaction rate is greatly,and the dye removal rate increased with temperature,indicating an endothermic reaction.Fig.11 depicts the regressions of Vant Hoff plot for thermodynamic parameters in this study.
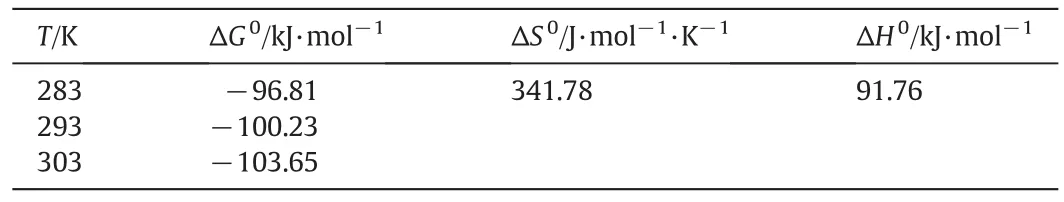
Table 4 Thermodynamic parameters calculated at various temperatures

Fig.11.Regressions of vant Hoff plot for thermodynamic parameters and the adsorption of the RR-198 onto ZVI/MWCNTs at(various temperatures,initial dye concentration=100 mg·L?1and pH=3).
4.Conclusions
With increasing the temperature up to 303 K the dye adsorption was increased. Also, RR198 removal followed Freundlich isotherm and Pseudo-second-order kinetic models. Among the parameters of a solution,pH was more emphasized in the present research. In the studied processes, as the pH decreased, the efficiency of dye removal process increased.Also, it is described here that iron powder provides ease of use,allows iron filings to stay in the environment, and can be continually utilized. As a result, the ZVI/MWCNT method as a fast-functioning method with high efficiency can help remove dyes from industrial wastewater. Therefore, application of this method should be investigated from the economic point of view.

Acknowledgments
This article was extracted from Sudabeh Pourfadakar's M.Sc.thesis and approved by Shiraz University of Medical Sciences,Shiraz,Iran(No91/6133).The authors are grateful for Ms.A.Keivanshekouh at the Research Improvement Center of Shiraz University of Medical Sciences for improving the use of English in the manuscript.
[1]J.Cao,L.Wei,Q.Huang,L.Wang,S.Han,Reducing degradation of azo dye by zerovalent iron in aqueous solution,Chemosphere 38(3)(1999)565–571.
[2]A.Geethakarthi,B.R.Phanikumar,Adsorption of reactive dyes from aqueous solutions by tannery sludge developed activated carbon:Kinetic and equilibrium studies,Int.J.Environ.Sci.Technol.8(3)(2011)561–570.
[3]Y.Fu,T.Viraraghavan,Fungal decolorization of dye waste waters:A review,Bioresour.Technol.79(2001)251–262.
[4]M.T.Ghaneian,G.Ghanizadeh,M.Gholami,F.Ghaderinasab,Application of eggshell as a natural sorbent for the removal of reactive red 123 dye from synthetic textile wastewater,J.Res.Med.Sci.11(4)(2010)25–35(In Persian).
[5]N.Supaka,K.Juntongjin,S.Damronglerd,M.L.Delia,P.Strehaiano,Microbial decolorization of reactive azo dyes in a sequential anaerobic–aerobic system,J.Chem.Eng.99(2004)169–176.
[6]N.Pramanpol,N.Nitayapat,Adsorption of reactive dye by eggshell and its membrane,Kasetsart J.(Nat.Sci.)40(2006)192–197.
[7]S.Chatterjee,S.R.Lim,S.H.Woo,Removal of reactive black 5 by zero-valent iron modified with various surfactants,J.Chem.Eng.160(2010)27–32.
[8]M.Solís,A.Solís,H.I.Pérez,N.Manjarrez,M.Flores,Microbial decolouration of azo dyes:A review,Process Biochem.47(2012)1723–1748.
[9]F.Gholami-Borujeni,A.H.Mahvi,S.Naseri,M.A.Faramarzi,R.Nabizadeh,M.Alimohammadi,Application of immobilized horseradish peroxidase for removal and detoxi fi cation of azo dye from aqueous solution,J.Res.Chem.Environ.15(2011)217–222.
[10]F.Gholami-Borujeni,A.H.Mahvi,S.Nasseri,M.A.Faramarzi,R.Nabizadeh,M.Alimohammadi,Enzymatic treatment and detoxification of acid orange 7 from textile wastewater,Appl.Biochem.Biotechnol.165(2011)1274–1284.
[11]M.C.Ncibi,B.Mahjoub,M.Seffen,Adsorptive removal of textile reactive dye using Posidonia oceanica(L.) fibrous biomass,Int.J.Environ.Sci.Technol.4(4)(2007)433–440.
[12]B.C.Oei,S.Ibrahim,S.Wang,H.M.Ang,Surfactant modified barley straw for removal of acid and reactive dyes from aqueous solution,Bioresour.Technol.100(2009)4292–4295.
[13]H.D.Choi,M.C.Shin,D.H.Kim,C.S.Jeon,K.Baek,Removal characteristics of reactive black 5 using surfactant-modified activated carbon,Desalination 223(1–3)(2008)290–298.
[14]M.Shirmardi,A.Mesdaghinia,A.H.Mahvi,S.Nasseri,R.Nabizadeh,Kinetics and equilibrium studies on adsorption of acid red 18(azo-dye)using multi wall carbon nanotubes(MWCNTs)from aqueous solution,J.Chem.9(4)(2012)2371–2383.
[15]T.Madrakian,A.Afkhami,M.Ahmadi,H.Bagheri,Removal of some cationic dyes from aqueous solutions using magnetic-modified multi-walled carbon nanotubes,J.Hazard.Mater.196(30)(2011)109–114.
[16]R.Q.Long,R.T.Yang,Carbon nanotubes as superior sorbent for dioxin removal,J.Am.Chem.Soc.123(9)(2001)2058–2059.
[17]S.Pourfadakari,A.H.Mahvi,Kinetics and equilibrium studies for removal of reactive red 198 from aqueous solutions using zero valent iron powder,J.Health Scope 3(2)(2014)1–8.
[18]N.Deng,F.Luo,F.Wu,M.Xiao,X.Wu,Discoloration of aqueous reactive dye solutions in the UV/Fe 0 system,Water Res.34(2000)2408–2411.
[19]S.Nam,P.G.Tratnyek,Reduction of azo dyes with zero-valent iron,Water Res.34(6)(2000)1837–1845.
[20]M.Zarrabi,M.Samarghndi,A.Rahmani,Kinetic study of acid red 18 and acid red 14 removal from aqueous solution using metallic iron,J.Health Hyg.Ardebil Iran.3(2011)31–40.
[21]L.Clesceri,A.Greenberg,A.Eaton,Standard methods for the examination of water and wastewater,APHA,AWWA,WEF,USA,2010 20.
[22]C.H.Wu,Adsorption of reactive dye onto carbon nanotubes:equilibrium,kinetics and thermodynamics,J.Hazard.Mater.144(1–2)(2007)93–100.
[23]H.Zhu,R.Jiang,L.Xiao,G.Zeng,Preparation,characterization,adsorption kinetics and thermodynamics of novel magnetic chitosan enwrapping nanosized γ-Fe2O3and multi-walled carbon nanotubes with enhanced adsorption properties for methyl orange,Bioresour.Technol.101(14)(2010)5063–5069.
[24]K.Barbusiński,J.Majewski,Discoloration of azo dye acidred 18 by Fenton reagent in the presence of iron powder,J.Environ.Stud.Pol.12(2)(2003)151–155.
[25]F.M.Machado,C.P.Bergmann,T.H.Fernandes,E.C.Lima,B.Royer,T.Calvete,et al.,Adsorption of reactive red M-2BE dye from water solutions by multi-walled carbon nanotubes and activated carbon,J.Hazard.Mater.192(3)(2011)1122–1131.
[26]Y.Yao,H.Bing,X.Feifei,C.Xiaofeng,Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes,J.Chem.Eng.170(2011)82–89.
[27]Z.Shahryari,A.S.Goharrizi,M.Azadi,Experimental study of methylene blue adsorption from aqueous solutions onto carbon nanotubes,J.Water Res.Environ.Eng.Int.2(2010)16–28.
[28]J.A.Mielczarski,G.M.Atenas,E.Mielczarski,Role of iron surface oxidation layers in decomposition of azo-dye water pollutants in weak acidic solutions,Appl.Catal.B Environ.56(4)(2005)289–303.
[29]J.L.Gong,B.Wang,G.M.Zeng,C.P.Yang,C.G.Niu,Q.Y.Niu,et al.,Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent,J.Hazard.Mater.164(2–3)(2009)1517–1522.
[30]S.Khorramfar,N.Mahmoodi,M.Arami,K.Gharanjig,Dye removal from colored textile wastewater using Tamarindus indica Hull:Adsorption isotherm and kinetics study,J.Color.Sci.Technol.3(2009)81–88.
[31]T.S.Natarajan,K.Natarajan,H.C.Bajaj,R.J.Tayade,Enhanced photocatalytic activity of bismuth-doped TiO2nanotubes under direct sunlight irradiation for degradation of Rhodamine B dye,J.Nanopart.Res.15(2013)1–18.
[32]S.T.Wong,Y.P.Tan,A.H.Abdullah,S.T.Ong,Removal of basic blue 3 and reactive orange 16 by adsorption onto quartenized sugar cane bagasse,J.Anal.Sci.Malays.13(2009)185–193.
[33]N.Dizge,C.Aydiner,E.Demirbas,M.Kobya,S.Kara,Adsorption of reactivedyesfrom aqueous solutions by fly ash:Kinetic and equilibrium studies,J.Hazard.Mater.150(3)(2008)737–746.
[34]J.Fan,Y.Guo,J.Wang,M.Fan,Rapid decolorization of azo dye methyl orange in aqueous solution by nanoscale zerovalent iron particles,J.Hazard.Mater.166(2–3)(2009)904–910.
[35]P.Palanisamy,A.Agalya,P.Sivakumar,Polymer composite—A potential biomaterial for the removal of reactive dye,J.Chem.9(4)(2012)1823–1834.
[36]N.Thinakaran,P.Baskaralingam,M.Pulikesi,P.Panneerselvam,S.Sivanesan,Removal of acid violet 17 from aqueous solutions by adsorption onto activated carbon prepared from sun flower seed hull,J.Hazard.Mater.151(2–3)(2008)316–322.
[37]M.Temkin,V.Pyzhev,Kinetics of ammonia synthesis on promoted iron catalysts,Acta Physicochim.URSS 12(1940)217–229.
[38]J.R.Baseri,P.Palanisamy,P.Sivakumar,Adsorption of reactive dye by a novel activated carbon prepared from Thevetia peruviana,J.Chem.Res.3(2012)36–41.
[39]D.Suteu,C.Zaharia,T.Malutan,Removal of orange 16reactive dye from aqueous solutions by waste sun flower seed shells,J.Serb.Chem.Soc.76(4)(2011)607–624.
[40]W.Konicki,I.Pe?ech,E.Mijowska,I.Jasińska,Adsorption of anionic dye directred 23 onto magnetic multi-walled carbon nanotubes-Fe3C nanocomposite:kinetics,equilibrium and thermodynamics,Chem.Eng.210(1)(2012)87–95.
[41]K.S.Lin,H.W.Cheng,W.R.Chen,C.F.Wu,Synthesis,characterization,and adsorption kinetics of titania nanotubes for basic dye wastewater treatment,J.Adsorpt.16(1)(2010)47–56.
[42]M.A.M.Khraisheh,Y.S.Al-Degs,S.I.Allen,M.N.Ahmad,Elucidation of controlling steps of reactive dye adsorption on activated carbon,J.Ind.Eng.Chem.Res.41(6)(2002)1651–1657.
[43]M.Kara,H.Yuzer,E.Sabah,M.S.Celik,Adsorption of cobalt from aqueous solutions onto sepiolite,J.Water Res.37(1)(2003)224–232.
[44]Y.T.Lin,C.H.Weng,F.Y.Chen,Effective removal of AB24 dye by nano/micro-size zero-valent iron,Sep.Purif.Technol.64(1)(2008)26–30.
 Chinese Journal of Chemical Engineering2016年10期
Chinese Journal of Chemical Engineering2016年10期
- Chinese Journal of Chemical Engineering的其它文章
- An investigation on dissolution kinetics of single sodium carbonate particle with image analysis method
- Rapid synthesis of CNTs@MIL-101(Cr)using multi-walled carbon nanotubes(MWCNTs)as crystal growth accelerator☆
- Hydrodynamic cavitation as an efficient method for the formation of sub-100 nm O/W emulsions with high stability
- Isobaric vapor–liquid equilibrium for binary system of aniline+methyl-N-phenyl carbamate☆
- Proposal and evaluation of a new norm index-based QSAR model to predict pEC50and pCC50activities of HEPT derivatives☆
- Correlation of the mean activity coefficient of aqueous electrolyte solutions using an equation of state
