Modified methods for isolation of pancreatic stellate cells from human and rodent pancreas
Liangtao Zhao, Baobao Cai, Zipeng Lu, Lei Tian, Song Guo, Pengfei Wu, Dong Qian, Qingcheng Xu, Kuirong Jiang, Yi Miao,?
1Pancreas Institute of Nanjing Medical University, Nanjing, Jiangsu 211166, China;
2Pancreas Center;3Lab for Department of General Surgery, the First Aff i liated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029, China.
Modified methods for isolation of pancreatic stellate cells from human and rodent pancreas
Liangtao Zhao1,3, Baobao Cai1,2,3, Zipeng Lu1,2,3, Lei Tian1,2,3, Song Guo1,3, Pengfei Wu1,2,3, Dong Qian1,3, Qingcheng Xu1,3, Kuirong Jiang1,2,3, Yi Miao1,2,3,?
1Pancreas Institute of Nanjing Medical University, Nanjing, Jiangsu 211166, China;
2Pancreas Center;3Lab for Department of General Surgery, the First Aff i liated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029, China.
Primary cultures of pancreatic stellate cells (PSCs) remain an important basis forin vitrostudy. However, effective methods for isolating abundant PSCs are currently lacking. We report on a novel approach to isolating PSCs from normal rat pancreases and human pancreatic ductal adenocarcinoma (PDAC) tissue. After anaesthesia and laparotomy of the rat, a blunt cannula was inserted into the pancreatic duct through the anti-mesentery side of the duodenum, and the pancreas was slowly infused with an enzyme solution until all lobules were fully dispersed. The pancreas was then pre-incubated, fnely minced and incubated to procure a cell suspension. PSCs were obtained after the cell suspension was fltered, washed and subject to gradient centrifugation with Nycodenz solution. Fresh human PDAC tissue was fnely minced into 1×1×1 mm3cubes with sharp blades. Tissue blocks were placed at the bottom of a culture plate with fresh plasma (EDTA-anti-coagulated plasma from the same patient, mixed with CaCl2) sprinkled around the sample. After culture for 5–10 days under appropriate conditions, activated PSCs were harvested. An intraductal perfusion of an enzyme solution simplifed the procedure of isolation of rat PSCs, as compared with the multiple injections technique, and a modifed outgrowth method signifcantly shortened the outgrowth time of the activated cells. Our modifcation in PSC isolation methods signifcantly increased the isolation effciency and shortened the culture period, thus facilitating future PSC-related research.
pancreatic stellate cells, isolation, modifcation
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most lethal malignancy in humans with an extremely poor prognosis[1-2]. Chronic pancreatitis (CP) is a known risk factor for PDAC[3-4], with desmoplasia a common and important pathophysiological characteristic for both CP and PDAC[5]. There is a growing body of evidence showing that excessive desmoplasia plays a crucial role in the aggressive behaviour of PDAC[6]. Pancreatic stellate cells (PSCs) have been identified as the principal source of excessive extracellular matrix observed in CP and PDAC, and can promote growth, enhance invasion, trigger tumour immune escape or induce chemotherapeutic and radiation resistance of pancreatic cancer cells[7-11]. PSCs have generated considerable scientificinterest over recent years in light of their importance in the progression of PDAC[12-14].
In normal physiological states, PSCs are quiescent and located in the periacinar area with vitamin A lipid droplets in their cytoplasm[15]. When exposed to tissue injury, inflammation, tumour, toxin or cultured in vitro, PSCs will transform into an activated state with myofibroblast-like characteristics, including loss of vitamin A droplets, enhanced proliferation and migration ability, production of abundant amounts of extracellular matrix proteins, including collagen, fibronectin, and laminin and remain distinguishable from fibroblasts with the expression of neuroectodermal markers, such as glial fibrillary acidic protein (GFAP)[16].
Various methods for isolating PSCs have been published, either utilising dispersed acini, enzymatic tissue digestion and density centrifugation, or the outgrowth of PSCs from pancreatic tissue[17-18]. These methods require fairly large amounts of tissue to obtain a sufficient number of cells[19]. Although immortalised cells have been generated by various study groups with numerous methods, due to differences between individuals, immortalised PSCs may not be an ideal model for all studies and different cell sources are also required[20-21]. Therefore, the successful isolation of primary culture remains essential to the study of PSCs.
There were many shortcomings in current isolation methods of PSCs, which limited the yield and cell vitality of PSCs, mainly caused by incomplete or over digestive of pancreas in the process of isolation. Especially, due to the complex process, the success rate of isolation was low. The aim of present study was to describe our modifications in current isolation methods of PSCs.
Materials and methods
The protocols for this study were approved by the Ethical Committee at the First Affiliated Hospital with Nanjing Medical University (Nanjing, China).
Gey's balanced salt solution (GBSS with NaCl) and protease were purchased from Sigma-Aldrich, St Louis, MO, USA; Nycodenz was purchased from Axis-Shield, Oslo, Norway; Collagenase P. and deoxyribonuclease were purchased from Roche, Mannheim, Germany; penicillin-streptomycin antibiotics were purchased from Thermo Scientific, Rockford, IL, USA; conical flasks and plastic Petri dishes were purchased from Corning, NY, USA. Dulbecco's Modified Eagle Medium nutrient mixF-12 (DF-12), foetal bovine serum (FBS) and L-glutamine were purchased from Gibco, Carlsbad, Calif, USA.
Mouse anti-desmin antibodies and mouse anti-GFAP antibodies were purchased from Cell Signaling Technology (CST), Danvers, MA, USA; mouse anti-vimentin antibodies, rabbit anti-α-SMA antibodies, rabbit anti-CK, anti-CD68 antibodies and mouse anti-Pan-CK antibodies were purchased from Abcam, Cambridge, MA, USA; fluorescence (CY3) goat antimouse secondary antibody or fluorescence (FITC) goat anti-rabbit secondary antibody, HRP-conjugated anti-mouse secondary antibody or HRP-conjugated anti-rabbit secondary antibody, immunology staining blocking buffer, Triton X-100, and Trypan Blue staining were purchased from Beyotime, Beijing, China; bovine serum albumin (BSA) were purchased from Sigma-Aldrich.
Isolation and culture of quiescent rat pancreatic stellate cells (rPSCs)
A 250 g male Sprague-Dawley rat (5–7 weeks) was chosen for a single isolation. The rat was anesthetised by intraperitoneal injection of pentobarbital. Our method used for the isolation of quiescent PSCs was based on that described by Apteet al.with some novel modifications, as detailed below[18]: The digestive enzyme solution for pancreatic tissues was made from 100 mL GBSS+NaCl with 65 mg collagenase P, 50 mg protease and 1 mg deoxyribonuclease. Once the abdomen was fully opened under sterile conditions and the duodenum exposed, a sterile blunt cannula (intravenous infusion catheter) was inserted into the pancreatic duct through the duodenum papilla, and fixed with silk thread and the rat pancreas was allowed to be slowly infused with 5–6 mL enzyme solution until all lobules were well dispersed. After the gland was fully infused, the pancreas was resected and transferred into a conical flask for pre-incubation with a 10 mL enzyme solution. The conical flask was incubated at 37°C for 7 minutes in a shaking water bath [high speed (120 cycles/minute) for 4 minutes and low speed (80 cycles/minute) for 3 minutes]. The partially digested tissue was then transferred into another glass dish and was cut into morsels using a pair of two-curved scissors. If the mixture was sticky, 20 μL deoxyribonuclease (4 mg/mL) was added. The mixture was then transferred back into the previous conical flask to undergo a second incubation for 7 minutes at 37°C in a shaking water bath at 80 cycles/minute. Twenty millilitres of complete culture medium was added to the flask and pipetted to mix thoroughly. The cell suspension was then filtered through a 250 μm nylon mesh into a 50 mL centrifuge tube. The tube was centrifuged at 450 g for 10 minutes at 4°C. After the supernatant was carefully aspirated, the cell pellet was washed with GBSS + NaCl containing 0.3% BSA and centrifuged at450 g for 10 minutes at 4°C. The supernatant was cautiously removedviapipetting and the cell pellet was thoroughly suspended with 9.5 mL GBSS + NaCl containing 0.3% BSA. Eight millilitres of 28.7% Nycodenz was added and mixed. Six millilitres of GBSS + NaCl with 0.3% BSA was placed into another centrifuge tube and the cell suspension was layered beneath using a 20 mL syringe, taking care not to disrupt the interface. The tube was centrifuged at 1400 g for 20 minutes at 4°C. The white thin band just above the interface was collected using a 5 mL transfer pipette without disturbing the density gradient layers. Cells were washed with GBSS + NaCl containing 0.3% BSA, centrifuged at 450 g for 10 minutes at 4°C, resuspended in DF-12 containing 10% FBS and counted. The harvested cells were cultured in a 60 mm plastic Petridish and incubated in a humidified atmosphere with 5% CO2at 37°C.
Isolation and culture of cancer associated pancreatic stellate cells (CaPSCs)
Human cancer associated PSCs were isolated via the outgrowth method, using histologically-proven fibrotic pancreatic tissue from patients with pancreatic cancer. Informed consent was obtained from the patients for the collection of all clinical tissues and blood samples. Detailed steps were as follows: Two millilitre blood samples were drawn from patients diagnosed with pancreatic cancer before surgery using EDTA-anticoagulant containers. After centrifuge at 1,300 rpm for 10 minutes, the plasma was collected and filtered with 0.45 μm mesh and stored at 4°C for further use. After surgical resection, pancreatic cancer tissue was collected and stored in a cold sterile 0.9% NaCl solution. Tissues were washed for three times with cold 0.9% NaCl and cut into 1×1×1 mm blocks in a glass dish with sharp scissors. Tissue blocks were washed 1–3 times until there was no oil on the liquid surface, and then seeded in two a 6-well culture plate with 5–8 pieces per well. Plasma collected from the same patient was mixed with 100 μmol/L calcium chloride (CaCl2) solution [200 μL CaCl2solution per 1000 μL plasma], and was dropped on the organisation blocks in one 6-well culture plate, while blocks in the other 6-well culture plate were not dropped with plasma as control. The plate was transferred into the incubator until the plasma coagulated and 2 mL total DF-12 medium was added to the plate. Blocks were further incubated in a humidified atmosphere with 5% CO2at 37°C. Once confluence reached 90%, the CaPSCs were trypsinised and replanted into another culture plate, cultured with total DF-12 medium and incubated in a humidified atmosphere with 5% CO2at 37°C.
Immunof l uorescence
rPSCs and CaPSCs were cultured in 24-well plates. Cells were washed twice with cold PBS, fixed in 4% paraformaldehyde, permeabilised in a 0.1% Triton X-100 PBS solution, blocked (immunology staining blocking buffer), and then followed by immunofluorescent staining for α-SMA, vimentin, GFAP, CK19, Pan-CK and CD68 at 4°C overnight. After wash with 0.1% Triton X-100 PBS solution, cells were incubated with secondary antibodies goat anti-mouse (CY3) or goat anti-rabbit (FITC) for 90 min at room temperature and nuclei/DNA counterstained with DAPI. Staining was evaluated using a Nikon Eclipse 80i microscope with a Nikon DS-Qi1 camera and NIS-Elements software.
Immunocytochemistry
rPSCs and CaPSCs were seeded in 24-well plates. Cells were washed twice with cold PBS, fixed in 4% paraformaldehyde and permeabilised with 0.1% Triton X-100 PBS solution, blocked (immunology staining blocking buffer), then incubated for α-SMA, vimentin, desmin, GFAP, CK19, CD68 and Pan-CK antibodies at 4°C overnight. After wash with 0.1% Triton X-100 PBS solution, cells were incubated with HRP-conjugated anti-rabbit or anti-mouse secondary antibody for 120 minutes at room temperature and nuclei/DNA counterstained with hematoxylin. Staining was evaluated using a Nikon Eclipse 80i microscope with a Nikon DS-Qi1 camera and NISElements software.
Results
Isolation and culture of rPSCs
With our trans -duodenal intraductal perfusion technique, solutions are well-infiltrated into the pancreas tissue, which can be demonstrated in our preliminary experiment with hematoxylin. Moreover, there was no leakage of the infused solution (Fig. 1A). When infused with digestive solution, the pancreas became transparent and increased to twice its original volume (Fig. 1B, C). Using the protocol described above, rPSCs yields were about 0.5–1×107/g pancreas. The viability of freshly isolated PSCs, using trypan blue staining, was approximately 90%. In early culture, quiescent rPSCs had a small size and globular appearance and contained multiple lipid droplets (Fig. 1D), with blue-green autofluorescence at 320 nm (Fig. 1E). For rPSCs, most cells were attached to the culture vessel 24 hours after isolation. After about 72–96 hours, rPSCs underwent auto-activation process, the cells gained appearance of myofibroblasts withloss of lipid droplets in their cytoplasm (Fig. 1F). We have tested our modified method on 25 rats, the successful rate reached 92%, and was more than that of traditional methods (about 43%,P<0.01).
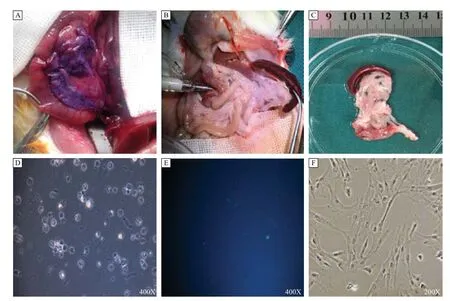
Fig. 1Isolation and culture of rPSCs. Rat pancreas was slowly infused with hematoxylin, the appearance was violet, and there was no leakage (A), and when infused with 5–6 mL enzyme solution through a sterile blunt cannula (B), the volume of the pancreas was twice than the original (C). The freshly isolated rPSCs were cultured in 24-well plates. After cells were adhered, quiescent rPSCs had globular appearance and contained multiple lipid droplets (D), with blue-green autofuorescence at 320 nm (E); after passage, the PSCs showed a myofbroblastic appearance (F). rPSCs: Rat pancreatic stellate cells.
Isolation and culture of CaPSCs
Five to ten days after human pancreatic cancer tissue blocks were seeded, CaPSCs could be observed to grow out from the block edges (Fig. 2A). Simultaneously, most of the tissue blocks remained adherent to plate, while there were more tissue blocks floating in the control plate. These cells showed an active state feature, with larger cell volume and loss of intracellular lipid droplets. After about 2–3 weeks, primary cells would reach 90% confluence and pass into next generation (Fig. 2B). With in vitro cell culture and subculture, CaPSCs could be further activated, with its appearance changed, including increased cell volume, a more apparent intracellular fibrous structure and more accelerated cell proliferation. In the control group without the plasma, the CaPSCs would need more than 2-weeks to grow out from the tissue blocks, usually taking 4–5 weeks for the first cell passage. We have tested our new method on 15 human tissues, the successful rates of our modified method for isolating human CaPSCs reached 93%, while the parallel traditional methods' successful rates was only 64% (P<0.01).
Expression of PSC markers α-SMA, vimentin, desmin and GFAP
Cells were assessed for positive PSCs markers: α-SMA, vimentin, desmin and GFAP.[18]Strong cytoplasmic positivity for vimentin and desmin was observed by immunofluorescence (Fig. 3A) and immunocytochemistry (Fig. 3B), while α-SMA and GFAP showed mild positive staining.
In order to exclude the potential contamination of epithelial cells or macrophages, CK19 and pan-CK (epithelial markers), and CD68 (macrophage marker) staining was also performed[22]. The PSCs isolated using our methods stained negatively for these markers (Fig. 3C, D).
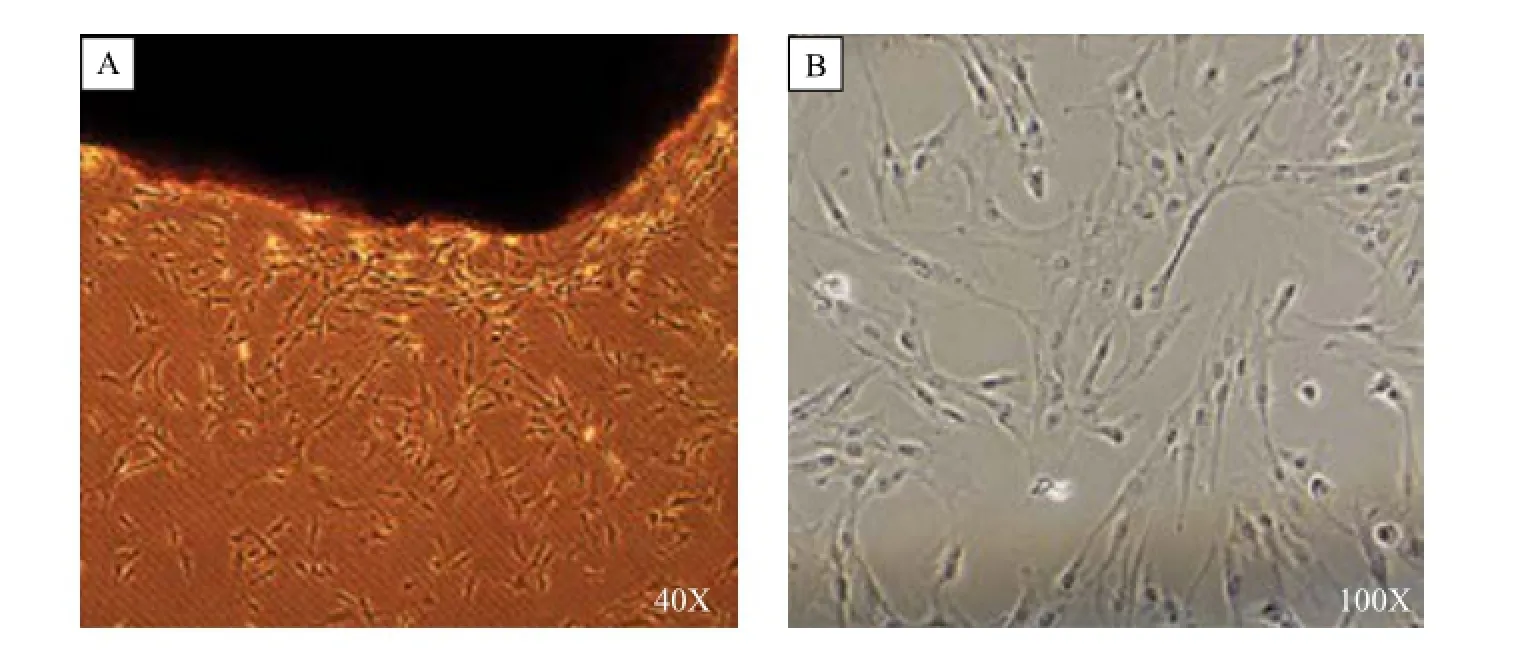
Fig. 2Isolation and culture of CaPSCs. Human pancreatic cancer tissue blocks were seeded in a 6-well plate and dropped around plasma, while the tissue blocks in the other 6-well plate were not dropped around plasma; after 5–10 days, cells grew out from the edges of tissue blocks (A), and about 2–3 weeks later, primary cells reached 90% confuence (B). CaPSCs: Cancer associated pancreatic stellate cells.
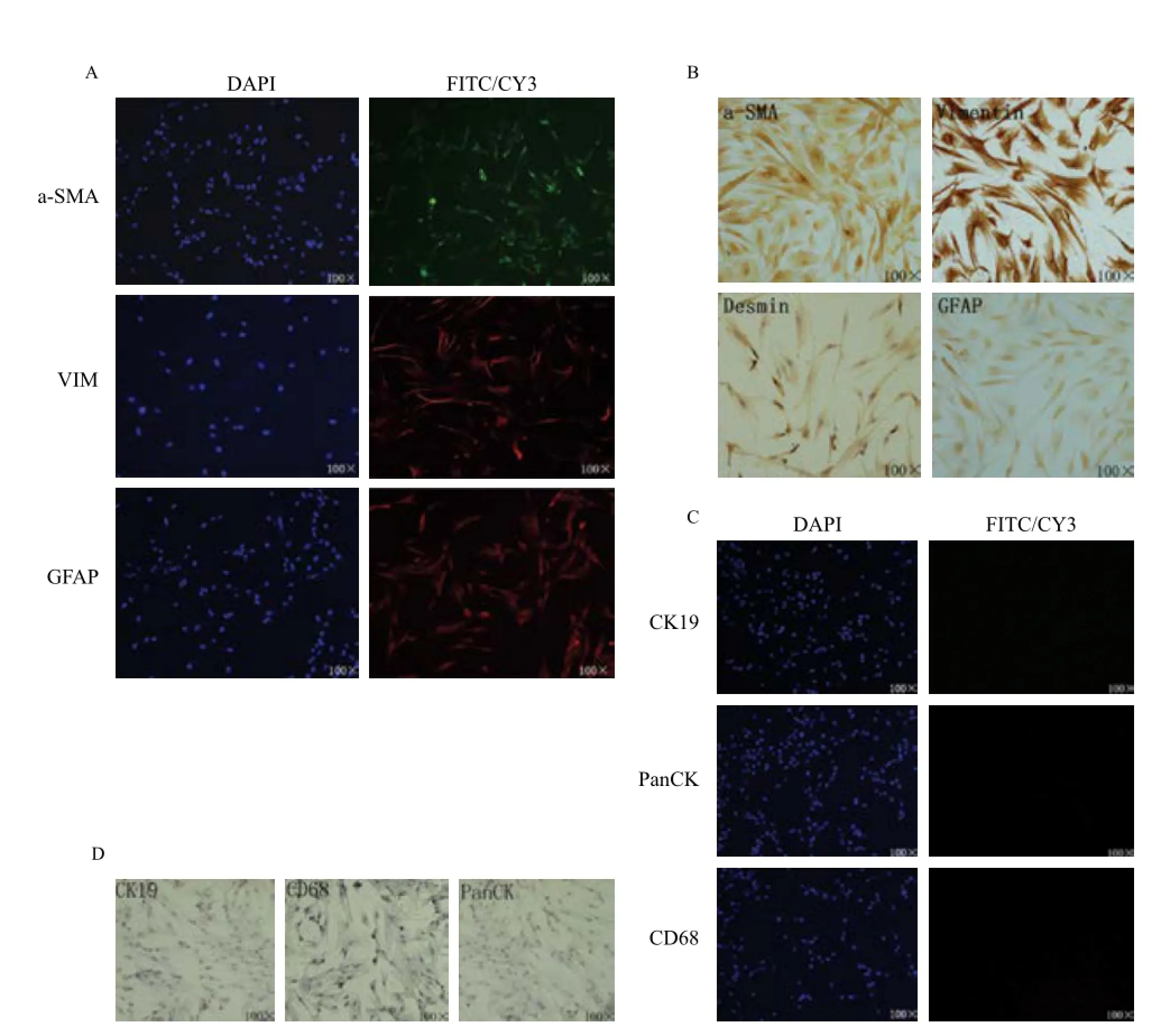
Fig. 3Expression of characteristic pancreatic stellate cell markers in isolated cells. A: Immunofuorescent staining of α-SMA, vimentin and GFAP; B: Immunocytochemistry staining of α-SMA, vimentin, desmin and GFAP; C: Immunofuorescent staining of CK19, Pan-CK and CD68; D: Immunocytochemistry staining of CK19, Pan-CK and CD68.
Discussion
The effective isolation and culture of PSCsin vitroandin vivois a key technological infrastructure necessary for the clarification of their role in the pathogenesis of CP and pancreatic cancer[23-24]. Although immortalised PSCs have been constructed and applied to current researches, individual differences remain a problem that cannot be ignored[25]. Therefore, primary isolation of PSCs will continue to serve an indispensable role in the field of PSC research[18].
The main method of isolating quiescent PSCs is via density gradient centrifugation with Nycodenz, Percoll, iohexol, and oriodixanol being the most common materials[15]. We chose Nycodenz for its low cytotoxicity and convenient preparation for gradient[26]. Reasonable digestion is the key point for the isolation of PSCs. Compared to previous studies, intraductal perfusion of the enzyme solution simplified the procedure and improved digestive conditions. Despite an increase in cold ischaemia time, intraductal perfusion resulted in a more even distribution of the digestion solution and adequate digestion of the pancreatic tissues. We also adjusted the concentration of digestive enzymes, shaking speed in water bath and digestion time. These improvements also resulted in decreased cell debris and significantly increased rPSCs yield. This indicates that suitable digestion is the key to the success of the enzymatic digestion-density gradient centrifugation method for isolating quiescent PSCs.
Explant techniques are mainly utilised for the isolation of activated state PSCs from CP or pancreatic tumours[27]. Compared to previous reports, the method used in our study (i.e. adding plasma) is simple and feasible. With the plasma coagulant, blocks firmly adhered to the culture plate, which significantly increased the yield of outgrowth cells. Plasma coagulant contains various growth factors, such as IL-2, IL-6, TGF-β and TNF-α, calcium and a fibrin three-dimensional skeleton structure, which provides an appropriate physical structure and physiological environment for PSCs to promote their maximal outgrowth[28]. With our improved method, and the time for cells to grow out was shortened from 2 weeks to 5–10 days. Moreover, the cells grow faster so that the time for first cell passage was shortened from 4–5 weeks to 2–3 weeks, while the phenotype of the cells did not have difference compared with the control cells. Nevertheless, in 2–5 generations, the cells are at the optimum state and provide an excellent basis for related experiments.
Primarily isolated PSCs, especially for cells isolated via the explant method, could inevitably be contaminated with other cells. Therefore, it is necessary to evaluate the purity of the isolat PSCs. Quiescent PSCs have as pecific morphology and structure, including large amounts of lipid droplets in the cytoplasm[17]. When subcultured, the PSCs were activated with a change in cell morphology and the lipid droplets disappeared[29]. Subsequently, isolated cells were immunostained by immunofluorescence and immunocytochemistry, and assessed for the presence of PSC markers, including α-SMA, vimentin, desmin and GFAP.[30]Strong cytoplasmic positivity for vimentin and vesmin were observed, while α-SMA and GFAP were moderately positive. We also evaluated the presence of epithelial markers (CK19, Pan-CK) and macrophage marker (CD68), which were all negatively immunostained, illustrating that no contamination with other types of cells had occurred.
However, due to the presence of dense stroma in human pancreatic tissue and individual heterogeneity, our method did not yield good results in isolating quiescent human PSCs; further studies are needed to improve the method.
In summary, this article reports effective modifications for the isolation of PSCs. Not only were the cell harvest and viability significantly increased, but cell purity and success rate were also ensured. This method is expected to benefit future in vitro PSC research.
Acknowledgments
This work was partially supported by the National Natural Science Foundation of China (81300351, 81272239, 81170336) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, JX10231801).
[1] Paulson AS, Tran Cao HS, Tempero MA, et al. Therapeutic advances in pancreatic cancer[J].Gastroenterology, 2013, 144: 1316-1326.
[2] Pandol S, Gukovskaya A, Edderkaoui M, et al. Epidemiology, risk factors, and the promotion of pancreatic cancer: role of the stellate cell[J].J Gastroenterol Hepatol, 2012, 27 Suppl 2: 127-134.
[3] Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis, the risk of pancreatic cancer. International Pancreatitis Study Group[J].N. Engl. J. Med, 1993, 328: 1433-1437.
[4] Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update[J].Dig Dis, 2010, 28: 645-656.
[5] Seymour AB, Hruban RH, Redston M, et al. Allelotype of pancreatic adenocarcinoma[J].Cancer Res, 1994, 54: 2761-2764.
[6] Whatcott CJ, Diep CH, Jiang P, et al. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer[J].Clin Cancer Res, 2015, 21: 3561-3568.
[7] Erkan M, Michalski CW, Rieder S, et al. The activated stroma index is a novel and independent prognostic markerin pancreatic ductal adenocarcinoma[J].Clin Gastroenterol Hepatol, 2008, 6: 1155-1161.
[8] Takikawa T, Masamune A, Hamada S, et al. miR-210 regulates the interaction between pancreatic cancer cells and stellatecells[J].Biochem Biophys Res Commun, 2013, 437: 433-439.
[9] Cabrera MC, Tilahun E, Nakles R, et al. Human Pancreatic Cancer-Associated Stellate Cells Remain Activated after in vivo Chemoradiation[J].Frontiers in oncology, 2014, 4: 102.
[10] McCarroll JA, Naim S, Sharbeen G, et al. Role of pancreatic stellate cells in chemoresistance in pancreatic cancer[J].Frontiers in physiology, 2014, 5: 141.
[11] Watt J, Kocher HM. The desmoplastic stroma of pancreatic cancer is a barrier to immune cell infiltration[J].Oncoimmunology, 2013, 2: e26788.
[12] Vonlaufen A, Phillips PA, Xu Z, et al. Pancreatic stellate cells and pancreatic cancer cells: anunholy alliance[J].Cancer Res, 2008, 68: 7707-7710.
[13] Hamada S, Masamune A, Takikawa T, et al. Pancreatic stellate cells enhance stem cell-like phenotypes in pancreatic cancer cells[J].Biochem Biophys Res Commun, 2012, 421: 349-354.
[14] Wu PF, Lu ZP, Cai BB, et al. Role of CXCL12/CXCR4 signaling axis in pancreatic cancer[J].Chin Med J (Engl), 2013, 126: 3371-3374.
[15] Apte MV, Haber PS, Applegate TL, et al. Periacinarstellate shaped cells in rat pancreas: identification, isolation, and culture[J].Gut, 1998, 43: 128-33.
[16] Erkan M, Adler G, Apte MV, et al. StellaTUM: current consensus and discussion on pancreatic stellate cell research[J].Gut, 2012, 61: 172-178.
[17] Bachem MG, Schneider E, Gross H, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans[J].Gastroenterology, 1998, 115: 421-432.
[18] Vonlaufen A, Phillips PA, Yang L, et al. Isolation of quiescent human pancreatic stellate cells: a promising in vitro tool for studies of human pancreatic stellate cell biology[J].Pancreatology, 2010, 10: 434-443.
[19] Apte MV, Xu Z, Pothula S, et al. Pancreatic cancer: The microenvironment needs attention too![J].Pancreatology, 2015, 15: S32-S38.
[20] Jesnowski R, Furst D, Ringel J, et al. Immortalization of pancreatic stellate cells as an in vitro model of pancreatic fibrosis: deactivation is induced by matrigel and N-acetylcysteine[J].Lab Invest, 2005, 85: 1276-1291.
[21] Rosendahl AH, Gundewar C, Said Hilmersson K, et al. Conditionally immortalized human pancreatic stellate cell lines demonstrate enhanced proliferation and migration in response to IGF-I[J].Exp Cell Res, 2015, 330: 300-310.
[22] Jaskiewicz K, Nalecz A, Rzepko R, et al. Immunocytes and activated stellate cells in pancreatic fibrogenesis[J].Pancreas, 2003, 26: 239-242.
[23] Pothula SP, Xu Z, Goldstein D, et al. Key role of pancreatic stellate cells in pancreatic cancer[J].Cancer Lett, 2015.
[24] Haqq J, Howells LM, Garcea G, et al. Pancreatic stellate cells and pancreas cancer: current perspectives and future strategies[J].Eur J Cancer, 2014, 50: 2570-2582.
[25] Sparmann G, Hohenadl C, Tornoe J, et al. Generation and characterization of immortalized rat pancreatic stellate cells[J].Am J Physiol Gastrointest Liver Physiol, 2004, 287: G211-G219.
[26] Watanabe S, Nagashio Y, Asaumi H, et al. Pressure activates rat pancreatic stellate cells[J].Am J Physiol Gastrointest Liver Physiol, 2004, 287: G1175-G1181.
[27] Kruse ML, Hildebrand PB, Timke C, et al. Isolation, longterm culture, and characterization of rat pancreatic fibroblastoid/stellate cells[J].Pancreas, 2001, 23(1): 49-54.
[28] Bao Y, Giovannucci EL, Kraft P, et al. Inflammatory plasma markers and pancreatic cancer risk: a prospective study of five U.S. cohorts[J].Cancer Epidemiol Biomarkers Prev, 2013, 22: 855-861.
[29] Mathison A, Liebl A, Bharucha J, et al. Pancreatic stellate cell models for transcriptional studies of desmoplasiaassociated genes[J].Pancreatology, 2010, 10: 505-516.
[30] Wehr AY, Furth EE, Sangar V, et al. Analysis of the human pancreatic stellate cell secreted proteome[J].Pancreas, 2011, 40: 557-566.
?Corresponding author: Professor Yi Miao, Pancreas Center, The First Affliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, Jiangsu 210029, China. E-mail: miaoyi@njmu.edu.cn.
? 2016 by the Journal of Biomedical Research. All rights reserved.
Received 10 March 2016, Revised 27 April 2016, Accepted 18 May 2016, Epub 15 June 2016
R576, Document code: A.
The authors reported no confict of interests.
10.7555/JBR.30.20160033
 THE JOURNAL OF BIOMEDICAL RESEARCH2016年6期
THE JOURNAL OF BIOMEDICAL RESEARCH2016年6期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Pathology and molecular characterization of recent Leucocytozoon caulleryi cases in layer f l ocks
- Retrograde traff i cking of VMAT2 and its role in protein stability in non-neuronal cells
- Protein inhibitor of activated STAT 4(PIAS4)regulates liver fi brosis through modulating SMAD3 activity
- Chronic intermittent hypoxia induces cardiac inf l ammation and dysfunction in a rat obstructive sleep apnea model
- Assessment of atrial electromechanical interval using echocardiography after catheter ablation in patients with persistent atrial f i brillation
- Elevated thyroid stimulating hormone levels are associated with metabolic syndrome in a Chinese community-based population of euthyroid people aged 40 years and older
