Optimal concentration and time window for proliferation and diff erentiation of neural stem cells from embryonic cerebral cortex: 5% oxygen preconditioning for 72 hours
Li-li Yuan, Ying-jun Guan, Deng-dian Ma, Hong-mei Du
1 Department of Histology and Embryology, Academy of Basic Medicine, Jining Medical University, Jining, Shandong Province, China
2 Department of Histology and Embryology, Academy of Basic Medicine, Weifang Medical University, Weifang, Shandong Province, China
3 Department of Otorhinolaryngology, Affi liated Hospital of Jining Medical University, Jining, Shandong Province, China
Optimal concentration and time window for proliferation and diff erentiation of neural stem cells from embryonic cerebral cortex: 5% oxygen preconditioning for 72 hours
Li-li Yuan1,2,*, Ying-jun Guan2,*, Deng-dian Ma3, Hong-mei Du2
1 Department of Histology and Embryology, Academy of Basic Medicine, Jining Medical University, Jining, Shandong Province, China
2 Department of Histology and Embryology, Academy of Basic Medicine, Weifang Medical University, Weifang, Shandong Province, China
3 Department of Otorhinolaryngology, Affi liated Hospital of Jining Medical University, Jining, Shandong Province, China
Hypoxia promotes proliferation and diff erentiation of neural stem cells from embryonic day 12 rat brain tissue, but the concentration and time of hypoxic preconditioning are controversial. To address this, we cultured neural stem cells isolated from embryonic day 14 rat cerebral cortex in 5% and 10% oxygen in vitro. MTT assay, neurosphere number, and immunofl uorescent staining found that 5% or 10% oxygen preconditioning for 72 hours improved neural stem cell viability and proliferation. With prolonged hypoxic duration (120 hours), the proportion of apoptotic cells increased. Thus, 5% oxygen preconditioning for 72 hours promotes neural stem cell proliferation and neuronal diff erentiation. Our fi ndings indicate that the optimal concentration and duration of hypoxic preconditioning for promoting proliferation and diff erentiation of neural stem cells from the cerebral cortex are 5% oxygen for 72 hours.
nerve regeneration; brain injury; neural stem cells; low oxygen; cerebral cortex; apoptosis; diff erentiation; microtubule-associated protein 2; glial fi brillary acidic protein; caspase-3; neural regeneration
Funding: This work was supported by the Science Foundation of Jining Science and Technology Bureau of China, No. 2012jnjc07.
Yuan LL, Guan YJ, Ma DD, Du HM (2015) Optimal concentration and time window for proliferation and diff erentiation of neural stem cells from embryonic cerebral cortex: 5% oxygen preconditioning for 72 hours. Neural Regen Res 10(9):1516-1522.
Introduction
Stem cells in the central nervous system are defi ned by the characteristics of self-renewal and diff erentiation into neurons, astrocytes (Gage and Temple, 2013), and oligodendrocytes (Zawadzka et al., 2010). The fate of central nervous system stem cells to specific cell lineages can be changed by a single extrinsic factor (Guerout et al., 2014). Central nervous system stem cells that are used in the treatment of common degenerative and ischemic diseases have become a major research focus (Cheng et al., 2015).
Recently, the role of oxygen in proliferation and diff erentiation of neural stem cells (NSCs) has been examined (Xiong et al., 2013). Low oxygen condition promotes survival and proliferation of NSCs from the mouse ganglionic eminence (Horie et al., 2008). However, controversy remains surrounding the proper length of time and concentration of lowered oxygen preconditioning for improved NSC diff erentiation. There are many NSCs in the cerebral cortex (Borrell and Reillo, 2012), yet little research exists concerning the eff ect of low oxygen concentration in vitro on NSCs derived from the cerebral cortex of embryonic rat. In this study, NSCs were isolated from the cerebral cortex of embryonic day 14 (E14) Sprague-Dawley rats, and cultured in vitro. Our aim was to examine the infl uence of low oxygen concentration and preconditioning time on cultured NSCs by comparing with normoxia, to provide a tool for NSC transplantation in the treatment of hypoxic-ischemic brain injury and degenerative diseases in the central nervous system.
Materials and Methods
Isolation and culture of NSCs
A total of 60 adult Sprague-Dawley rats, aged 3–6 months and weighing 220–250 g with a male to female ratio of 1:2, were housed and allowed free access to food and water under a 12-hour light/dark cycle, in accordance with the institutional guidelines on the care and handling of experimental animals (License No. SCXK (Lu) 20130001). Male and female rats were placed in the same cage at 8 p.m. E0 day was defi ned when sperm was found in the vagina of a female rat. E14 rat cerebral cortex was isolated for cell subculture. All
protocols were approved by the Institutional Animal Care and Use Committee of Jining Medical University, China and performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Proliferation and diff erentiation of NSCs
After incubation in trypsin, cells dissected from E14 rat cerebral cortices were mechanically dissociated, plated at a density of 5 × 105/mL into 5 mL cell culture dishes in serum-free medium containing Dulbecco’s modifi ed Eagle’s medium/ F12 medium (Hyclone, Logan, UT, USA), 20 ng/mL of epidermal growth factor and basic fi broblast growth factor (Sigma, St. Louis, MO, USA), and 2% B27 (Gibco BRL, Ground Island, NY, USA), then placed into a saturated humidity incubator (Heraeus, Hanau, Germany) at 37°C with 5% CO2. The medium was fi rst changed after 24 hours, then every other day. After culturing for 7 days, cells were passaged by mechanical beating with a glass pipette, trypsin digested, and reseeded as single cells at 5 × 105/mL in serum-free medium (fi rst passage cells). Cells were passaged three times according to the above method. Third passage cells were processed for subsequent experiments.
Passage 3 neurosphere cell suspensions were centrifuged and the supernatant discarded. Next, 10% fetal bovine serum was added to plastic 6-well plates with poly-L-lysine-coated coverslips. Cells were treated with 50 mg/L 5-bromo-2′-deoxyuridine (BrdU) (Boster, Wuhan, China) and cultured in a saturated humidity incubator at 37°C with 5% CO2for 6 hours. Cells mounted on coverslips were fi xed for 10 minutes using cold acetone, and nestin (Chemicon, Billerica, MA, USA) and BrdU (Accurate Chemical, Westbury, NY, USA) double immunofl uorescence staining performed. The remaining slides were cultured for 7 days in medium containing serum, then fi xed and prepared for immunofl uorescence staining using microtubule associated protein-2 (MAP2) and glial fi brillary acidic protein (GFAP) (Chemicon). Slides were observed by inverted fl uorescence microscopy (Leica, Wetzlar, Germany).
Hypoxic preconditioning
Third passage NSCs derived from E14 rat cerebral cortex were first cultured in serum-free medium for 7 days, including treatment with 5% O2for 72 or 120 hours then 20% O2, or with 10% O2for 72 or 120 hours then 20% O2. All groups were cultured in medium containing serum at 20% O2. A normal control group (always 20% O2) was also set up. Thus, NSCs were divided into groups of 5% O272-hours, 5% O2120-hours, 10% O272-hours, 10% O2120-hours, and a normal control, according to low oxygen concentration and intervention time. In low oxygen conditions, NSCs were housed in a tri-gas incubator (Heraeus,) at 37°C with 5% CO2, with diff erent oxygen concentrations set using nitrogen gas. In the normal control group, cells were placed in an incubator at normal humidity (i.e., 37°C) with 5% CO2. Immunofluorescent analysis of NSCs was performed using a laser scanning confocal microscope (Hitachi, Tokyo, Japan).
Electron microscopy analysis of NSCs
After culturing for 7 days in serum-free medium, neurospheres from each group were collected separately and centrifuged at 1,500 × g for 15 minutes. After supernatant removal, neurospheres were fixed in 3% glutaraldehyde and 1% osmium tetroxide, dehydrated, resin embedded, and then sliced into ultrathin sections. Ultrathin sections were double-stained using uranyl acetate and lead citrate, and imaged with a transmission electron microscope (Hitachi).
3-(4,5-Cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay for NSC viability
Single cell suspensions of passage 3 NSCs were centrifuged, diluted to 1 × 105/mL, and seeded into 96-well plates. In each plate, each group used four wells with a blank control group also. The wells were fi lled with 200 μL serum-free suspension and the NSCs were observed every day. After 7 days, NSCs were divided into two portions, with one portion used for the MTT assay and the other for neurosphere counting. Optical density values of NSCs were measured by MTT assay. MTT (5 g/L, 20 μL) was added to each well (of 96-well plates) and NSCs placed in an incubator of saturated humidity (37°C) at 5% CO2for 4 hours, then centrifuged and collected. Each well was treated with 150 μL dimethyl sulfoxide and oscillated for 10 minutes. Finally, optical density values were automatically detected at 490 nm using a microplate reader (Thermo Electric, Shanghai, China).
Neurosphere counting
Neurospheres were observed using an inverted microscope at 200× magnifi cation. The neurosphere number in each group was counted. Eight fi elds from each well (of 96-well plates) were randomly selected and four wells from each group were counted. The average number of neurospheres in each group was determined.
Apoptosis and diff erentiation of NSCs
In low-oxygen conditions, neurospheres were cultured in serum-free medium for 7 days, then centrifuged and the supernatant was removed. Next, neurospheres were resuspended in medium containing serum at a density of 0.5–1 × 105/mL and cultured in 24-well plates with poly-L-lysine-coated coverslips. The diff erentiation and fi xation methods were the same as before. After diff erentiation for 4 hours, four coverslips from each group were randomly selected and fi xed for immunofluorescent staining of rabbit anti-caspase-3. Cell numbers of positive staining and Hoechst staining were counted using an inverted phase contrast microscope at 200× magnifi cation. Five random fi elds from each coverslip were counted. After culturing for a further 7 days in medium containing serum, the remaining cells were used for immunofl uorescent staining of MAP2. The observation index and MAP2 calculation method were the same as for caspase-3. All counts were performed by observers blinded to the experimental conditions.
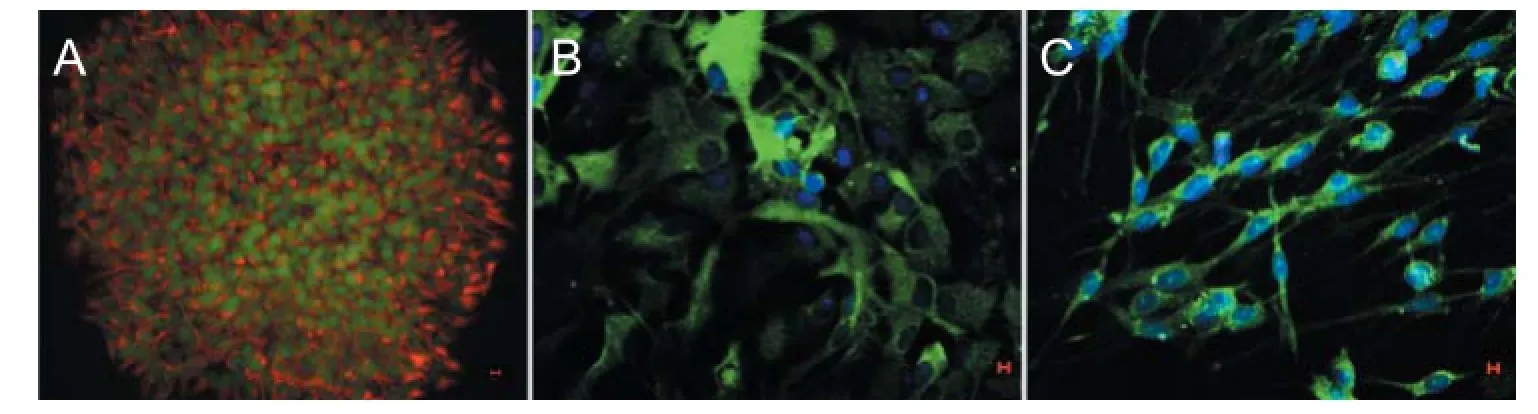
Figure 1 Expression of markers related to neural stem cell proliferation and diff erentiation (immunofl uorescent staining, laser scanning confocal microscope).
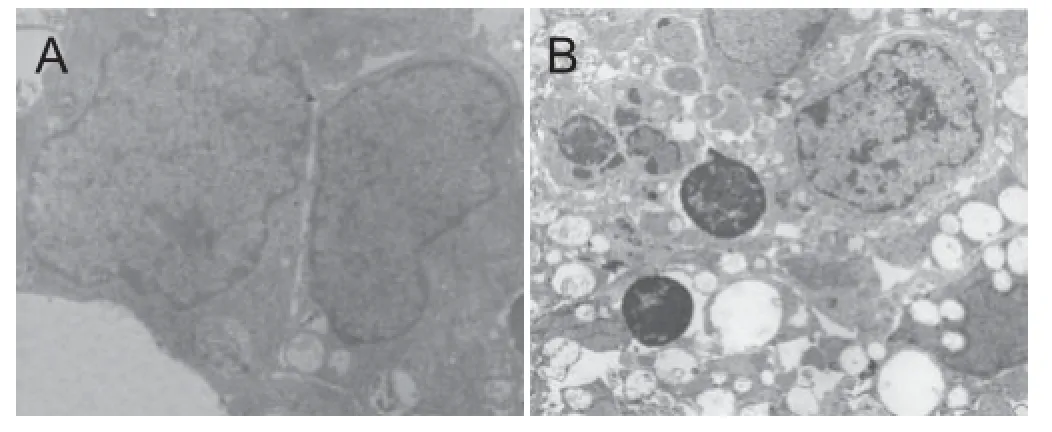
Figure 2 Infl uence of hypoxic preconditioning on the ultrastructure of neural stem cells from embryonic rat cerebral cortex (uranyl acetate and lead citrate double staining, transmission electron microscope).
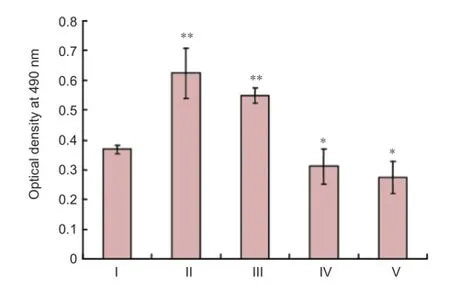
Figure 3 Eff ect of low oxygen on neural stem cell viability (MTT assay).
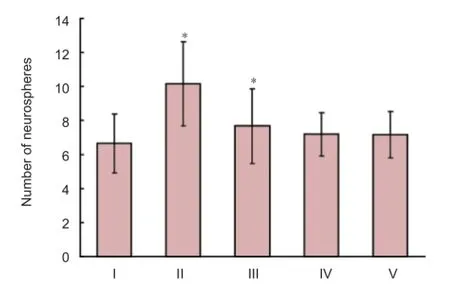
Figure 4 Distinct promotion of neural stem cell proliferation in the low oxygen 72-hour group as shown by neurosphere number.
Immunofl uorescent staining
Fixed cells on coverslips were washed three times for 5 minutes in PBS containing 0.1% Triton X-100, followed by 2 M HCl incubation for 30 minutes at 37°C. After three PBS washes (this step was only used to detect BrdU), sheep serum was added to the coverslips for 30 minutes at room temperature. After serum removal, mixed antibodies composed of mouse anti-nestin monoclonal antibody (1:200; Chemicon) and mouse anti-BrdU polyclonal antibody (1:200; Accurate Chemical), or rabbit anti-caspase-3 monoclonal antibody (1:100; Boster) were added to neurospheres on coverslips; or mouse anti-MAP2 polyclonal antibody (1:200; Chemicon) or mouse anti-GFAP polyclonal antibody (1:400; Chemicon) was added to diff erentiated cells on coverslips, then incubated overnight at 4°C. Coverslips were washed three times with PBS, and the cells were incubated with the corresponding fl uorescent secondary antibody: Cy3 (sheep anti-mouse IgG; 1:80) or FITC (sheep anti-rat IgG; 1:200) (Sigma), or a mixture of both, for 60 minutes at 37°C in the dark. After three PBS washes, Hoechst 33258 (10 μg/mL)
was added for 30 minutes at 37°C in the dark, followed by a further three PBS washes. Images were obtained and the number of positive cells was counted using a confocal laser scanning microscope (Zeiss, Jena, Germany) or fl uorescence microscope (Leica, Solms, Germany). The calculation method was the same as before.
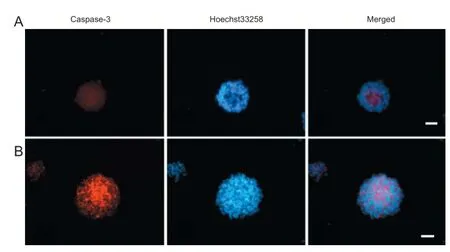
Figure 5 Eff ect of hypoxic preconditioning on neural stem cell apoptosis (immunofl uorescent staining, fl uorescence microscopy).
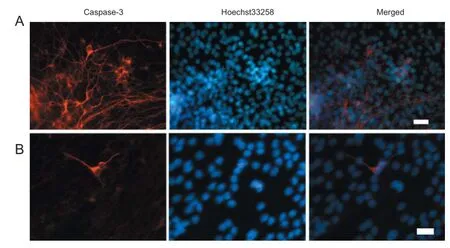
Figure 6 Infl uence of 5% O2preconditioning on neural stem cell diff erentiation (immunofl uorescent staining, fl uorescence microscopy).
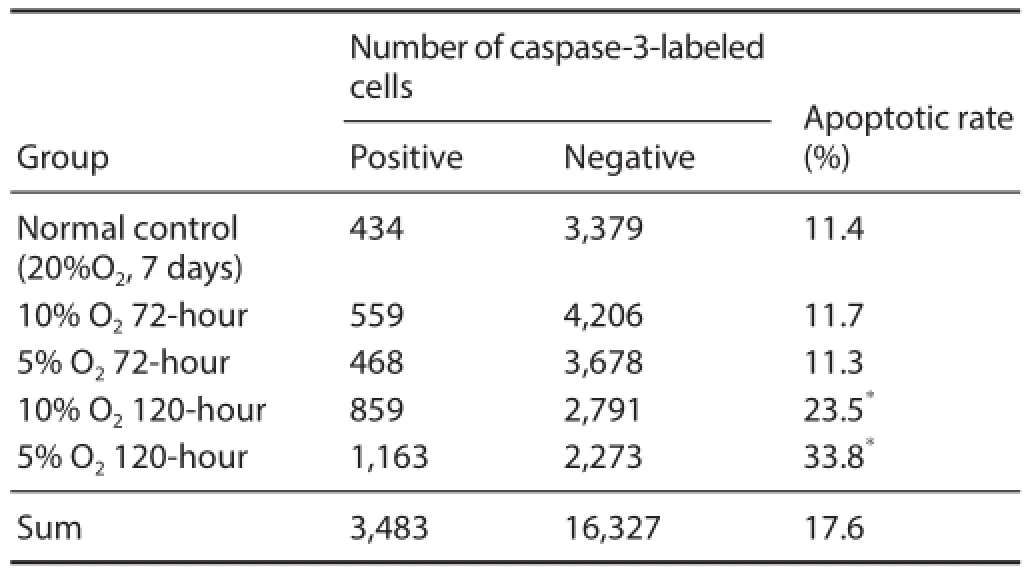
Table 1 Influence of hypoxic preconditioning on neural stem cell apoptosis as detected by immunofl uorescent caspase-3 staining
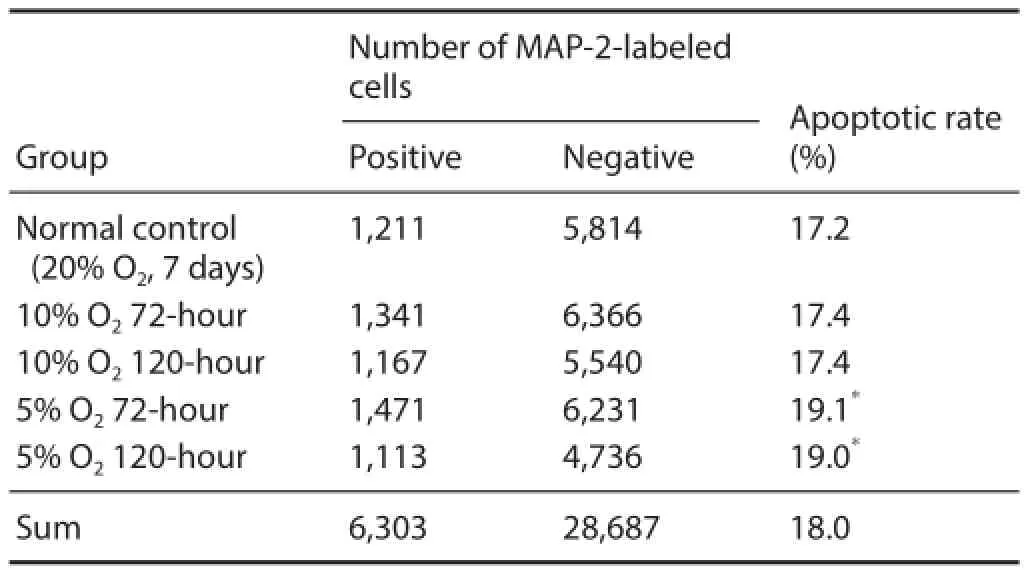
Table 2 Eff ect of hypoxic preconditioning on neuronal diff erentiation of neural stem cells as detected by immunofl uorescent MAP2 staining
Statistical analysis
Statistical analysis was performed using SPSS 11.7 software (SPSS, Chicago, IL, USA). The measurement data results were expressed as the mean ± SD. Comparisons of measurement data between groups were performed using Student’s t-test. Numeration data were expressed as percentages. Comparison of numeration data between groups was performed by chi-square test (α = 0.05).
Results
Characteristics of NSCs from embryonic rat cerebral cortex
Cells isolated from embryonic rat cerebral cortex were cultured in vitro and grown to neurospheres suspended in growth medium. Immunofl uorescent staining (Figure 1A) showed that all cells within neurospheres were nestin/BrdU double positive cells. After culturing in medium containing serum for 7 days, neurosphere-derived cells were positive for GFAP or MAP2 (Figure 1B, C). These results indicate that cells derived from the cerebral cortex can be regarded as NSCs.
Eff ect of hypoxic preconditioning on the ultrastructure of NSCs derived from embryonic rat cerebral cortex
After culturing for 7 days in growth medium, neurospheres were collected from each group for electron microscopy. Pyknosis and condensed chromatin near the nuclear membrane were distinct in the 5% O2120-hour group (Figure 2). In contrast, only modest changes in the morphological signs of apoptosis were detected in cells in the other low oxygen groups and normal control group.
Eff ect of hypoxic preconditioning on viability of NSCs from embryonic rat cerebral cortex
After culturing in growth medium for 7 days, NSC viability was determined by MTT assay. Signifi cantly higher optical density values were observed in NSCs from the 10% O272-hour and 5% O272-hour groups compared with the normal control group (20% O2, 7 days) (P < 0.01; Figure 3). In contrast, signifi cantly lower optical density values were observed in the 10% O2120-hour and 5% O2120-hour groups compared with the normal control group (P < 0.05).
Infl uence of low oxygen on proliferation of NSCs from embryonic rat cerebral cortex
The average number of neurospheres in each group was calculated, and was signifi cantly higher in the low O272-hour group compared with the normal control group (P < 0.05; Figure 4).
Low oxygen treatment for 120 hours promoted NSC apoptosis
After culturing for 4 hours in medium containing serum, neurospheres were fi xed and caspase-3 immunofl uorescent staining was performed. Caspase-3-immunoreactive cells were mainly located in neurosphere centers (Figure 5). Moreover, the rate of caspase-3-immunoreactive cells was signifi cantly higher in the 10% O2120-hour and 5% O2120-hour groups compared with the normal control group (P <0.05; Table 1).
5% O2treatment enhanced NSC diff erentiation into neurons
After culturing in medium containing serum for 7 days, immunofl uorescent staining of NSCs was performed. A signifi cantly higher proportion of MAP2-immunoreactive cells was observed in the 5% O272-hour and 120-hour groups compared with the normal control group (P < 0.05; Figure 6 and Table 2).
Discussion
Here, we have investigated the impact of lower oxygen levels than the 20% traditionally used to culture NSCs in vitro. Our major fi ndings on NSCs derived from E14 rat cerebral cortex are: (1) hypoxic preconditioning for 72 hours enhances NSC viability and proliferation; (2) neuronal differentiation of NSCs is notably promoted with 5% O2preconditioning; and (3) after hypoxic preconditioning for 120 hours, the apoptotic rate of NSCs is strikingly elevated.
In previous studies addressing the eff ect of low oxygen on NSCs in vitro, the cells were isolated from the adult subependyma, E14 ganglionic eminence, and mesencephalon (Horie et al., 2008, Vernon et al., 2011). However, there are known NSCs in the cerebral cortex (Tao et al., 2006; Nakagomi et al., 2009), and the cells we used were derived from embryonic rat cerebral cortex (Shen and Zhang, 2003). Immunofl uorescent staining for nestin, BrdU, MAP-2, and GFAP demonstrated that our in vitro cultured cells are NSCs.
Proliferation and diff erentiation of NSCs are a complicated process regulated by many internal and external factors (Yang et al., 2003; Zechel et al., 2007; Kim et al., 2015), and its exact control mechanisms are not clear. Change in oxygen concentration can aff ect biological characteristics of NSCs, including proliferation and diff erentiation (Horie et al., 2008). In previous studies, low oxygen concentration levels of mean tissue levels in vivo were examined (Hall et al., 1996). To follow a similar physiological environment, NSCs were cultured in vitro in decreased oxygen levels (5% oxygen), which are closer to physiological oxygen levels. The other oxygen level (10% O2) was also used in the experimental groups, while 20% oxygen was used after hypoxic preconditioning.
Compared with the normal control group (20% O2), in the low O272-hour group after culturing for 7 days in serum-free medium, the neurosphere number increased notably, and may be due to increased viability and proliferation. This is consistent with previous studies (Studer et al., 2000;
Clarke and van der Kooy, 2009). Proliferation of NSCs increased with hypoxic preconditioning in culture, partially by inhibition of caspase-dependent apoptosis. A previous study found that culture in low (4%) oxygen promotes survival of primitive NSCs by inhibiting apoptosis-inducing factor-dependent cell death, yet primitive NSCs undergo both apoptosis-inducing factor- and caspase-mediated cell death in 20% oxygen (Clarke and van der Kooy, 2009). In contrast, NSC survival in low oxygen is increased by inhibition of caspase-dependent cell death (Clarke and van der Kooy, 2009).
Moreover, we demonstrated that continually sustained lowered oxygen conditions (120 hours) are not benefi cial during in vitro culture of embryonic rat cortical NSCs. Neurosphere number and NSC viability did not increase signifi cantly between the low O2120-hour and normal control groups. We also found visibly lower optical density values after low oxygen preconditioning for 120 hours compared with the normal control group. These results indicate that stem cell viability decreases with lowered oxygen preconditioning for 120 hours. Furthermore, in the low oxygen 120-hour group, the viability and number of neurospheres decreased, while in some neurosphere cells (after culturing NSCs in serum-free medium for 7 days including in 5% or 10% oxygen for 120 hours, then in 20% oxygen for 2 days), electron microscopy showed pyknosis and condensed chromatin near the nuclear membranes. Therefore, NSC apoptosis was detected by more than one method. Caspase-3 is important for apoptosis during cerebral ischemia and hypoxia (Lackner et al., 2007; Xu et al., 2007; Rodrigues et al., 2010). The high proportion of caspase-3-positive immunofl uorescent cells in the 5% or 10% O2120-hour groups suggests that NSC apoptosis is promoted by continual hypoxic preconditioning (120 hours). Our NSC culture procedure is not exactly the same as the previous study, which cultured NSCs in consistently low (4%) oxygen (or another low oxygen concentration) without 20% oxygen treatment. In our study, with culture of the low oxygen 120-hour group there are many possible mechanisms for inducing apoptosis: caspase-3 may act as an apoptotic agent and be more expressed; or erythropoietin may act as an anti-apoptotic agent or anti-oxidant and be less expressed, although we did not directly test the latter. Oxidative activity may be conducive to apoptosis in the low oxygen 120-hour group after restoring cultures to normal oxygen (20%) conditions. With low oxygen preconditioning for 120 hours, nitric oxide may cause NSC apoptosis by a p38 mitogen-activated protein kinase-dependent mechanism (Cheng et al., 2001; Yang et al., 2005; Song et al., 2013, 2014).
Low oxygen not only maintains self-renewal states of NSCs, but also aff ects cell-fate diff erentiation. Some studies have shown that low oxygen promotes neuronal diff erentiation of NSCs (Zhao et al., 2007; Bai et al., 2008). Our MAP2 immunofl uorescent staining results show an increased proportion of neurons with 5% oxygen preconditioning during diff erentiation of NSCs derived from embryonic rat cortex. In our study, lowered oxygen preconditioning of NSCs was only present in the growth culture. Thus, the 5% oxygen conditions may enhance neuronal production by a mechanism that acts before neuronal diff erentiation. The Wnt signaling pathway, especially the Wnt/β-catenin signaling pathway, plays an essential role in neuronal specifi cation of NSCs (Haegele et al., 2003; Cui et al., 2011; Zhang et al., 2013; Bengoa-Vergniory et al., 2014). In the 5% O2group cultures, the Wnt signaling pathway may be activated and contribute to neuronal diff erentiation of NSCs, although we did not directly test this.
Altogether, hypoxic preconditioning with diff erent oxygen concentrations and times has diff erential eff ects on the proliferation, apoptosis, and neuronal differentiation of in vitro NSCs derived from the cerebral cortex of E14 rats. Accordingly, 5% O2preconditioning for 72 hours not only promotes NSC proliferation but also contributes to their neuronal diff erentiation. With prolonged time (120 hours) of hypoxic preconditioning, NSCs distinctly become apoptotic. This study provides basic technology research for culturing NSCs in vitro before cell transplantation in treatment of central nervous system diseases.
Acknowledgments: We are very grateful to Professor You-hua Kong and Bao-hua Cheng from Jining Medical University in China for pre-reviewing this paper.
Author contributions: YJG and LLY designed the study. LLY and HMD performed experiments. DDM and LLY participated in the immunofluerence measurements and statistical analysis. LLY wrote the paper. All authors approved the fi nal version of the paper.
Confl icts of interest: None declared.
Bai J, Luan Z, Zhou CL, Qu SQ, Jiang Y, Wang ZY (2008) Effect of hyperbaric oxygenation on the diff erentiation of implanted human neural stem cells into neurons in vivo. Zhongguo Dang Dai Er Ke Za Zhi 10:195-198.
Bengoa-Vergniory N, Gorrono-Etxebarria I, Gonzalez-Salazar I, Kypta RM (2014) A switch from canonical to noncanonical Wnt signaling mediates early diff erentiation of human neural stem cells. Stem Cells 32:3196-3208.
Borrell V, Reillo I (2012) Emerging roles of neural stem cells in cerebral cortex development and evolution. Dev Neurol 72:955-971.
Cheng A, Chan SL, Milhavet O, Wang S, Mattson MP (2001) p38 MAP kinase mediates nitric oxide-induced apoptosis of neural progenitor cells. J Biol Chem 276:43320-43327.
Cheng Y, Zhang J, Deng L, Johnson NR, Yu X, Zhang N, Lou T, Zhang Y, Wei X, Chen Z, He S, Li X, Xiao J (2015) Intravenously delivered neural stem cells migrate into ischemic brain, diff erentiate and improve functional recovery after transient ischemic stroke in adult rats. Int J Clin Exp Pathol 8:2928-2936.
Clarke L, van der Kooy D (2009) Low oxygen enhances primitive and defi nitive neural stem cell colony formation by inhibiting distinct cell death pathways. Stem Cells 27:1879-1886.
Cui XP, Xing Y, Chen JM, Dong SW, Ying DJ, Yew DT (2011) Wnt/ beta-catenin is involved in the proliferation of hippocampal neural stem cells induced by hypoxia. Ir J Med Sci 180:387-393.
Gage FH, Temple S (2013) Neural stem cells: generating and regenerating the brain. Neuron 80:588-601.
Guerout N, Li X, Barnabe-Heider F (2014) Cell fate control in the developing central nervous system. Exp Cell Res 321:77-83.
Haegele L, Ingold B, Naumann H, Tabatabai G, Ledermann B, Brandner S (2003) Wnt signalling inhibits neural diff erentiation of embryonic stem cells by controlling bone morphogenetic protein expression. Mol Cell Neurosci 24:696-708.
Hall JE, Guyton AC, Brands MW (1996) Pressure-volume regulation in hypertension. Kidney Int Suppl 55:S35-41.
Horie N, So K, Moriya T, Kitagawa N, Tsutsumi K, Nagata I, Shinohara K (2008) Eff ects of oxygen concentration on the proliferation and diff erentiation of mouse neural stem cells in vitro. Cell Mol Neurobiol 28:833-845.
Kim BJ, Lee YA, Kim KJ, Kim YH, Jung MS, Ha SJ, Kang HG, Jung SE, Kim BG, Choi YR, Do JT, Ryu BY (2015) Eff ects of paracrine factors on CD24 expression and neural diff erentiation of male germline stem cells. Int J Mol Med 36:255-262.
Lackner P, Burger C, Pfaller K, Heussler V, Helbok R, Morandell M, Broessner G, Tannich E, Schmutzhard E, Beer R (2007) Apoptosis in experimental cerebral malaria: spatial profi le of cleaved caspase-3 and ultrastructural alterations in diff erent disease stages. Neuropathol Appl Neurobiol 33:560-571.
Nakagomi T, Taguchi A, Fujimori Y, Saino O, Nakano-Doi A, Kubo S, Gotoh A, Soma T, Yoshikawa H, Nishizaki T, Nakagomi N, Stern DM, Matsuyama T (2009) Isolation and characterization of neural stem/ progenitor cells from post-stroke cerebral cortex in mice. Eur J Neurosci 29:1842-1852.
Rodrigues CA, Diogo MM, da Silva CL, Cabral JM (2010) Hypoxia enhances proliferation of mouse embryonic stem cell-derived neural stem cells. Biotechnol Bioeng 106:260-270.
Shen LH, Zhang JT (2003) Culture of neural stem cells from cerebral cortex of rat embryo and eff ects of drugs on the proliferation ability of stem cells. Yao Xue Xue Bao 38:735-738.
Song J, Cho KJ, Cheon SY, Kim SH, Park KA, Lee WT, Lee JE (2013) Apoptosis signal-regulating kinase 1 (ASK1) is linked to neural stem cell diff erentiation after ischemic brain injury. Exp Mol Med 45:e69. Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R (2000) Enhanced proliferation, survival, and dopaminergic diff erentiation of CNS precursors in lowered oxygen. J Neurosci 20:7377-7383.
Tao K, Chen J, Wang G, Shu X (2006) Culture and identification of monoclonal neural stem cells derived from cerebral cortex. J Huazhong Univ Sci Technolog Med Sci 26:451-454.
Vernon AC, Smith EJ, Stevanato L, Modo M (2011) Selective activation of metabotropic glutamate receptor 7 induces inhibition of cellular proliferation and promotes astrocyte diff erentiation of ventral mesencephalon human neural stem/progenitor cells. Neurochem Int 59:421-431.
Xiong YJ, Yin B, Xiao LC, Wang Q, Gan L, Zhang YC, Zhang SM (2013) Proliferation and differentiation of neural stem cells co-cultured with cerebral microvascular endothelial cells after oxygen-glucose deprivation. J Huazhong Univ Sci Technolog Med Sci 33:63-68.
Xu Q, Wang S, Jiang X, Zhao Y, Gao M, Zhang Y, Wang X, Tano K, Kanehara M, Zhang W, Ishida T (2007) Hypoxia-induced astrocytes promote the migration of neural progenitor cells via vascular endothelial factor, stem cell factor, stromal-derived factor-1alpha and monocyte chemoattractant protein-1 upregulation in vitro. Clin Exp Pharmacol Physiol 34:624-631.
Yang M, Donaldson AE, Jiang Y, Iacovitti L (2003) Factors infl uencing the differentiation of dopaminergic traits in transplanted neural stem cells. Cell Mol Neurobiol 23:851-864.
Yang SR, Cho SD, Ahn NS, Jung JW, Park JS, Jo EH, Hwang JW, Kim SH, Lee BH, Kang KS, Lee YS (2005) The role of p38 MAP kinase and c-Jun N-terminal protein kinase signaling in the diff erentiation and apoptosis of immortalized neural stem cells. Mutat Res 579:47-57.
Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, Rowitch DH, Kessaris N, Suter U, Richardson WD, Franklin RJ (2010) CNS-resident glial progenitor/ stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 6:578-590.
Zechel C, Moeckel S, Stoerchel P, Pawlak E, Tronnier V (2007) Infl uence of growth factors, neurotrophic factors and proteins of the extracellular matrix on expansion and diff erentiation of adult neural stem cells. J Stem Cells Regen Med 2:198-199.
Zhang X, Chen L, Wang Y, Ding Y, Peng Z, Duan L, Ju G, Ren Y, Wang X (2013) Macrophage migration inhibitory factor promotes proliferation and neuronal diff erentiation of neural stem/precursor cells through Wnt/beta-catenin signal pathway. Int J Biol Sci 9:1108-1120. Zhao T, Zhang CP, Zhu LL, Jin B, Huang X, Fan M (2007) Hypoxia promotes the diff erentiation of neural stem cells into dopaminergic neurons. Sheng Li Xue Bao 59:273-277.
Copyedited by James R, Raye W, Wang J, Qiu Y, Li CH, Song LP, Zhao M
*Correspondence to:
Li-li Yuan or Ying-jun Guan, Ph.D., liliyuan06@126.com or
guanyj@wfmc.edu.cn.
orcid:
0000-0002-3689-7847 (Li-li Yuan)
0000-0002-9578-0497 (Ying-jun Guan)
10.4103/1673-5374.165526
http://www.nrronline.org/
Accepted: 2015-07-12
- 中國神經(jīng)再生研究(英文版)的其它文章
- Lactulose enhances neuroplasticity to improve cognitive function in early hepatic encephalopathy
- Elastic modulus aff ects the growth and diff erentiation of neural stem cells
- Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a case report of improvement in relapsing auto-immune optic neuropathy
- Repair of peripheral nerve defects with chemically extracted acellular nerve allografts loaded with neurotrophic factors-transfected bone marrow mesenchymal stem cells
- Polylactic-co-glycolic acid microspheres containing three neurotrophic factors promote sciatic nerve repair after injury
- Transplantation of erythropoietin gene-modifi ed neural stem cells improves the repair of injured spinal cord

