Polylactic-co-glycolic acid microspheres containing three neurotrophic factors promote sciatic nerve repair after injury
Qun Zhao, Zhi-yue Li, Ze-peng Zhang, Zhou-yun Mo, Shi-jie Chen, Si-yu Xiang, Qing-shan Zhang, Min Xue
1 Health Management Center, Third Xiangya Hospital of Central South University, Changsha, Hunan Province, China
2 Department of Orthopedics, Third Xiangya Hospital of Central South University, Changsha, Hunan Province, China
3 Department of Orthopedics, Yiyang Municipal Central Hospital, Yiyang, Hunan Province, China
4 Department of Gynecology , Third Xiangya Hospital of Central South University, Changsha, Hunan Province, China
Polylactic-co-glycolic acid microspheres containing three neurotrophic factors promote sciatic nerve repair after injury
Qun Zhao1, Zhi-yue Li2, Ze-peng Zhang2, Zhou-yun Mo3, Shi-jie Chen2, Si-yu Xiang2, Qing-shan Zhang2, Min Xue4,*
1 Health Management Center, Third Xiangya Hospital of Central South University, Changsha, Hunan Province, China
2 Department of Orthopedics, Third Xiangya Hospital of Central South University, Changsha, Hunan Province, China
3 Department of Orthopedics, Yiyang Municipal Central Hospital, Yiyang, Hunan Province, China
4 Department of Gynecology , Third Xiangya Hospital of Central South University, Changsha, Hunan Province, China
A variety of neurotrophic factors have been shown to repair the damaged peripheral nerve. However, in clinical practice, nerve growth factor, neurotrophin-3 and brain-derived neurotrophic factor are all peptides or proteins that may be rapidly deactivated at the focal injury site; their local eff ective concentration time following a single medication cannot meet the required time for spinal axons to regenerate and cross the glial scar. In this study, we produced polymer sustained-release microspheres based on the polylactic-co-glycolic acid copolymer; the microspheres at 300-μm diameter contained nerve growth factor, neurotrophin-3 and brain-derived neurotrophic factor. Six microspheres were longitudinally implanted into the sciatic nerve at the anastomosis site, serving as the experimental group; while the sciatic nerve in the control group was subjected to the end-to-end anastomosis using 10/0 suture thread. At 6 weeks after implantation, the lower limb activity, weight of triceps surae muscle, sciatic nerve conduction velocity and the maximum amplitude were obviously better in the experimental group than in the control group. Compared with the control group, more regenerating nerve fi bers were observed and distributed in a dense and ordered manner with thicker myelin sheaths in the experimental group. More angiogenesis was also visible. Experimental fi ndings indicate that polylactic-co-glycolic acid composite microspheres containing nerve growth factor, neurotrophin-3 and brain-derived neurotrophic factor can promote the restoration of sciatic nerve in rats after injury.
nerve regeneration; biological compatibility; microspheres; nerve injury; nerve repair; polylactic-co-glycolic acid copolymer; nerve growth factor; neurotrophin-3; brain-derived neurotrophic factor
Funding: This study was fi nancially supported by a grant from the Natural Science Foundation of Hunan Province of China, No. 13JJ6016.
Zhao Q, Li ZY, Zhang ZP, Mo ZY, Chen SJ, Xiang SY, Zhang QS, Xue M (2015) Polylactic-co-glycolic acid microspheres containing three neurotrophic factors promote sciatic nerve repair after injury. Neural Regen Res 10(9):1491-1497.
Introduction
The diffi culty in nerve restoration surgery is achieving the correct para-position of the nerve bundle due to the complex structure of the peripheral nerve. This is despite the advances in microsurgical techniques, nerve bundle map, neuroelectrical stimuli, cholinesterase immunohistochemistry and nerve bundle staining. Failures in nerve bundle para-position cause axonal growth and domination in the wrong direction, hinder the recovery of physiological function, and restrict its clinical application (Pabari et al., 2010). Autologous nerve transplantation can off er nerve growth factors necessary for axonal growth and induces little immunological rejection; therefore, it is regarded as the gold standard for peripheral nerve repair. However, for the thick and long-segmental peripheral nerve defects, such as brachial plexus injury, autologous nerve transplantation is not eff ective because of permanent denervation and nerve dysfunction, limited sources of donor nerves, and neuroma formation at the recipient area (Alluin et al., 2009). Variant and heterogeneous cell transplantation risks immunological rejection, low success rate of transplantation, infection and tumor formation (Ijpma et al., 2008). Scholars have previously proposed the application of natural materials and synthetic materials, such as arteries and veins, amniotic membrane, fallopian tubes and nerve allograft, but they are not ideal substitutes for autologous nerve (Yao et al., 2010). Therefore, a three-dimensional nerve conduit complex of nerve cells and biological materials, based on tissue engineering technology, has become a potential tool for nerve injury repair. It consists of four components: seed cells, stent materials, extracellular matrix, induction and growth factors. Seed cells include Schwann cells and stem cells, which can promote
the maturation of regenerated axons, provide appropriate growth environment for neurons and myelin regeneration, and play a crucial role in regeneration after peripheral nerve injury (Mohammadi et al., 2012; Tang et al., 2012; Carriel et al., 2013; Zhang et al., 2013). Stents are mainly biodegradable materials, such as polyglycolic acid, polylactic acid and polylactic-co-glycolic acid (PLGA), which have been widely applied for preparing nerve conduits in animal experiments and clinical studies (Dellon et al., 1988; Mackinnon et al., 1990).
The growth of neural axons largely depends on the microenvironment around axons, rather than their inherent characteristics, and neurotrophic factors are the key components of the microenvironment. Previous studies have shown that, single application of exogenous neurotrophic factors such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and glial-derived neurotrophic factor, can promote axonal growth. However, the protective eff ects of neurotrophic factors on nerve axons and neurons are often unsatisfactory because of either the application alone, the limited action time and slow blood drug concentration, the unfavorable microenvironment for nerve and muscle regeneration when the long-term denervation causes muscle atrophy and fi brosis. Each of these may hinder the restoration of neurological function (Purves et al., 2004; Fiore et al., 2009).
Neurotrophic factors are proteins synthesized from the target tissue, which can be transported retrogradely to the neuron cell body under physiological conditions, provide nutritional support and promote neural development (Ascano et al., 2012). The commonly used neurotrophic factors include NGF, BDNF, NT-3, glial-derived neurotrophic factor and insulin-like growth factor. Different administration methods, concentrations or combinations of neurotrophic factors resulted in diff erent therapeutic eff ects in promoting the regeneration of peripheral nerve (McRae et al., 2012; Jin et al., 2013; Wood et al., 2013). NGF, NT-3 and BDNF are all peptides or proteins that may be rapidly deactivated at the focal injury site, therefore a single administration cannot maintain an eff ective concentration over the time required for spinal axons to regenerate and cross the glial scar.
We looked for a convenient, stable and safe scheme to solve the above shortcomings. Could we combine these neurotrophic factors into a vector, achieving both the activity and long-term concentration? A variety of natural or synthetic materials have been used in research to address the regeneration after spinal cord and peripheral nerve injury; in addition to the bridging eff ect, they also maintained the stability of the drugs and achieve long-term release (Patist et al., 2004). Among them, PLGA sustained-release microspheres have good compatibility with brain tissue (Fournier et al., 2003), and have been used in the treatment of nerve degenerative diseases (Parkinson’s disease and Alzheimer’s disease) and for local chemotherapy in experimental brain tumors (Jollivet et al., 2004).
In this study, we produced PLGA sustained-release microspheres, containing NGF, NT-3 and BDNF, and observed the restoration of sciatic nerve function in the Sprague-Dawley rat model of sciatic nerve injury. This is part of a broader attempt to provide evidence for the contribution of polymer sustained-release microspheres containing various neurotrophic factors in the repair after peripheral nerve injury.
Materials and Methods
Preparation of experimental microspheres
The diameter of PLGA microspheres was 300 μm, which was 1/3–1/4 of sciatic nerve diameter in Sprague-Dawley rats, commensurate with the gap between the sciatic nerve and the muscle. PLGA microspheres containing NGF, NT-3 and BDNF were produced with the multiple emulsion-solvent evaporation method, as previously described (Erickson et al., 2012). In brief, 2-g PLGA (Jinan Daigang Biomaterials Co., Ltd., Jinan, China) were completely dissolved in 15-mL methylene chloride for 30 minutes, reserving, the oil phase. NGF (5 μg), NT-3 (3 μg) and BDNF (5 μg) lyophilized powders (Shanghai Solarbio Bioscience & Technology Co., Ltd., Shanghai, China) were dissolved in 5-mL phosphate buff er, reserving the internal phase. Subsequently the oil phase was mixed with the internal phase for 1 minute and emulsifi ed using an ultrasonic emulsifi cation probe for 12 seconds. The emulsifi ed liquid was added to medical polyvinyl alcohol and emulsifi ed for an additional 12 seconds, then mixed at 900 r/min for 3 hours to evaporate the methylene chloride. The emulsion was repeatedly fi ltered using a 300-μm mesh, centrifuged for 10 minutes at 1,500 r/min and rinsed three times with distilled water to obtain the polymer microspheres (Figure 1A). After irregular microspheres were excluded, a total of 100 microspheres were obtained and stored at ?20°C.
Determination of in vitro release eff ect of PLGA microspheres
The in vitro release effect of PLGA microspheres was determined with the ELISA method. A total of 30-mg microspheres were weighed and suspended in 3-mL PBS solution in a dialysis bag; they were then placed in a PBS-contained glass bottle and oscillated at 37°C. Every 5 days, 100-μL supernatant was collected and supplemented with equal amount of PBS. The NGF, NT-3 and BDNF content in the supernatant were determined according to the instructions of the ELISA kit (Shanghai Solarbio Bioscience & Technology Co., Ltd.). Based on the ELISA results, the release curves of NGF, NT-3, BDNF were plotted (Winn et al., 1999; Ozbas et al., 2002).
Establishment of sciatic nerve mutilation injury models and microsphere transplantation
Thirty healthy, clean adult Sprague-Dawley rats, irrespective of gender, weighing 200–250 g, were provided by the Laboratory Animal Center of Central South University (Changsha, Hunan Province, China; animal license No. SYXK (Xiang) 2014-0013). Experimental protocols were approved by the Medical Ethics Committee, Third Xiangya Hospital of Central South University, under the approval number of
2014S012. The 30 rats were randomly divided into an experimental group and a control group, with 15 rats in each group. A 2-cm-long sciatic nerve segment, 1 cm below the piriformis muscle of the left lower limb of rats, was cut and subjected to the end-to-end anastomosis. Rats in the experimental group had six polymer microspheres inserted in the sciatic nerve after anastomosis, while rats in the control group received the end-to-end anastomosis using 10/0 silk thread.
Following previously described methods (Isaacs et al., 2013; Wang et al., 2014), after the rats were anesthetized with 10% chloral hydrate through intraperitoneal injection (400 mg/kg), the left hind limb was disinfected and secured. A 2.5-cm-long longitudinal incision was made in the posterolateral left thigh, then the skin, subcutaneous tissue and fascia were cut; the muscle was bluntly dissected, exposing the sciatic nerve. The wounds were rinsed with normal saline after hemostasis, the sciatic nerve was cut 1 cm below the piriformis muscle and subjected to the end-to-end anastomosis using 10/0 silk thread. After the wounds were sutured, the damaged sciatic nerve was implanted with six sustained-release microspheres, three on each side (Figure 1B). After injury, rats were not able to place their left feet on the ground owing to left claudication nor perform left plantar fl exion, without obvious stabbing pain, these activities indicated the success of the modeling. Rats of the control group were anesthetized, the skin was disinfected and sciatic nerve was cut, but no microspheres were implanted. The wounds in each group were treated with a small amount of diluted gentamicin solution. In the experimental group, the muscle was sutured to wrap the sciatic nerve and the microspheres so the microspheres did not press on the sciatic nerve. The fascia and skin of rats in each group were fi nally sutured using No. 1 thread. After surgery, Sprague-Dawley rats were housed in separate cages. Previous studies have found that, the NGF-contained PLGA microspheres released NGF for at least 6 weeks, and in vitro release experiments of PLGA microspheres in this study showed that the release of NGF began to decrease after 45 days. Therefore, we chose the time point of 6 weeks postoperatively for the observation of experimental animals (Fisher et al., 2001; Yang et al., 2005).
General observation
Within 6 weeks postoperatively, the wound healing, gait, response to external stimuli (We used the needle under the same pressure to stimulate rat feet and observed whether rats had limb twitched and withdrawn), swelling and muscle atrophy of the left lower limb were observed.
Electrophysiological detection
Sciatic nerve conduction velocity and amplitude on the ipsilateral (injury) side of rats in each group were detected and recorded using an electrophysiological instrument (Dantec Dynamics A/S, Copenhagen, Denmark). Rat sciatic nerve was exposed under the 10% chloral hydrate anesthesia; two stimulation electrodes were placed on the nerve stem proximal and distal to the suture at an interval of 10 mm. The recording electrode was anchored on the triceps muscle. The nerve stems were fi rst stimulated and the latency was recorded as T1 and T2 at the proximal and distal ends, respectively. Nerve conduction velocity was calculated according to the formula: nerve conduction velocity (m/s) = distance between stimulation points (cm) × 10 / the diff erence of latency (ms). The stimulating current was given for a certain period to record the stable waveform, the diff erence between the upper and lower wave crest was the maximal amplitude. All rats were killed under anesthesia at 6 weeks post-surgery and sciatic nerve specimens were harvested for the following detections.
Measurement of wet weight in triceps surae
At 6 weeks post-surgery, rats were anesthetized with 10% chloral hydrate through intraperitoneal injection, and bilateral triceps surae was completely resected, the triceps muscle wet weight was measured and recorded. Triceps weight ratio (%) = triceps weight on the ipsilateral side/triceps weight on the contralateral side × 100%.
Histological observation
At 6 weeks post-surgery, after electrophysiological test was completed, the suture was withdrawn and 2-cm-long sciatic nerve was fi xed with 4% paraformaldehyde (Jintong Letai Chemical Product (Beijing) Co., Ltd., Beijing, China) for 24 hours, embedded, sliced to 60-nm-thick sections and stained with toluidine blue (Jintong Letai Chemical Product (Beijing) Co., Ltd.). Buffer solution (pH 3.7) containing 0.63-g citric acid, 0.3-g disodium hydrogen phosphate and 400-mL double-distilled water was mixed with toluidine blue. Nerve sections were stained with toluidine blue, cemented and observed under an optical microscope (Shanghai Optical Instrument Factory, Shanghai, China).
Statistical analysis
Measurement data were expressed as the mean ± SD and analyzed using SPSS 18.0 software (SPSS, Chicago, IL, USA). The diff erence of mean value between two groups was compared using two-sample t-test. α = 0.05 was considered statistically signifi cant.
Results
General observation of sciatic nerve injury rats
One rat in the experimental group died of postoperative incisional infection at 7 days post-surgery, and two rats in the control group died of unknown causes at 12 and 18 days, respectively. These three deaths were excluded from the fi nal analysis.
Within postoperative 2 weeks: All the incisions appeared swollen, to diff erent degrees, at 12 hours that subsequently attenuated by 3–5 days. During the 10 days post-surgery, rats suff ered no or slow stabbing pain in the left lower limb in response to pressure, and muscle atrophy appeared in the operated legs.
At postoperative 2–4 weeks: Stabbing pain was detected in rats of the experimental group at 16 days and in rats of the
control group at 20 days. Rat claudication was still present and muscle atrophy had aggravated.
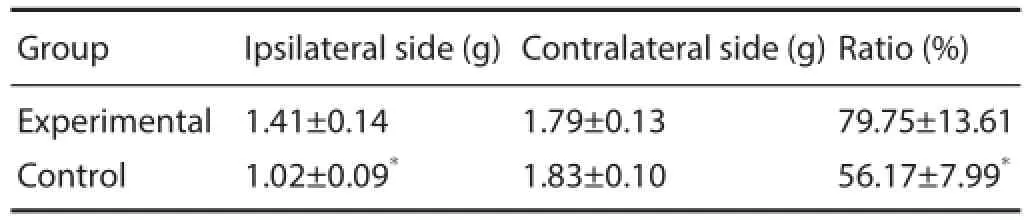
Table 1 Triceps wet weight and wet weight ratio in rats at 6 weeks after surgery
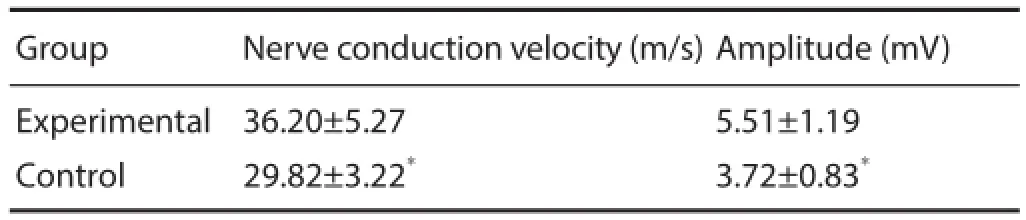
Table 2 Electrophysiological measurements of rat sciatic nerve at 6 weeks after surgery
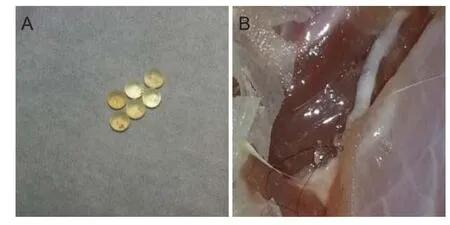
Figure 1 Polylactic-co-glycolic acid (PLGA) microsphere image (A) and implantation of sustained-release microspheres into the injured sciatic nerve (B).
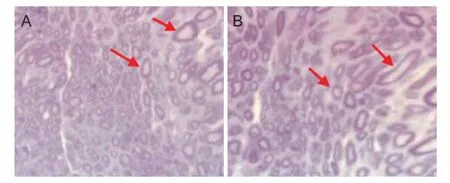
Figure 2 Eff ect of composite microsphere transplantation on histological change of rat sciatic nerve (hematoxylin-eosin staining, light microscope, × 400).
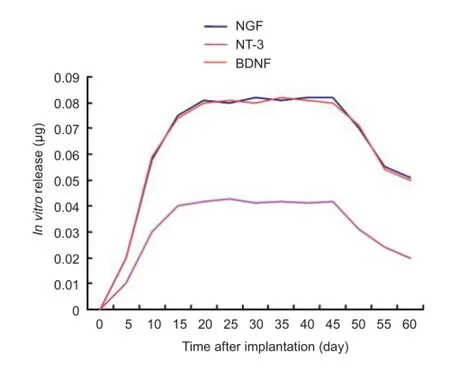
Figure 3 In vitro release curves of various neurotrophic factors over 60 days following implantation of microspheres.
At postoperative 4–6 weeks: Muscle atrophy had ceased and regrowth began to restore at 4 weeks in the experimental group. No signifi cant recovery was found in rats of the control group. At 5 weeks postoperatively, stabbing pain was sensitive and the claudication was attenuated in the experimental group; in the control group muscle atrophy began to reverse and the claudication was still present. At 6 weeks postoperatively, the sensitivity of response to stabbing pain had obviously improved, but was still lower than in the experimental group.
Eff ect of microspheres transplantation on sciatic nerve function of sciatic nerve injury rats
At 6 weeks after surgery, nerve conduction velocity and amplitude in the experimental group were higher than in the control group (P < 0.01; Table 1).
Eff ect of microsphere transplantation on triceps wet weight of sciatic nerve injury rats
At 6 weeks after surgery, triceps surae in all Sprague-Dawley rats showed atrophy to diff erent degrees, triceps wet weight and wet weight ratio in the experimental group were higher than in the control group (P < 0.01; Table 2).
Eff ect of microsphere transplantation on the pathomorphology of rat sciatic nerve
At 6 weeks postoperatively, diff erent levels of nerve fi ber growth within sciatic nerve sections were observed under an optical microscope, but the density, diameter and quantity showed notable diff erences between the two groups. Nerve fi bers in the experimental group were dense and abundant, and distributed uniformly, newborn blood capillaries and thick myelin sheath were clearly visible (Figure 2A). Compared with the experimental group, the number of nerve fi bers in the control group was signifi cantly less, nerve fi bers were distributed unevenly and myelin sheaths were thinner (Figure 2B).
In vitro release of composite microspheres
PLGA microspheres can sustain the release of neurotrophic factors in an aqueous solution. The release of each factor from the microspheres was determined using ELISA method every 5 days. The released amounts were at a low level within the fi rst 5 days, signifi cantly increased after 5 days, reached their peak at 10–15 days, and decreased after 45 days, but some release of neurotrophic factors was still detected at 60 days (Figure 3).
Discussion
As the pathological process after peripheral nerve injury is very complex, nerve regeneration is slow and nerve functional recovery is unsatisfactory (Siemionow et al., 2009). The existing treatments include direct surgical repair, tissue engineering technology, neural transplantation and gene therapy. Neurotrophic factors play key roles in the development of tissue engineering, they can not only provide nutrition for the nerve, but also promote axonal growth, adjust the survival of nerve cells, and promote cell growth and diff erentiation. However, most previous studies emphasize the application of a single neurotrophic factor or use the synergic eff ect of two neurotrophic factors in nerve injury repair (Skaper et al., 2012). In this study, the prepared PLGA microspheres contained three neurotrophic factors, NGF, NT-3 and BDNF, which have been shown to promote the regeneration of peripheral nerve (Crigler et al., 2006). NGF is a secreted protein that has an important eff ect on the growth of neurons and triggers the extensions of axons and their branching (Madduri et al., 2009). NGF also promotes the regeneration of the peripheral nerve in rats and facilitates the repair of the myelin sheath (Althaus et al., 2004; Sun et al., 2009). NT-3 is a small molecular basic protein encoded by the NTF3 gene, which can not only promote the survival of the damaged neurons and glial cells in vivo, but also induce the diff erentiation of in vitro cultured cells to nerve cells. In addition, NT-3 contributes to promote the axonal regeneration and myelinization, guide the axonal growth, assist in neuronal survival and diff erentiation, and promote the growth and diff erentiation of new neurons and synapses (Tessarollo et al., 1994; Kamei et al., 2007). BDNF is mainly synthesized in the central nervous system and functions to promote neuronal survival and axonal growth, improve nerve regeneration and synaptic reconstruction, participate in glial cell damage and promote the growth and diff erentiation of new neurons (Galko et al., 2000; Huang et al., 2001). NGF, NT-3 and BDNF can be simultaneously expressed after peripheral nerve injury; diff erent combinations and dosages of neurotrophic factors promote diff erent outcomes of nerve repair (Ichim et al., 2012; Sand et al., 2012). These neurotrophic factors contribute to regeneration after peripheral nerve injury, but no study has reported the eff ect of application of the combination of NGF, NT-3 and BDNF and compared that with a single application. In this novel study, we applied three factors and used sustained-release microspheres for treating sciatic nerve injury in rats. Results showed that the triceps wet weight, nerve conduction velocity and wave amplitude in the experimental group were higher than in the control group. Pathological observation results showed that nerve fi bers were denser and distributed uniformly in the experimental group, with newborn capillaries and thick myelin sheath, whereas the nerve fi bers were arranged unevenly, thinly myelinated and there were signifi cantly fewer in the control group. Our fi ndings indicate that, NGF, NT-3 and BDNF have synergic eff ects in the restoration of neurons after peripheral nerve injury.
Previous methods used for nerve repair were mainly the injection and oral administration of neurotrophic factors after suture and transplantation, but the focal drug concentration and action time were not satisfactory, leading to poor prognosis, although they promoted some regeneration of the damaged nerve. The currently proposed treatment favors continuous administration and that microsphere technology is superior to traditional treatment in the target tissue and
selectivity. Microspheres can be injected into the lesions in a minimally invasive way and release therapeutic substances sustainedly, and could improve, even cure, patients. Mithun et al. (2010) found that local drug delivery, using microsphere technology, with radiation therapy could act directly on the tumor, and signifi cantly reduced systemic adverse reactions. Wu et al. (2011) demonstrated that transplanting the BDNF-contained microcapsules into rats with optic nerve injury was more eff ective than BDNF injection alone. Ideal microspheres should possess the following properties: permeability, no (low) biological toxicity, stable physical-chemical properties, and natural degradability (National et al., 2008; Liu et al., 2010).
PLGA has been regarded as a good potential material owing to its excellent biodegradability, biocompatibility, controllable degradation speed, controlled porosity, good ball, cyst or membrane formation. Once PLGA enters the human body, it is naturally degraded to lactic acid and glycolic acid, natural by-products in various metabolic pathways. PLGA has been widely applied in the food and medical industry, and biomedical device production (Mohammad et al., 2010). In this study, we prepared sustained-release microspheres containing NGF, NT-3 and BDNF with the emulsion-solvent evaporation method, taking PLGA as the basement material, and formed the microspheres in vitro. Results found that, the PLGA microspheres sustainedly released the three factors over a long time, and the released amounts attained a stable level after 10 days, only decreased after 45 days, and were still detected at 60 days. This evidence indicates that PLGA is a suitable candidate of slow-release materials and contributes to solve low drug concentration and short action time for the treatment of peripheral nerve injury by using tissue engineering technology.
In this study, we combined PLGA with NGF, NT-3 and BDNF to obtain microspheres slow-release system; the microspheres gradually hydrolyzed, released NGF, NT-3 and BDNF, and promoted the restoration after sciatic nerve injury in rats. Results showed that the PLGA-based sustained-release microspheres could release various neurotrophic factors over a long period and clearly improved the recovery of neurological function after sciatic nerve injury. In addition, triceps wet weight, electrophysiological test and histological observation results in the experimental group were signifi cantly better than in the control group. PLGA microspheres compounded with neurotrophic factors can promote the restoration of sciatic nerve after injury in Sprague-Dawley rats.
The present study uses an innovative method of combining three neurotrophic factors in a microsphere for treating sciatic nerve injury in rats. However, the synergic effects among three factors, molecular mechanisms, dose-eff ect relationship and toxic eff ects remain unclear and deserve further exploration. Choice of other materials as the carrier of the sustained-release microspheres, their in vitro release properties, biocompatibility and toxic reactions in vivo also need further research.
Acknowledgments: We would like to thank Sheng-hui Liao from School of Information Science and Engineering, Central South University, China for providing great help and support in the data processing, Hai-lin Yang from Powder Metallurgy Research Institute of Central South University, China for the preparation of specimens, and Teachers Hu and Ding from Experimental Animal Center, Third Xiangya Hospital of Central South University, China for the animal feeding.
Author contributions: ZYL was responsible for the study concept and design, supervising animal experiments, collecting and analyzing experimental data, and writing the paper. QZ provided information and data support, instructed the study and performed data analysis. ZPZ implemented animal experiments, collected the data and wrote the paper. ZYM, SJC, XYX and QSZ participated in the establishment of animal model, collection of experimental data, statistical analysis and literature retrieval. All authors approved the fi nal version of the paper.
Confl icts of interest: None declared.
Alluin O, Wittmann C, Marqueste T, Garcia S, Lavaut MN, Guinard D, Feron F, Decherchi P (2009) Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biomaterials 30:363-373.
Althaus HH (2004) Remyelination in multiple sclerosis: a new role for neurotrophins. Prog Brain Res 146:415-432.
Ascano M,Bodmer D,Kuruvilla R (2012) Endocytic traffi cking of neurotrophins in neural development. Trends Cell Biol 22:266-273.
Carriel V, Garrido-Gomez J, Hernández-Cortés P, Garzón I, García-García S, Sáez-Moreno JA, Del Carmen Sánchez-Quevedo M, Campos A, Alaminos M (2013) Combination of fi brin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. J Neural Eng 10:026022.
Crigler L, Robey RC, Asawachaichrn A, Gaupp D, Phinney DG (2006) Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol 1:54-64.
Dellon AL, Mackinnon SE (1988) An alternative to the classical nerve graft for the management of the short nerve gap. Plast Reconstr Surg 82:849-856.
Erickson IE, Kestle SR, Zellars KH, Dodge GR, Burdick JA, Mauck RL (2012) Improved cartilage repair via in vitro pre-maturation of MSC-seeded hyaluronic acid hydrogels. Biomed Mater 7:024110.
Farazuddin M, Chauhan A, Khan RM, Owais M (2010) Amoxicillin bearing microparticles: potential in treatment of Listeria monocytogenes infection in Swiss albino mice. Biosci Rep 31:265-272.
Fiore M, Chaldakov GN, Aloe L (2009) Nerve growth factor as a signaling molecule for nerve cells and also for the neuroendocrine-immune systems. Rev Neurosci 20:133-145.
Fisher J, Levkovitch-Verbin H, Schori H, Yoles E, Butovsky O, Kaye JF, Ben-Nun A, Schwartz M (2001) Vaccination for neuroprotection in the mouse optic nerve: implications for optic neuropathies. J Neurosci 21:136-142.
Fournier E, Passirani C, Montero-Menei CN, Benoit JP (2003) Biocompatibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibility. Biomaterials 24:3311-3331.
Galko MJ, Tessier-Lavigne M (2000) Function of an axonal chemoattractant modulated by metalloprotease. Science 289:1365-1367.
Huang EJ, Reichardt (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677-736.
Ichim G, Tauszig-Delamasure S, Mehlen P (2012) Neurotrophins and cell death. Exp Cell Res 318:1221-1228.
Ijpma FF, Van De Graaf RC, Meek MF (2008) The early history of tubulation in nerve repair. J Hand Surg Eur Vol 33:581-586.
Jin Y, Kondo K, Ushio M, Kaga K, Ryan AF, Yamasoba T (2013) Developmental changes in the responsiveness of rat spiral ganglion neurons to neurotrophic factors in dissociated culture: diff erential responses for survival, neuritogenesis and neuronal morphology. Cell Tissue Res 351:15-27.
Jollivet C, Aubert-Pouessel A, Clavreul A, Venier-Julienne MC, Montero-Menei CN, Benoit JP, Menei P (2004) Long-term effect of intra-striatal glial cell line-derived neurotrophic factor-releasing microspheres in a partial rat model of Parkinson’s disease. Neurosci Lett 356:207-210.
Kamei N, Tanaka N, Oishi Y, Hamasaki T, Nakanishi K, Sakai N, Ochi M (2007) BDNF, NT-3 and NGF released from transplanted neural progenitor cells promote corticospinal axon growth in organotypic cocultures. Spine 32:1272-1278.
Liu G, Keeler BE, Zhukareva V, Houlé JD (2010) Cycling exercise aff ects the expression of apoptosis-associated microRNAs after spinal cord injury in rats. Exp Neurol 226:200-206.
Lsaacs J, Mallu S, Wo Y, Shah S (2013) A rodent model of partial muscle re- innervation. J Neurosci Methods 219:183-187.
Mackinnon SE, Dellon AL (1990) Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast Reconstr Surg 85:419-424. Madduri S, Papalo?zos M, Gander B (2009) Synergistic eff ect of GDNF and NGF on axonal branching and elongation in vitro. Neurosci Res 65:88-97.
McRae BR,Shew M, Aaron GP, Bijangi-Vishehsaraei K, Halum SL (2012) A rapid, novel model of culturing cranial nerve X-derived motoneurons for screening trophic factor outgrowth response. Neurol Res 34:564-575.
Mohammadi R,Azizi S,Delirezh N, Hobbenaghi R, Amini K, Malekkhetabi P (2012) The use of undiff erentiated bone marrow stromal cells for sciatic nerve regeneration in rats. Int J Oral Maxillofac Surg 41:650-656.
National Spinal Cord Injury Statistical Center B, Alabama (2008) Spinal cord injury: facts and fi gures at a glance. J Spinal Cord Med 31:357-358.
Ozbas TS, Akbuga J, Aral C (2002) Controlled release of interleukin-2 from chitosan microsphere. Pharm Sci 91:1245-1251.
Pabari A, Yang SY, Seifalian AM, Mosahebi A (2010) Modern surgical management of peripheral nerve gap. Reconstr Aesthet Surg 63:1941-1948.
Patist CM, Mulder MB, Gautier SE, Maquet V, Jér?me R, Oudega M (2004) Freeze-dried poly (D, L-lactic acid) macroporous guidance scaff olds impregnated with brain-derived neurotrophic factor in the transected adult rat thoracic spinal cord. Biomaterials 25:1569-1582.
Purves D, Augustine G, Fitzpatrick D, Hall W, LaMantia A, McNamara J, White L (2004) Neuroscience. Sunderland, Mass: Sinauer, UK.
Rajput MS, Agrawal P (2010) Microspheres in cancer therapy. Indian J Cancer 47:458-468.
Sand PG, Langguth B, Schecklmann M, and Kleinjung T (2012) GDNF and BDNF gene interplay in chronic tinnitus. Int J Mol Epidemiol Genet 3:245-251.
Siemionow M,Brzezicki G(2009)Current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol 87:141-172.
Skaper SD(2012)The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol 846:1-12.
Sun W, Sun C, Lin H, Zhao H, Wang J, Ma H, Chen B, Xiao Z, Dai J (2009) The eff ect of collagen-binding NGF-beta on the promotion of sciatic nerve regeneration in a rat sciatic nerve crush injury model. Biomaterials 30:4649-4656.
Tang X, Xue C, Wang Y, Ding F, Yang Y, Gu X (2012) Bridging peripheral nerve defects with a tissue engineered nerve graft composed of an in vitro cultured nerve equivalent and a silk fi broin-based scaff old. Biomaterials 33:3860-3867.
Tessarollo L, Vogel K, Palko M, Reid S, Parada L (1994) Targeted mutation in the neurotrophin-3 gene results in loss of muscle sensory neurons. Proc Natl Acad Sci U S A 91:11844-11848.
Wang Y, Wang Q (2014) Advance of animal model of peripheral nerve injury. Zhongguo Kangfu Lilun yu Shijian 20:537-539.
Winn SR, Uludag H, Hollinger JO (1999) Carrier systems for bone morphogenetic proteins. Clin Orthop Relat Res 367:S95-106.
Wood MD, Gordon T, Kemp SW, Liu EH, Kim H, Shoichet MS, Borschel GH (2013) Functional motor recovery is improved due to local placement of GDNF microspheres after delayed nerve repair. Biotechnol Bioeng 110:1272-1281.
Wu HP, Wang NL, Zeng MB, Chen JC, Fan ZG (2011) The protecting eff ect of chitosan/alginate microcyst encapsulating BDNF to damaged optic nerve in SD rat. Guangzhou Yixueyuan Xuebao 42:56-58.
Yang Y, De Laporte L, Rives CB, Jang JH, Lin WC, Shull KR, Shea LD (2005) Neurotrophin releasing single and multiple lumen nerve conduits. J Control Release 104:433-446.
Yao L, de Ruiter GC, Wang H, Knight AM, Spinner RJ, Yaszemski MJ, Windebank AJ, Pandit A (2010) Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials 31:5789-5797.
Zhang YG, Sheng QS, Qi FY, Hu XY, Zhao W, Wang YQ, Lan LF, Huang JH, Luo ZJ (2013) Schwann cell-seeded scaff old with longitudinally oriented micro-channels for reconstruction of sciatic nerve in rats. J Mater Sci Mater Med 24:1767-1780.
Copyedited by Dawes E, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
*Correspondence to:
Min Xue, Ph.D., xuemin5908@sina.com orcid:
0000-0003-4369-8747 (Min Xue)
10.4103/1673-5374.165522
http://www.nrronline.org/
Accepted: 2015-06-07
 中國(guó)神經(jīng)再生研究(英文版)2015年9期
中國(guó)神經(jīng)再生研究(英文版)2015年9期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Lactulose enhances neuroplasticity to improve cognitive function in early hepatic encephalopathy
- Elastic modulus aff ects the growth and diff erentiation of neural stem cells
- Optimal concentration and time window for proliferation and diff erentiation of neural stem cells from embryonic cerebral cortex: 5% oxygen preconditioning for 72 hours
- Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a case report of improvement in relapsing auto-immune optic neuropathy
- Repair of peripheral nerve defects with chemically extracted acellular nerve allografts loaded with neurotrophic factors-transfected bone marrow mesenchymal stem cells
- Transplantation of erythropoietin gene-modifi ed neural stem cells improves the repair of injured spinal cord
