Repair of peripheral nerve defects with chemically extracted acellular nerve allografts loaded with neurotrophic factors-transfected bone marrow mesenchymal stem cells
Yan-ru Zhang, Ka Ka Ge-chen Zhang, Hui Zhang, Yan Shang, Guo-qiang Zhao, Wen-hua Huang
1 Medical College, Henan Polytechnic University, Jiaozuo, Henan Province, China
2 Institute of Clinical Anatomy, Southern Medical University, Guangzhou, Guangdong Province, China
3 Department of Orthopedics, First Affi liated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
4 Department of Laboratory Medicine, Children’s Hospital of Zhengzhou, Zhengzhou, Henan Province, China
5 School of Basic Medical Sciences, Zhengzhou University, Zhengzhou, Henan Province, China
Repair of peripheral nerve defects with chemically extracted acellular nerve allografts loaded with neurotrophic factors-transfected bone marrow mesenchymal stem cells
Yan-ru Zhang1,2,*, Ka Ka1, Ge-chen Zhang3, Hui Zhang3, Yan Shang4, Guo-qiang Zhao5, Wen-hua Huang2,*
1 Medical College, Henan Polytechnic University, Jiaozuo, Henan Province, China
2 Institute of Clinical Anatomy, Southern Medical University, Guangzhou, Guangdong Province, China
3 Department of Orthopedics, First Affi liated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
4 Department of Laboratory Medicine, Children’s Hospital of Zhengzhou, Zhengzhou, Henan Province, China
5 School of Basic Medical Sciences, Zhengzhou University, Zhengzhou, Henan Province, China
Chemically extracted acellular nerve allografts loaded with brain-derived neurotrophic factor-transfected or ciliary neurotrophic factor-transfected bone marrow mesenchymal stem cells have been shown to repair sciatic nerve injury better than chemically extracted acellular nerve allografts alone, or chemically extracted acellular nerve allografts loaded with bone marrow mesenchymal stem cells. We hypothesized that these allografts compounded with both brain-derived neurotrophic factor- and ciliary neurotrophic factor-transfected bone marrow mesenchymal stem cells may demonstrate even better eff ects in the repair of peripheral nerve injury. We cultured bone marrow mesenchymal stem cells expressing brain-derived neurotrophic factor and/or ciliary neurotrophic factor and used them to treat sciatic nerve injury in rats. We observed an increase in sciatic functional index, triceps wet weight recovery rate, myelin thickness, number of myelinated nerve fi bers, amplitude of motor-evoked potentials and nerve conduction velocity, and a shortened latency of motor-evoked potentials when allografts loaded with both neurotrophic factors were used, compared with allografts loaded with just one factor. Thus, the combination of both brain-derived neurotrophic factor and ciliary neurotrophic factor-transfected bone marrow mesenchymal stem cells can greatly improve nerve injury.
nerve regeneration; peripheral nerve injury; brain-derived neurotrophic factor; ciliary neurotrophic factor; chemically extracted acellular nerve allografts; bone marrow mesenchymal stem cells; peripheral nerve; neural regeneration
Zhang YR, Ka K, Zhang GC, Zhang H, Shang Y, Zhao GQ, Huang WH (2015) Repair of peripheral nerve defects with chemically extracted acellular nerve allografts loaded with neurotrophic factors-transfected bone marrow mesenchymal stem cells. Neural Regen Res 10(9):1498-1506.
Introduction
The local concentration gradient of nerve growth factor is important for guiding proximal growth cones. Brain-derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF) not only maintain neuronal morphology, but also promote neuronal growth (Krase et al., 2014). In recent years, with the advance in biotechnology, gene and cell-replacement therapy has gradually become an expanding area (Min et al., 2014). However, it is not known whether target genes are effi ciently expressed in vivo either long-term or permanently. A key issue is the ability to select appropriate target cells in which introduced cells can maintain self-renewal (Tiago et al., 2014).
Bone marrow mesenchymal stem cells (BMSCs) are adult stem cells that possess self-renewal, multi-directional differentiation properties and can be gene-transfected mature carrier cells (Mamun et al., 2013). Our previous studies investigated chemically extracted acellular nerve allograft (CEANA) compounded with BDNF-transfected BMSCs and CEANA compounded with ciliary neurotrophic factor (CNTF)-transfected BMSCs for repair of rat peripheral nerve injury and resulted in remarkable curative effects (Zhang et al., 2013, 2014a, b). We hypothesized that CEANA compounded with both BDNF- and CNTF-transfected BMSCs for repair of peripheral nerve injury would achieve better curative eff ects than CEANA compounded just one neurotrophic factor with transfected BMSCs. We constructed eukaryotic expression vectors carrying BDNF and CNTF genes and acquired BMSCs strains containing BDNF-CNTF genes. CEANA compounded with BDNF-CNTF gene-transfected BMSCs were transplanted by end-to-end anastomosis, thus creating space for regeneration. After being transfected
into BMSCs, BDNF and CNTF have been shown to be consistently expressed in the local microenvironment, greatly reducing neuronal death and promoting neurite regeneration after peripheral nerve injury (Hudson et al., 2004). This method also enhanced the promotional eff ects of CEANA and promoted the improvement of morphological changes of the injured nerve.
Materials and Methods
Animals
This study included 65 male 8-week-old Sprague-Dawley rats weighing 200–300 g, which were provided by Henan Province Laboratory Animal Center, China (license No. SCXK (Yu) 2010-0002). All eff orts were made to minimize the number of animals used and the suff ering caused by the procedures used in the present study. The whole experimental procedure was approved by the Ethics Committee of Zhengzhou University of China, and in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1986). All rats were housed at controlled temperature (22°C) in a 12-hour light-dark cycle with free access to food and water.
Preparation of acellular nerve allograft
Ten Sprague-Dawley rats were anesthetized with 10% chloral hydrate (0.4 mL/100 g) and the skin at the surgical area was shaved and disinfected according to methods described by Zheng et al. (2000). Under sterile conditions, rat bilateral sciatic nerves, 10 mm in length, were cut 3 mm away from the inferior margin of periformis muscle, and adipose tissue on the surface of the nerve was removed under the microscope. Rat sciatic nerves were immersed in distilled water for 13 hours, oscillated in 3.0% Triton X-100 solution for 12 hours at room temperature and in sodium deoxycholate for 24 hours at room temperature (Sondell et al., 1998). The above steps were repeated once. The sciatic nerves were then washed in distilled water for 0.5 hours and preserved in sterile PBS solution (pH 7.4) at 4 °C for later use (Mligiliche et al., 2002).
Preparation of BDNF and/or CNTF-transfected BMSCs
Femoral bone marrow was taken from fi ve Sprague-Dawley rats. The harvested BMSCs were cultured in a 5% CO2-containing environment at 37 °C. After 24 hours, the culture medium was replaced for the fi rst time. The cells were washed three times with PBS to remove the non-adherent cells, and the culture medium was replaced with an equal amount of fresh L-Dulbecco’s Modifi ed Eagle Medium (L-DMEM) and refreshed once every 3 or 4 days. When cells reached 80–90% confl uency after 8–10 days of culture, they were digested with 0.25% trypsin. When the cells became round-shaped and began to depart from the bottom of the fl ask, the culture was terminated with L-DMEM complete culture solution and prepared into a single cell suspension. The single cell suspension was centrifuged at 1,100 r/min for 5 minutes. After removal of supernatant, cell suspension was seeded at 1 × 105/mL in a 6-well culture plate and culture medium was refreshed every 3 or 4 days. Cell growth was observed daily. When cells reached nearly complete confl uency, they were digested with trypsin. BMSCs could be sub-cultured for several passages.
Passage 3 BMSCs were transfected with different recombinant plasmids to form four groups: pIRES-BDNF, pIRES-CNTF, pIRES-BDNF-CNTF and empty vector pIRES. A blank control group in which cells were not transfected by any plasmid was also designated. Eukaryotic expression vector pIRES (Clontech Laboratories, Mountain View, CA, USA) can highly express two target genes in eukaryotic mammalian cells. Because these two target genes are isolated by IRES sequence, the two target proteins are expressed interdependently. Cytomegalovirus eukaryotic expression promoters were used for the vectors. Kanamycin was used for prokaryotes, but geneticin was required for eukaryotes.
Recombinant plasmids pIRES-BDNF, pIRES-CNTF and pIRES-BDNF-CNTF (School of Basic Medical Sciences, Zhengzhou University, China) were electrically transferred to BMSCs. The BMSC suspension (1 mL) prepared in the above procedure was inserted into a 4-mm electrical transfer glass (Bio-Rad, Hercules, CA, USA), followed by addition of 12 μg pIRES-BDNF (or pIRES-CNTF, pIRESBDNF-CNTF or pIRES) plasmid. The mixture was electrically transferred using the following electrical transfer parameters: voltage 300 V, capacitance 980 μF, second pulse, and square wave/cyclotron wave. The cells were subjected to electric shock within 5 minutes, immediately placed in an ice bath, and then cultured with DMEM medium containing 15% fetal bovine serum at 37 °C in a 5% CO2incubator.
Detection of BDNF and CNTF genes in transfected cells
The plasmid pCMV-SPORT6-BDNF (or CNTF) which encodes full-length cDNA of BDNF or CNTF gene was taken as a PCR template. PCR amplifi cation was performed with primers specifi c for the BDNF gene.
The primer information is shown as follows:
Identifi cation of recombinant plasmids
BDNF: Brain-derived neurotrophic factor; CNTF: ciliary neurotrophic factor.
After BDNF (or CNTF) was conjugated with plRES and transformed on LB agar plates with ampicillin, several positive colonies appeared. Two positive colonies were randomly selected and subjected to PCR amplifi cation using primers specifi c for sequences of BDNF and CNTF gene. PCR product was electrophoresed on a 1.5% agarose gel to determine whether target genes BDNF and CNTF were inserted into plRES.
After CNTF was conjugated with pIRES-BDNF and transformed on LB agar plates with ampicillin, a large number of positive colonies appeared. Two positive colonies were randomly selected and inoculated in the LB broth containing ampicillin. PCR amplifi cation of extracted plasmids was performed using primers specific for sequences of BDNF and CNTF gene (dilution 1:100).
Based on PCR amplifi cation, recombinant plasmid DNA was digested by restriction enzymes Xba I, Sal I, Xho I and Mlu I (New England Biolabs, Ipswich, MA, USA). PCR products were visualized by gel electrophoresis using a 1.5% agarose gel. The size of enzyme-digested products was determined by a DNA marker and the products were observed by a gel image system under a wavelength of 256 nm to confirm the recombinant BDNF and CNTF mRNA-expressing vectors pIRES-BDNF-CNTF mRNA. pIRES-BDNF-CNTF protein expression was detected by western blot assay.
Preparation of rat models of sciatic nerve defects
The remaining 50 Sprague-Dawley rats were anesthetized using 10% chloral hydrate (0.4 mL/100 g) for 2–2.5 hours. Under sterile conditions, the right sciatic nerve was exposed and a length of 7 mm sciatic nerve was cut 3 mm lateral to the inferior margin of the piriformis muscle, allowing sciatic nerve retraction (one more sciatic nerve resection was necessary if 10 mm sciatic nerve defect was not achieved). Therefore, a 10 mm sciatic nerve defect model was created.
The 50 models of sciatic nerve defect were randomly divided into five groups, with 10 rats per group: CEANA, CEANA + BDNF/BMSCs, CEANA + CNTF/BMSCs, CEANA + BDNF + CNTF/BMSCs and CEANA/BMSCs. In the CEANA group, sciatic nerve defect was repaired with 10 mm CEANA through end-to-end anastomosis. In the CEANA + BDNF/BMSCs, CEANA + CNTF/BMSCs, CEANA + BDNF + CNTF/BMSCs and CEANA/BMSCs groups, sciatic nerve defect was repaired with 10 mm CEANA through end-toend anastomosis and then 0.1 mL of 33 mg/L BDNF/BMSCs, CNTF/BMSCs, BDNF + CNTF/BMSCs and BMSCs was injected into the tissue around the transplanted site.
General condition of rats after surgery
The mental state, wound healing, muscle atrophy and ulceration at the surgical site, complications, presence of a dragging or crouching walk were observed.
Morphology of distal anastomotic stoma of nerve allografts
At 8 weeks after surgery, a segment of sciatic nerve comprising the tissue 1 mm away from the proximal and distal anastomotic stoma of CEANA was resected (mainly to observe whether regenerated axons passed through the anastomotic scar), fi xed with 10% formaldehyde, embedded with paraffi n, and successively sliced into sections (each section spanning the area 2 mm away from the proximal and distal anastomotic stoma). The transverse sections were routinely stained with hematoxylin-eosin, cut into semi-thin sections, stained with toluidine blue and observed under the optical microscope (Olympus, Tokyo, Japan). A 3 mm-long sciatic nerve segment was cut 3 mm away from distal anastomotic stoma of the sciatic nerve on the surgical side. An identical sciatic nerve segment from the control side was cut as a normal control. The specimen was rinsed, fi xed with glutaraldehyde, embedded with epon 812, sliced into ultrathin cross-sections, counterstained with uranium-lead and observed under transmission electron microscope (CM120, Oxoid, Basingstoke, UK). Using the Image-Pro Plus image analysis system (Media Cybernetics, Bethesda, MD, USA), the total number of myelinated nerve fi bers was counted and the thickness of myelin sheath was measured.
Rat sciatic functional index (SFI) after surgery
Before surgery and at 2, 4, 6 and 8 weeks after surgery, according to the method described by Nakazato et al. (2007), total spreading (toe spread), print length, and distance between intermediate toes (intermediary toe spread) were measured. Rat SFI was calculated using the Bain formula: SFI = ?38.3 (EPL?NPL)/NPL + 109.5 (ETS?NTS)/NTS + 13.3 (EIT?NIT)/NIT ? 8.8, where EPL is the print length of experimental feet; NPL is the print length of normal feet; ETS is the toe spread of experimental feet; NTS is the toe spread of normal feet; EIT is the distance between intermediary toe spread of experimental feet; and NIT is the distance between intermediary toe spread of normal feet. SFI = 0 indicates normal sciatic nerve function and SFI = ?100 indicates complete loss of sciatic nerve function.
Determination of triceps wet weight recovery rate
At 8 weeks after surgery, rat bilateral triceps surae were harvested and weighed after surface blood blot was removed using gauze. Triceps wet weight recovery rate was calculated as (triceps wet weight on the surgical side /triceps wet weight on the control side) × 100%.
Electrophysiology tests
At 8 weeks after surgery, rat sciatic nerve on the surgical side was exposed. Gastrocnemius muscle surgery was performed using an SXP-1 operating microscope (Shanghai Medical Equipment Co., Shanghai, China). The recording electrodes were placed in the middle of the calf muscle and pricked into the muscle at an appropriate depth. The upper part of the proximal anastomotic stoma and lower part of the distal anastomotic stoma were stimulated using stimulating electrodes to record latency and motor-evoked potential amplitude (NDI-200P EMG evoked potentiometer; Shanghai Haishen Medical Electronic Instrument Co., Shanghai, China). The distance between the two points of stimulation was measured to calculate nerve conduction velocity.

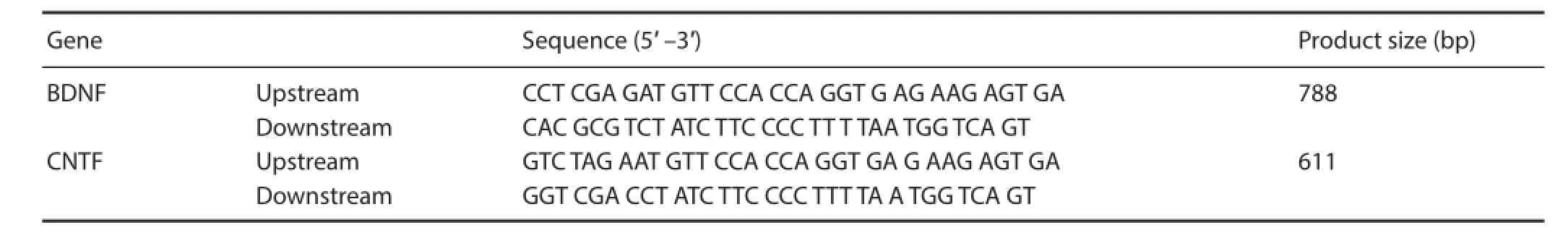
Figure 1 Electrophoretogram of the amplifi ed products of targeted genes brain-derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF) in the transfected cells.
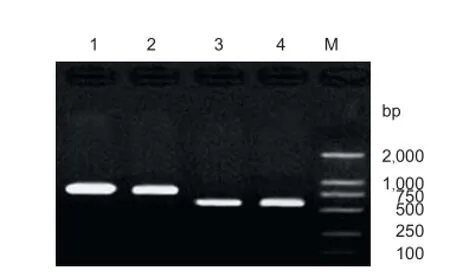
Figure 2 PCR identification of recombinant plasmids pIRES-BDNF and pIRES-CNTF.

Figure 3 Identifi catiion of PCR products by enzymatic digestion.
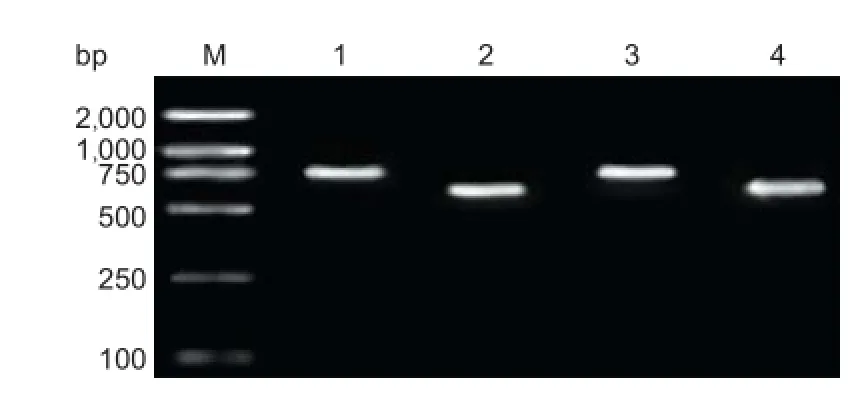
Figure 4 Identifi cation of double-gene recon by PCR.
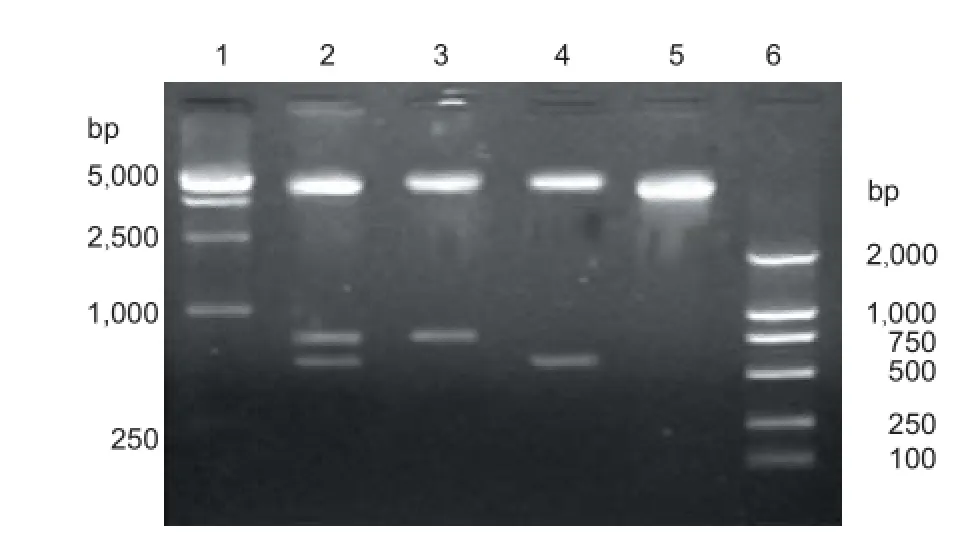
Figure 5 Identification of recombinant plasmid pIRES-BDNF-CNTF by enzymatic digestion.
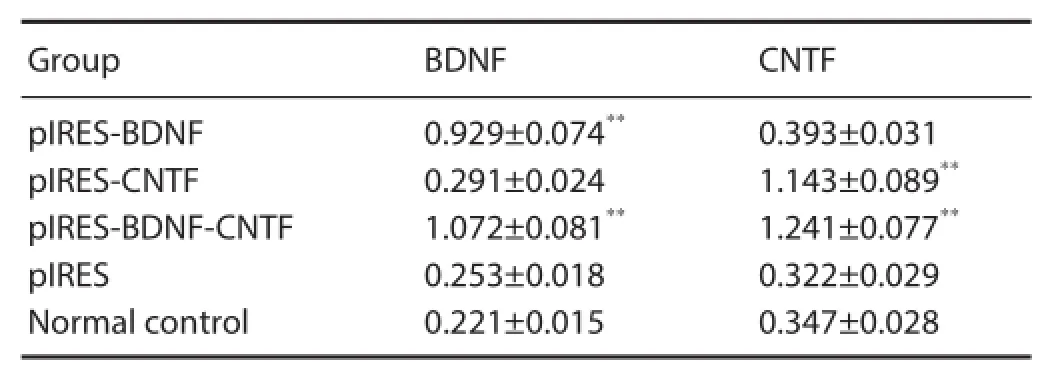
Table 1 mRNA expression (relative optical density value) of BDNF and CNTF in BMSCs after transfection (RT-PCR)
Figure 6 Morphology of BMSCs stably transfected with BDNF and CNTF mRNA (hematoxylin-eosin staining, optical microscope, × 400).
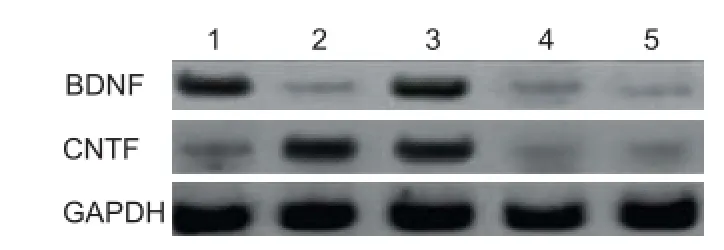
Figure 7 BDNF and CNTF protein expression in BMSCs after transfection (western blot assay).
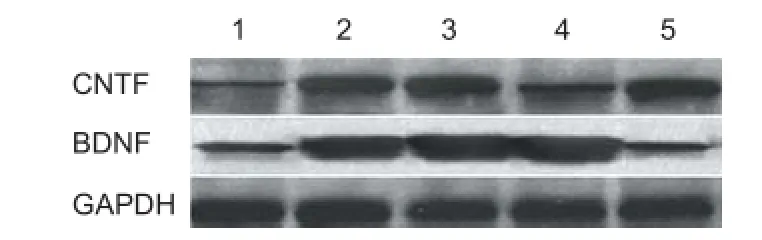
Figure 10 BDNF and CNTF protein expression in the rat nerve tissue harvested from transplantation site (western blot assay).
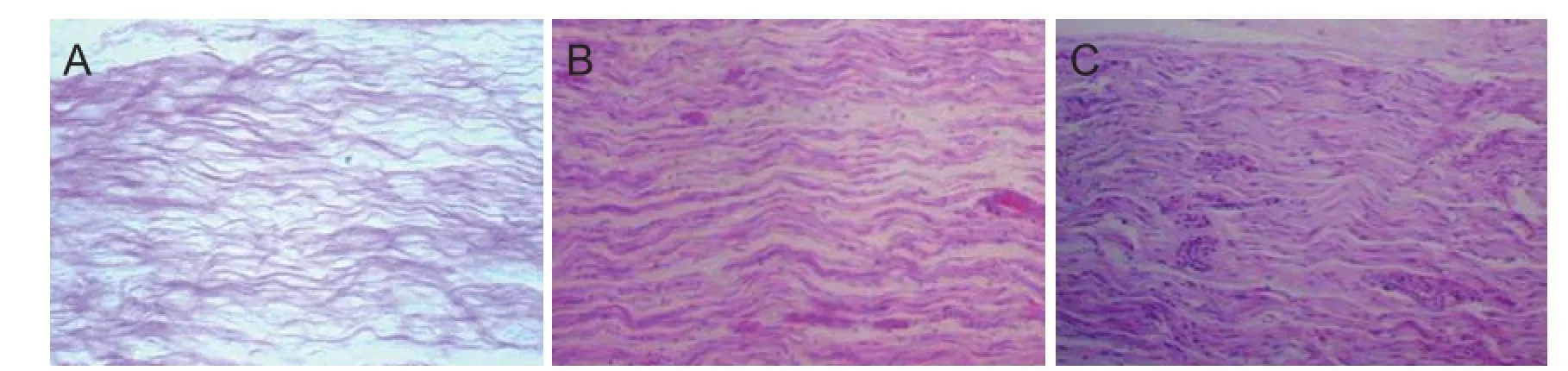
Figure 8 Morphology of distal nerve anastomotic sites at 8 weeks after transplantation of CEANA loaded with neurotrophic factors-transfected BMSCs shown on longitudinal sections (hematoxylin-eosin staining, optical microscope, × 40).
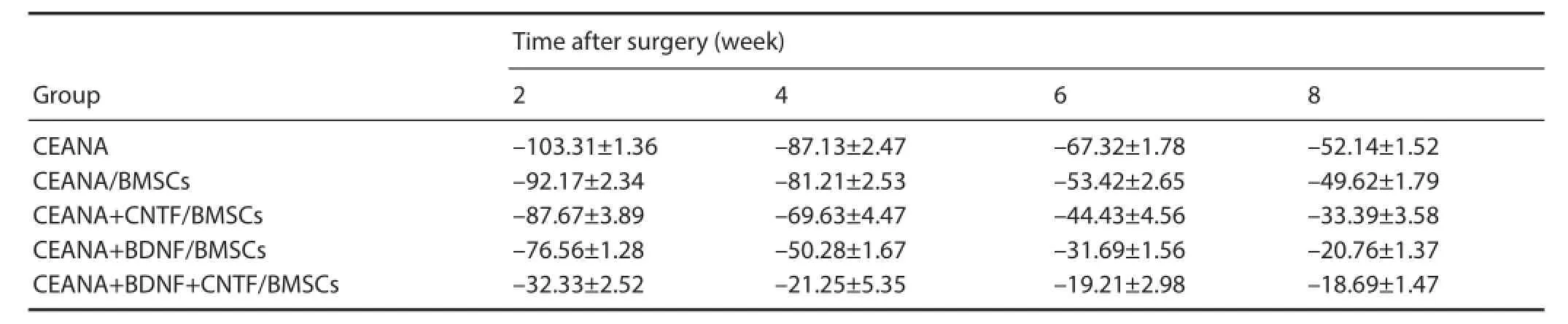
Table 2 Rat sciatic functional index (SFI) after transplantation of CEANA loaded with neurotrophic factors-transfected BMSCs
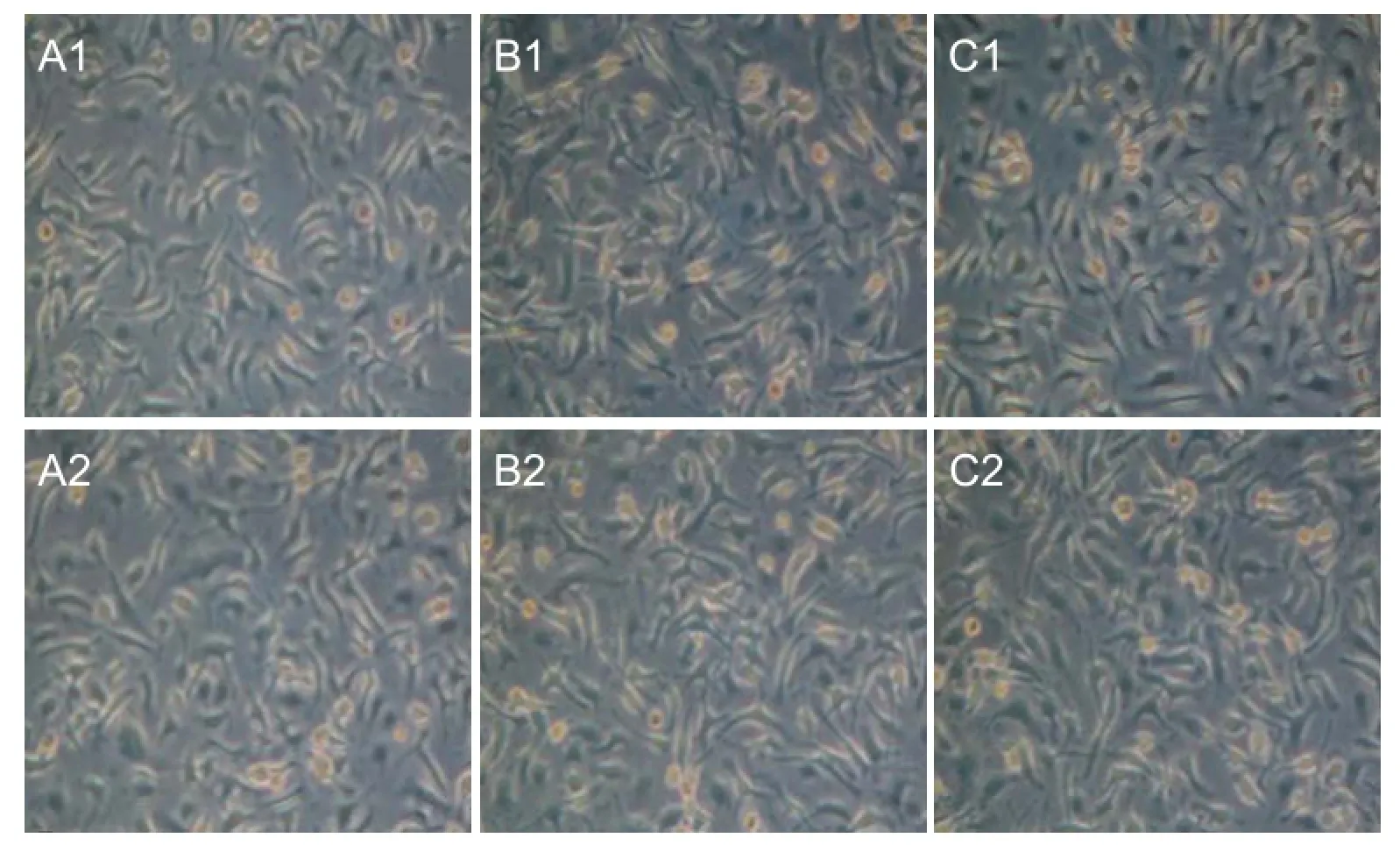
Figure 9 Morphology of distal nerve anastomotic sites at 8 weeks after transplantation of CEANA loaded with neurotrophic factorstransfected BMSCs shown on transverse sections (toluidine blue staining, optical microscope, × 40).
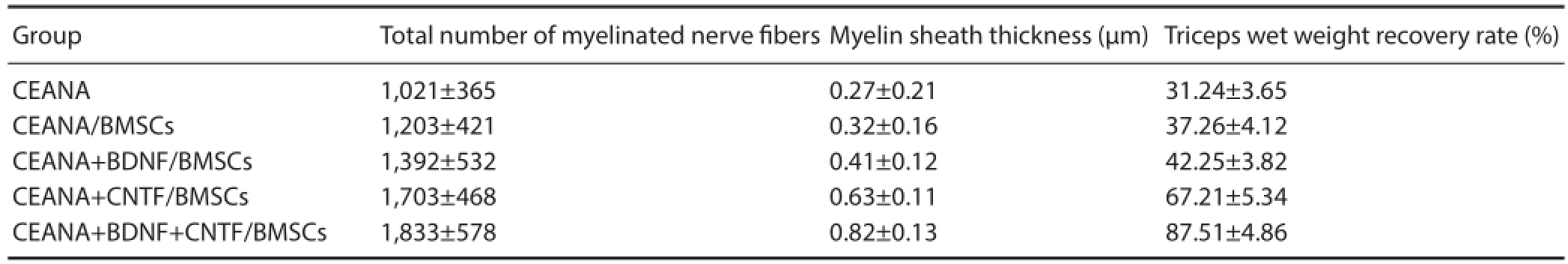
Table 3 Triceps wet weight recovery rate, total number of myelinated nerve fi bers and myelin thickness at 8 weeks after transplantation of CEANA loaded with neurotrophic factors-transfected BMSCs
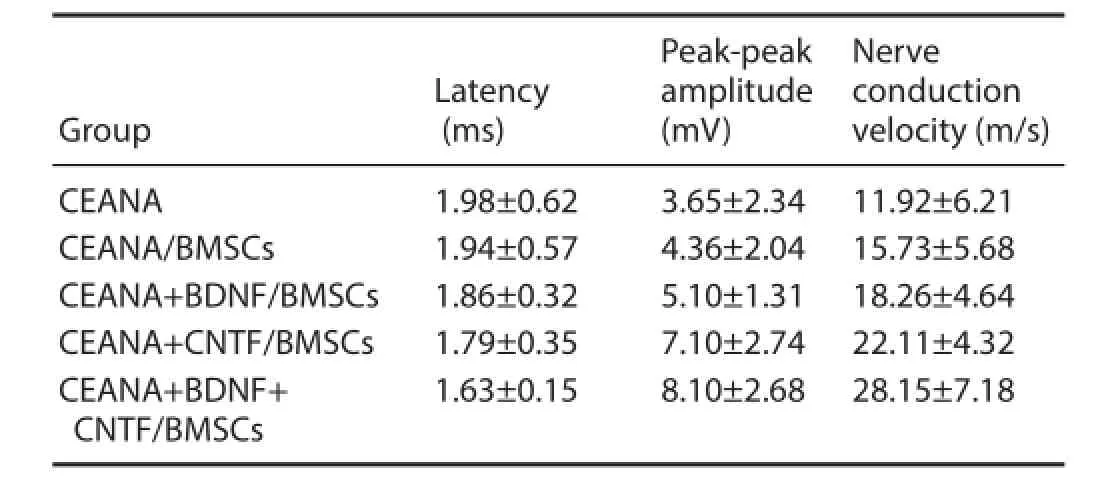
Table 4 Rat gastrocnemius muscle motor-evoked potential at 8 weeks after transplantation of CEANA loaded with neurotrophic factorstransfected BMSCs
Western blot assay
At 8 weeks after surgery, a 100-g tissue sample was harvested from the transplantation site in each group, homogenized at a low temperature and protein extracted. After protein concentration determination, electrophoresis, and transfer to membrane, protein samples were placed in antibody solution overnight at 4°C. Reagents were added in the following order: rabbit anti-mouse BDNF and rabbit anti-mouse CNTF antibodies (dilution 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), goat anti-rabbit IgG antibody (dilution 1: 500; Santa Cruz Biotechnology) and alkaline phosphatase at appropriate concentration, and protein samples were incubated for 1 hour. There were three TBST washes for 5 minutes each between each step. Thereafter, protein samples were developed with BCIP/NBT for 30–60 minutes, treated with distilled water and naturally dried. Protein expression of BDNF and CNTF in the tissue samples was detected by western blot assay using a gel image system (Bio-Rad).
Statistical analysis
All data were statistically processed using SPSS 13.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance and the least signifi cance diff erence test were used for intergroup comparison. A level of P < 0.05 was considered statistically signifi cant.
Results
BDNF and CNTF mRNA expression levels after transfection
PCR products of BDNF and CNTF target gene bands were consistent with that of the expected size (Figure 1).
Identifi cation of recombinant plasmids pIRES-BDNF and pIRES-CNTF
Recombinant plasmids pIRES-BDNF and pIRES-CNTF were preliminarily confi rmed by the presence of a 788 bp-sized BDNF band and a 611 bp-sized CNTF target gene band (Figure 2).
Identifi cation of PCR products by enzymatic digestion
Recombinant plasmid pIRES-CNTF, which was preliminarily confi rmed positive by PCR, was digested by Xba I and Sal I. Two fragments, a 597-bp-long target gene CNTF fragment and an approximately 6.1-kb-long pIRES carrier fragment, were acquired (Figure 3), which suggests that the recombinant plasmid was pIRES-CNTF. Recombinant plasmid pIRES-BDNF, which was preliminarily confi rmed as positive by PCR, was digested by Xho I and Mlu I. Two fragments, a 774-bp-long target gene BDNF fragment and an approximately 6.1-kb-long pIRES carrier fragment, were acquired (Figure 4), which suggests that the recombinant plasmid was pIRES-BDNF.
DNA sequencing
The MCS-A insertion sequence of the recombinant plasmid pIRES-BDNF, which was confirmed by PCR amplification and Xho I and Mlu I digestion, was analyzed and its homology with the sequence of NM_007540 BDNF gene in the GenBank was compared. The MCS-A insertion sequence was completely consistent with the designed. This suggests that the acquired recombinant plasmid was pIRES-BDNF. The MCS-B insertion sequence of the recombinant plasmid pIRES-CNTF, which was confirmed by PCR amplification and Xba I and Sal I digestion, was analyzed and its homology with the sequence of NM_170786 CNTF gene in the GenBank was compared. The MCS-B insertion sequence was completely consistent with the designed. This suggests that the acquired recombinant plasmid was pIRES-CNTF.
Reconstruction of recombinant plasmid pIRES-BDNF-CNTF
PCR identification: In Figure 4, there was a 611-bp-long target gene CNTF, indicating that target gene CNTF was inserted into the MCS-B sequence of recombinant plasmid pIRES-BDNF. At the same time, a 788-bp-long target gene BDNF also appeared on the vector pIRES. Therefore, double-gene recombinant plasmid pIRES-BDNF-CNTF was preliminarily confi rmed.
Enzymatic digestion: In Figure 5, there was a 6.1-kb-long vector band, a 774-bp-long BDNF gene band and a 597-bplong CNTF gene band. This suggests that the double-gene recombinant plasmid is pIRES-BDNF-CNTF.
Recombinant plasmid expression in BMSCs after transfection
At 24 hours after pIRES-BDNF-CNTF was electrically transferred to BMSCs, DMEM containing 400 μg/mL G418 was added. About 3 weeks later, stable BDNF and CNTF mRNA-transfected BMSCs were acquired (Figure 6) and the cells were maintained with DMEM containing 400 μg/mL G418.
BDNF and CNTF mRNA expression after transfection
RT-PCR results showed that in the pIRES-BDNF group (or pIRES-CTNF group) and pIRES-BDNF-CNTF group, pIRES-BDNF (or pIRES-CTNF group) and pIRES-BDNFCNTF expressed by cells can highly transcribe BDNF (or CTNF) mRNA (P < 0.01, vs. plRES group and blank control group) (Table 1).
BDNF and CNTF protein expression after transfection
Western blot assay showed an intense BDNF band in BMSCs from the plRES-BDNF and plRES-BDNF-CNTF groups, and a much lighter BDNF band in the plRES-CNTF, plRES and blank control groups (Figure 7). Thus pIRES-BDNF and pIRES-BDNF-CNTF transfected in BMSCs from the pIRES-BDNF and pIRES-BDNF-CNTF groups can highly express BDNF protein and confi rms that BMSCs strains highly expressing BDNF, CNTF and BDNF-CNTF were acquired.
General condition of rats after surgery
At 8 weeks after surgery, rat wound healing was good and without infection, and the ulcer at the surgical site disappeared completely. There was no obvious diff erence in surgical surface before and after surgery in the CEANA + BDNF/ BMSCs, CEANA + CNTF/BMSCs, CEANA + BDNF + CNTF/ BMSCs, and CEANA/BMSCs groups. In the CEANA + BDNF + CNTF/BMSCs group, rat feet could completely straighten when walking and there was no obvious diff erence in locomotor activity between the left and right sides. Rats in the CEANA and CEANA/BMSCs groups bent their bodies when walking.
Morphology of distal anastomotic stoma of nerve allografts
Hematoxylin-eosin staining: At 8 weeks after surgery, myelinated nerve fi bers were unevenly distributed in the CEANA + BDNF/BMSC, CEANA + CNTF/BMSCs and CEANA + BDNF + CNTF/BMSCs groups (Figure 8). Toluidine blue staining showed a high number of large-sized regenerated nerve fi bers with a thick myelin sheath. The number of myelinated nerve fi bers and myelin thickness were greater in the CEANA + BDNF + CNTF/BMSCs group than in the CEANA + BDNF/BMSCs and CEANA + CNTF/BMSCs groups
(Figure 9). The number of myelinated nerve fi bers and myelin thickness in the CEANA and CEANA/BMSCs groups were inferior to those in the CEANA + BDNF/BMSC, CEANA + CNTF/BMSCs and CEANA + BDNF + CNTF/BMSCs groups.
Rat SNFI after surgery
Before and 2, 4, 6 and 8 weeks after surgery, wounds healed well. SNFI in the CEANA + BDNF/BMSCs, CEANA + CNTF/BMSCs, and CEANA + BDNF + CNTF/BMSC groups increased with time. At 8 weeks after surgery, SNFI was highest in the CEANA + BDNF + CNTF/BMSCs group, followed by CEANA + CNTF/BMSCs, CEANA + BDNF/BMSCs, CEANA/BMSCs groups and lowest in the CEANA group (P< 0.05; Table 2).
Triceps wet weight recovery rate, total number of
myelinated nerve fi bers and myelin thickness after surgery At 8 weeks after surgery, triceps wet weight recovery rate, total number of myelinated nerve fi bers and myelin thickness in the CEANA + BDNF + CNTF/BMSCs group were signifi cantly higher than in the other groups (P < 0.05). A large number of large-sized regenerated nerve fi bers were observed in the CEANA + BDNF/BMSCs, CEANA + CNTF/ BMSCs and CEANA + BDNF + CNTF/BMSCs groups. Triceps wet weight recovery rate, total number of myelinated nerve fi bers, and myelin thickness were highest in the CEANA + BDNF + CNTF/BMSCs group, followed by CEANA + CNTF/BMSCs, CEANA + BDNF/BMSCs, CEANA/BMSCs groups and lowest in the CEANA group (P < 0.05; Table 3).
Electrophysiological index changes of injured rat sciatic nerve after surgery
At 8 weeks after surgery, rat gastrocnemius muscle motor-evoked potential amplitude in the CEANA+ CNTF/ BMSCs group was signifi cantly higher than in the CEANA + BDNF /BMSCs group, but it was signifi cantly lower than in the CEANA + BDNF + CNTF/BMSCs group (both P < 0.05). In each group, motor-evoked potential latency was gradually shortened, amplitude was gradually increased, and nerve conduction velocity was gradually increased after surgery (all P < 0.05; Table 4).
BDNF and CNTF protein expression in the rat nerve tissue harvested from transplantation site
Western blot assay results of the CEANA, CEANA + BDNF/ BMSCs, CEANA + CNTF/BMSCs, CEANA + BDNF + CNTF/BMSCs, and CEANA/BMSCs group are shown in Figure 10. BDNF expression in the rat nerve allografts from CEANA + BDNF/BMSCs and CEANA + BDNF + CNTF/ BMSCs was stronger than in the other groups, indicating that recombinant plasmids pIRES-BDNF and pIRES-BDNF-CNTF were normally expressed in the nerve allografts. CNTF expression in the rat nerve allografts from CEANA + CNTF/BMSCs and CEANA + BDNF + CNTF/BMSCs was stronger than in the other groups, indicating that recombinant plasmids pIRES-CNTF and pIRES-BDNF-CNTF were normally expressed in the nerve allografts.
Discussion
Neurotrophic factors in the microenvironment play an important role in peripheral nerve regeneration (Zhang et al., 2013). Strong evidence exists that dissoluble protein can promote the synthesis of some nerve regeneration-related enzymes and exhibit eff ects on neuronal regeneration and survival by inhibiting neuronal apoptosis (Kubo et al., 2001; Zhang et al., 2014a, b). BDNF and CNTF are important neurotrophic factors which aff ect central and peripheral neurons and not only increase the survival rate of chick embryo sensory neurons, but also promote neurite growth (Mohammad et al., 2000; Mligilich et al., 2002).
BMSCs are a kind of non-hematopoietic adult stem cell that exhibits multidirectional diff erentiation potential, can express Schwann cell markers after in vitro culture and differentiation, and promote the repair of peripheral nerve defects after transplantation (Lassner et al., 1989; Matsumoto et al., 2005). Taken together, BMSCs are characterized by a rich source, strong proliferative capacity and low immunogenicity (Sun et al., 2006). BMSCs can migrate widely and integrate into surrounding tissue easily, and better transfect and express exogenous genes than other stem cells. All these features indicate that BMSCs are ideal carrier cells for gene therapy (Hudson et al., 2004a, b).
CEANA exhibits extremely low immunogenicity, reduces post-transplantation immunological rejection, creates an ideal microenvironment for nerve regeneration, guides the neurite of regenerated peripheral nerves to grow from the proximal disassociated nerve end toward the distal part, reduces the opportunities of aberrant nerve regeneration, ensures selective regeneration, and contributes to growth and functional recovery of regenerated nerves (Zhang et al., 2014a).
In this study, we constructed genetically engineered cells by transfecting BMSCs with BDNF and CNTF using eukaryotic expression vectors. We detected BDNF and CNTF mRNA expression after transfection and thus, BMSCs strains highly expressed BDNF mRNA, CNTF mRNA and BDNF-CNTF mRNA. We used CEANA compounded with BDNF and CNTF-transfected BMSCs to repair sciatic nerve defects. Results showed that in the CEANA + BDNF + CNTF/BMSCs group, tissue adhesion was milder than that in the other groups, no obvious infl ammatory reaction was observed, and only a small amount of scar tissue formed. The number of myelinated nerve fi bers, myelin thickness, sciatic functional index, and triceps wet weight recovery rate were highest in the CEANA + BDNF + CNTF/BMSCs group, followed by CEANA + CNTF/BMSCs group, CEANA + BDNF/BMSCs group, CEANA/BMSCs group, and lowest in the CEANA group. In the CEANA + BDNF + CNTF/BMSCs group, neurite density, nerve regeneration velocity, and motor-evoked potential amplitudes in the soleus muscle were greater compared with the other groups. Western blot assay results showed that at 8 weeks after surgery, pIRES-BDNF and pIRES-BDNF-CNTF were normally expressed in the
nerve allografts. The CNTF in the nerve allografts from the CEANA + BDNF + CNTF/BMSCs and CEANA + CNTF/ BMSCs groups was stronger than that in the nerve allografts from the CEANA + BDNF/BMSCs, CEANA + BMSCs and CEANA groups. These results indicate that after CNTF and CNTF mRNA transfection in BMSCs, recombinant plasmids pIRES-CNTF and pIRES-BDNF-CNTF are normally expressed in the nerve allografts.
This suggests that BDNF- and CNTF-transfected BMSCs injected into CEANA exhibit effects on peripheral motor neurons; that is, they fi rst inhibit the irreversible degeneration of soma and maintain neuronal growth, then induce regenerated neurites to extend and pass through the injured site, and lastly promote neurite growth cone to enter the target organ, diff erentiate into new neurites and advance nerve regeneration. We also found that the treatment with BDNF and CNTF together shows better therapeutic eff ects than that involving just one neurotrophic factor. Therefore, it is presumed that BDNF and CNTF show synergistic effects. Results from this study also suggest that BDNF and CNTF mRNA-transfected BMSCs secrete a large amount of BDNF and CNTF and promote the regeneration of peripheral tissue cells through paracrine action. After transplantation, the carrier cells not only secrete a large amount of neurotrophic factors, but also compensate for neurons lost during ischemic injury. CEANA compounded with BDNF and CNTF-transfected BMSCs for repair of sciatic nerve injury is better than CEANA singularly compounded with BDNF or CNTF-transfected BMSCs.
Author contributions: WHH and YRZ designed this study and analyzed experimental data. YRZ, HZ and KK performed experiments. GCZ, GQZ and YS participated in molecular biology experiments. All authors approved the fi nal version of this paper.
Confl icts of interest: None declared.
Hudson TW, Liu SY, Schmidt CE (2004a) Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng 10:1346-1358.
Hudson TW, Zawko S, Deister, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE (2004b) Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng 10:1641-1651.
Krase A, Abedian R, Steck E, Hurschler C, Richter W (2014) BMP activation and Wnt-signalling aff ect biochemistry and functional biomechanical properties of cartilage tissue engineering constructs. Osteoarthritis Cartilage 22:284-292.
Kubo M, Sonoda Y, Muramatsu R, Usui M (2001) Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci 42:1539-1546.
Lassner F, Schaller E, Steinhoff G, Wonigeit K, Walter G F, Berger A (1989) Rejection and regeneration in peripheral nerve allografts. Transplantation 48:386-392.
Mamun MA, Khan AAM, Alles N, Matsui M, Tabata Y, Ohya K, Aoki K (2013) Gelatin hydrogel carrier with the W9-peptide elicits synergistic eff ects on BMP-2-induced bone regeneration. J Oral Biosci 55:217-223.
Matsumoto K, Ohnishi K, Kiyotani T, Muramatsu R (2005) Polyglycolic acid(pga)-collagen tube fi lled.with lamin in coated collagen fi bers: a histological and eleetrophysiological evaluation of regeneration. Brain Res 868:315-328.
Min HK, Oh SH, Lee JM, Im GI, Lee JH (2014) Porous membrane with reverse gradients of PDGF-BB and BMP-2 for tendon-to-bone repair: In vitro evaluation on adipose-derived stem cell diff erentiation. Acta Biomaterialia 10:1272-1279.
Mligiliche N, Endo K, Okamoto K, Fujimoto E, Ide C (2002) Extracellular matrix of human amnion manufactured into tubes as conduits for peripheral nerve regeneration. J Biomed Mater Res 63:591-600.
Mohammad J, Shenaq J, Rabinovsky E, Shenaq S (2000) Modulation of peripheral nerve regeneration: a tissue-engineering approach. The role of amnion tube nerve conduit across a 1-centimeter nerve gap. Plast Keconstr Surg 105:660-666.
Nakazato K, Song H, Waga T (2007) Dietary apple polyphenols enhance gastrocnemius function in Wistar rats. Med Sci Sports Exerc 39:934-940.
Prall WC, Haasters F, Heggeb? J, Polzer H, Schwarz C, Gassner C, Grote S, Anz D, J?ger M, Mutschler W, Schieker M (2013) Mesenchymal stem cells from osteoporotic patients feature impaired signal transduction but sustained osteoinduction in response to BMP-2 stimulation. Biochem Biophys Res Commun 440:617-622.
Sondell M, Lundborg G, Kanje M (1998) Regeneration of the rat sciaticnerve into allografi s acellular through chemical extraction. Brain Res 795:44-54.
Sun MX, Tang JS, Xu WJ (2006) Experimental study on chemical extracted acellular nerve allograft. Chin J Orthop 26:267-271.
Tiago DM, Marques CL, Roberto VP, Cancela ML, Laizé V (2014) Mir-20a regulates in vitro mineralization and BMP signaling pathway by targeting BMP-2 transcript in fi sh. Arch Biochem Biophys 543:23-30.
Zhang YR, Zhang GC, Liu QH, Wang YS (2013) Effect of amnion-wrapped allogenic nerve bridging on peripheral nerve injury. Int J Morphol 31:980-985.
Zhang YR, Zhang H, Zhang GH, Ka K, Huang WH (2014a) Chemically extracted acellular allogeneic nerve graft combined with ciliary neurotrophic factor promotes sciatic nerve repair. Neural Regen Res 9:1635-1638.
Zhang YR, Zhang H, Zhang GC, Ka K, Huang WH (2014b) Combining acellular nerve allografts with brain-derived neurotrophic factor transfected bone marrow mesenchymal stem cells restores sciatic nerve injury better than either intervention alone. Neural Regen Res 9:1814-1819.
Zheng GF, Xin C, An GL (2000) Cold Storage allograft nerve receptors in plasma bridging nerve defects morphological study. J Hand Surg 2:123-125.
Copyedited by Paul P, Robens J, Wang J, Li CH, Song LP, Zhao M
*Correspondence to:
Wen-hua Huang, Ph.D. or Yan-ru Zhang, jiaoxueban2010@126.com.
orcid:
0000-0001-6965-5898 (Wen-hua Huang)
10.4103/1673-5374.165523
http://www.nrronline.org/
Accepted: 2015-07-05
- 中國神經(jīng)再生研究(英文版)的其它文章
- Lactulose enhances neuroplasticity to improve cognitive function in early hepatic encephalopathy
- Elastic modulus aff ects the growth and diff erentiation of neural stem cells
- Optimal concentration and time window for proliferation and diff erentiation of neural stem cells from embryonic cerebral cortex: 5% oxygen preconditioning for 72 hours
- Stem Cell Ophthalmology Treatment Study (SCOTS) for retinal and optic nerve diseases: a case report of improvement in relapsing auto-immune optic neuropathy
- Polylactic-co-glycolic acid microspheres containing three neurotrophic factors promote sciatic nerve repair after injury
- Transplantation of erythropoietin gene-modifi ed neural stem cells improves the repair of injured spinal cord

