Paclitaxel-etoposide-carboplatin/cisplatin versus etoposidecarboplatin/cisplatin as first-line treatment for combined small-cell lung cancer: a retrospective analysis of 62 cases
Yue-Ya Li, Chan Zhou, Deng-Xia Yang, Jing Wang, Zhu-Jun Liu, Xin-Yue Wang, Kai Li
Department of Thoracic Oncology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin Lung Cancer Diagnosis and Treatment Center, Tianjin 300060, China
ORIGNAL ARTICLE
Paclitaxel-etoposide-carboplatin/cisplatin versus etoposidecarboplatin/cisplatin as first-line treatment for combined small-cell lung cancer: a retrospective analysis of 62 cases
Yue-Ya Li, Chan Zhou, Deng-Xia Yang, Jing Wang, Zhu-Jun Liu, Xin-Yue Wang, Kai Li
Department of Thoracic Oncology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin Lung Cancer Diagnosis and Treatment Center, Tianjin 300060, China
Objective: To compare the efficacy and adverse effects of paclitaxel-etoposide-carboplatin/cisplatin (TEP/TCE) regimen with those of etoposide-carboplatin/cisplatin (EP/CE) regimen as first-line treatment for combined small-cell lung cancer (CSCLC).
Methods: A retrospective study was conducted on 62 CSCLC patients who were treated at Tianjin Medical University Cancer Institute and Hospital from July 2000 to April 2013 and administered with TEP/TCE regimen (n=19) or EP/ CE regimen (n=43) as first-line CSCLC treatment. All patients received more than two cycles of chemotherapy, and the response was evaluated every two cycles. The primary endpoint was overall survival (OS), and the secondary endpoints were progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), and adverse effects.
Results: ORR between the TEP/TCE and EP/CE groups showed a statistical difference (90% vs. 53%, P=0.033). Both groups failed to reach a statistical difference in DCR (100% vs. 86%, P=0.212). The median PFS and OS of the TEP/TCE group were slightly longer than those of the EP/CE group, although both groups failed to reach a statistical difference (10.5 vs. 8.9 months, P=0.484; 24.0 vs. 17.5 months, P=0.457). However, stratified analysis indicated that the PFS of patients with stages III and IV CSCLC showed marginally significant difference between the TEP/TCE and EP/CE groups (19.5 vs. 7.6 months; P=0.071). Both rates of grade IV bone marrow depression and termination of chemotherapy in the TEP/TCE group were significantly higher than those in the EP/CE group (26.3% vs. 7.0%, P=0.036; 31.6% vs. 14.7%, P=0.004).
Conclusion: The TEP/TCE regimen may not be preferred for CSCLC, and this three-drug regimen requires further exploration and research. To date, the EP/CE regimen remains the standard treatment for CSCLC patients.
Small cell lung carcinoma (SCLC); chemotherapy; CE regimen; adverse effects; survival analysis; prognosis
Introduction
Small-cell lung cancer (SCLC) accounts for approximately 20% of all lung cancers worldwide1,2. Combined small-celllung cancer (CSCLC) is a subtype of SCLC, representing 2%-28% of SCLC cases3,4. According to the 2004 World Health Organization (WHO)/International Association for the Study of Lung Cancer classification of lung and pleural tumors5, CSCLC is defined as SCLC combined with non-small cell lung cancer (NSCLC) components, which usually include adenocarcinoma (Ad), squamous-cell carcinoma, large-cell carcinoma6-8, and spindle cell carcinoma9,10. Despite the rare incidence of CSCLC, this malignancy not only grows fast but also resists chemotherapy. Thus, this cancer is taken seriouslyin clinical studies. Unfortunately, no standard regimen has been determined for CSCLC; therefore, its treatment mainly refers to the therapeutic regimens of pure SCLC, such as etoposidecarboplatin/cisplatin (EP/CE)8. However, CSCLC often yields poor prognosis because the combined NSCLC components may be insensitive to such chemotherapy regimens. Given that the majority of combined components of CSCLC were Ad8,10,11. Zhu et al.12added paclitaxel to the EP/CE regimen, thereby forming paclitaxel-etoposide-carboplatin/cisplatin (TEP/TCE) regimen for CSCLC. Nevertheless, a consensus on whether the efficiency and security of TEP/TCE regimen is superior to the standard EP/CE regimen remains unclear. The present retrospective study included 62 CSCLC patients, who were diagnosed pathologically. These patients underwent complete follow-up sessions and received initial treatment at the Tianjin Medical University Cancer Institute and Hospital from July 2006 to April 2013. On the basis of the chemotherapy regimens administrated, 62 patients were classified into two-drug group (receiving EP/CE regimen) and three-drug group (receiving TCE/TEP regimen) to compare the tumor response, survival benefits, and adverse effects of the two groups.
Materials and methods
Eligibility of patients
A total of 62 primary CSCLC patients who were treated at the Cancer Hospital of Tianjin Medical University from July 2006 to April 2013 were enrolled in this study. The following inclusion criteria were applied: (I) patients were diagnosed with CSCLC, which was confirmed via pathology or cytology; (II) patients were previously naive to chemotherapy, radiotherapy, or surgery; (III) patients exhibited no other malignancies; (IV) the lesions of patients can be evaluated via imaging; (V) their ages ranged from 34-79, and Karnofsky Performance Status (KPS) score was ≥60; (VI) the results of their blood, routine urine, electrolyte, liver function, kidney function, and electrocardiogram tests were within normal range; (VII) patients underwent complete follow-up sessions.
Chemotherapy
The two regimens were TEP/TCE (paclitaxel 135 mg/m2, intravenous on day 1; etoposide 100 mg/m2, intravenous on days 1-3; carboplatin calculated at the area under the curve (AUC) =5, intravenous on day 1 or cisplatin 25 mg/m2, intravenous on days 1-3) and EP/CE (etoposide 100 mg/m2, intravenous on days 1-3; carboplatin calculated at AUC =5, intravenous on day 1 or cisplatin 25 mg/m2, intravenous on days 1-3).
Evaluation of response
CT or MRI scan was performed to evaluate tumor response every two chemotherapeutic cycles and at the end of treatment. Patients were examined monthly within 3 months after the end of treatment, every 2 months within 1 year after the end of treatment, and every 3-6 months thereafter. Unidirectional measurements were conducted in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 to evaluate short-term effects. Following the RECIST1.1, tumor response to treatment was classified as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).
Objective response rate (ORR) was defined as the proportion of patients evaluated as CR and PR, whereas disease control rate (DCR) was calculated as the proportion of patients evaluated as CR, PR, and SD. Overall survival (OS) was defined as the time from the start of treatment until death caused by any cause or until the last follow-up date. Moreover, progression-free survival (PFS) was defined as the time from the start of treatment to disease progression or death.
Toxicity
Chemotherapy-related adverse reactions were divided into five degrees (0-IV) based on the WHO classification of acute and subacute toxicity performance and indexing standards.
Statistical analysis
Statistical analyses were performed with SPSS 13.0. A P value≤0.05 was considered statistically significant. Kaplan-Meier method was used to estimate the PFS and OS. Multivariate analysis of the prognostic factors was performed using Cox’s regression model. Categorical variables were analyzed via χ2test, and measurement data were analyzed using t-test.
Results
A total of 540 CSCLC patients existed in 2,371 SCLC cases; thus, the incidence of CSCLC was 22.78%. Finally, we collected the data of 62 CSCLC patients who have met our inclusion criteria. Among 62 CSCLC patients, 49 were males and 13 were females, and the ratio of male patients to female patients was 3.85:1. The age of patients ranged from 34-79 years old, and the median age was 60. Smoking history was confirmed in 51 cases. In accordance with TNM staging, 5 patients were categorized in stage I, 5 patients were in stage II, and 52 patientswere in advanced stage (25 patients were in stage III and 27 patients were in stage IV). The two chemotherapy regimen groups showed no significant differences in the baseline data (Table 1).
A total of 15 patients at stages I, II, and IIIa (T1-3N2M0) received surgery and adjuvant chemotherapy with 3-drug regimen or 2-drug regimen. Other patients received radiationcombined with chemotherapy or chemotherapy alone. Among the 62 patients, 19 received TEP/TCE regimen and 43 received EP/CE regimen.

Table 1 General condition of 62 primary CSCLC patients, n (%)
Both chemotherapy regimens were administered at an interval of 3 weeks, and each patient completed at least 2 cycles of chemotherapy. Some patients accepted thoracic radiotherapy within 2 to 4 cycles of chemotherapy with a total dose of 50 Gy, which was administered with 2 Gy per fraction and conducted 5 d a week. Some other patients received prophylactic cranial irradiation with a total dose of 30 Gy, which was administered with 3 Gy per fraction and conducted 5 d a week after the chemotherapy was completed.
Effects
The TEP/TCE and EP/CE groups showed a statistical difference in ORR (90% vs. 53%, P=0.033, χ2=4.552). However, both groups failed to reach a statistical difference in DCR (100% vs. 86%, P=0.212, χ2=1.558) (Table 2).
Survival analysis
All patients were followed up until November 28, 2013, and the median follow-up time was 12.7 months (range, 2-73 months). A total of 30 patients were alive at the end of follow-up, which comprised 11 patients from the TEP/TCE group and 19 patients from the EP/CE group. The median PFS and OS of the TEP/ TCE group were slightly longer than those of the EP/CE group, although both groups failed to reach a statistical difference (10.5 vs. 9.8 months, P=0.484, χ2=0.489; 24 vs. 17.5 months, P=0.457, χ2=0.554) (Figures 1,2). However, stratified analysis indicated that in patients with stages III and IV CSCLC, the median PFS nearly reached a statistical difference between the TEP/TCE and EP/CE groups (19.5 vs. 7.6 months,P=0.071, χ2=3.259), whereas the median OS failed to reach a statistical difference (22.8 vs. 14.3 months, P=0.269, χ2=1.224) (Figures 3,4). However, no significant difference existed between the two groups at stages I and II (Figures 5,6).

Table 2 Comparison of response between two groups, n (%)
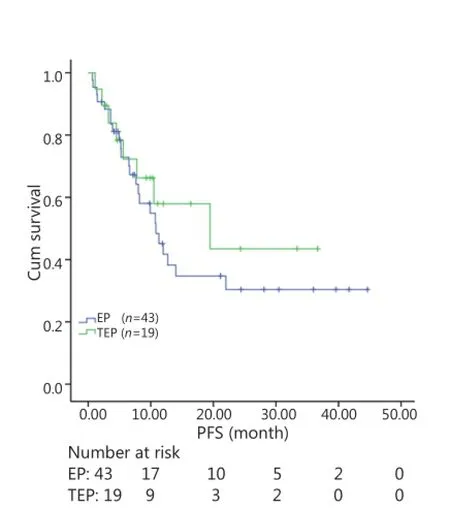
Figure 1 Kaplan-Meier curves for PFS of 62 patients with stages I-IV CSCLC. The median PFS of 3-drug group was not significantly longer than that of 2-drug group (10.5 vs. 9.8 months, P=0.484). PFS, progression-free survival; CSCLC, combined small-cell lung cancer.
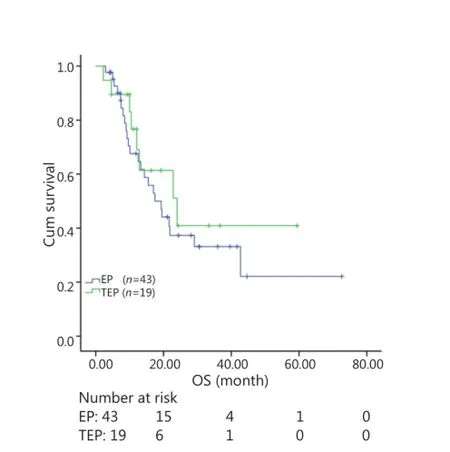
Figure 2 Kaplan-Meier curves for OS of 62 patients with stages I-IV CSCLC. The median OS of 3-drug group was not significantly longer than that of 2-drug group (24.0 vs. 17.5 months, P=0.457). OS, overall survival; CSCLC, combined small-cell lung cancer.
Univariate analysis
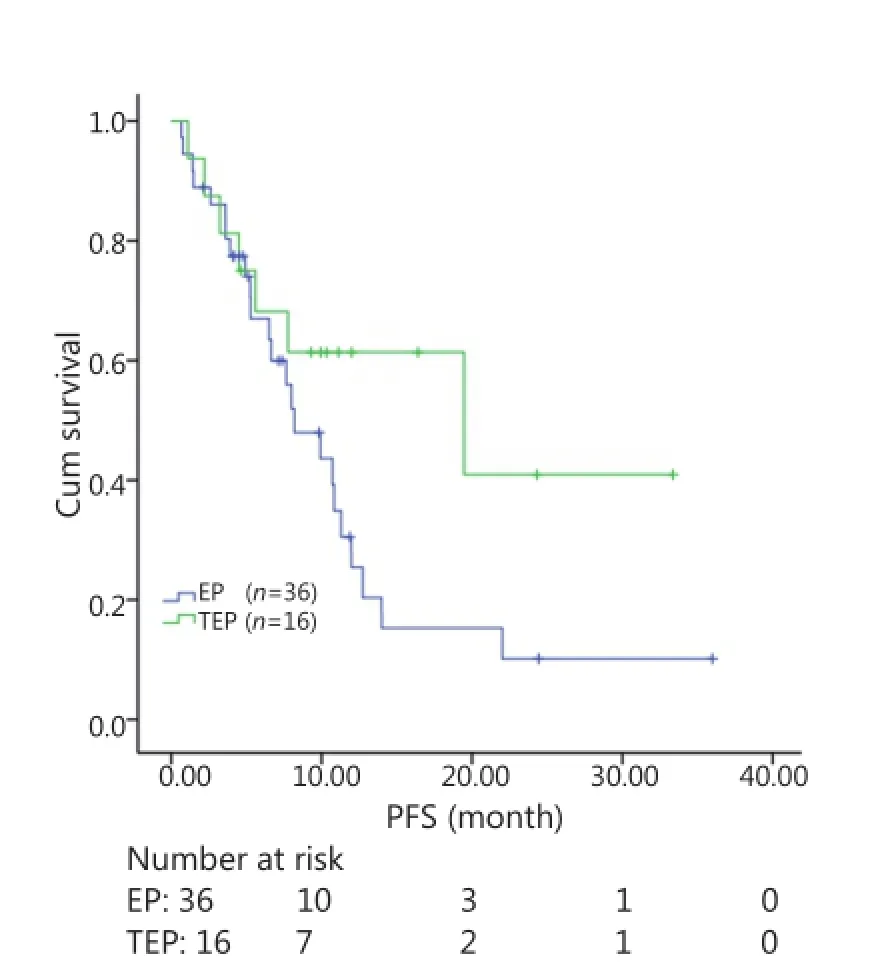
Figure 3 Kaplan-Meier curves for PFS of 52 patients with stages III-IV CSCLC. The median PFS of the two groups nearly reached a significant difference (19.5 vs. 7.6 months, P=0.071). PFS, progression-free survival; CSCLC, combined small-cell lung cancer.
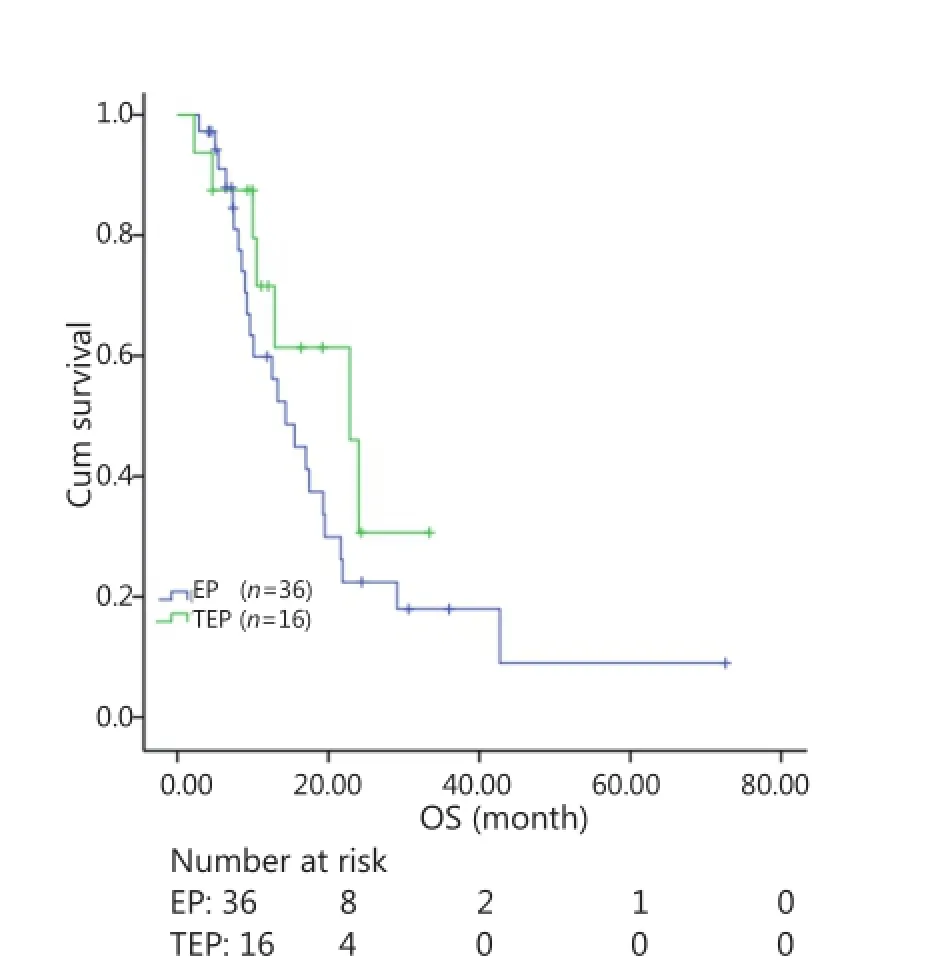
Figure 4 Kaplan-Meier curves for OS of 52 patients with stages IIIIV CSCLC. The difference in median OS between the two groups was marginally significant (22.8 vs. 14.3 months, P=0.269). OS, Overall survival; CSCLC, Combined small-cell lung cancer.
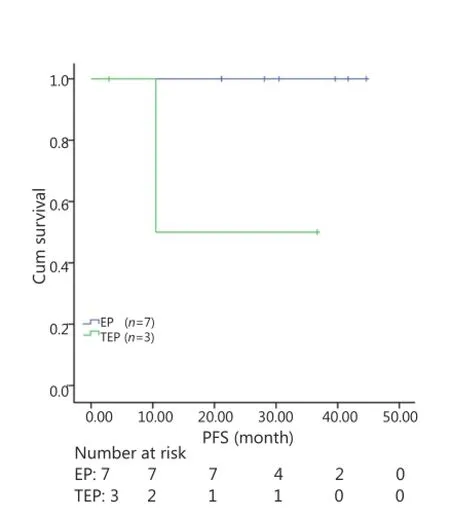
Figure 5 Kaplan-Meier curves for PFS of 10 patients with stages I-II CSCLC. The median PFS of 3-drug group was not significantly longer than that of 2-drug group (10.5 vs. 30.5 months, P=0.061). PFS, progression-free survival; CSCLC, combined small-cell lung cancer.
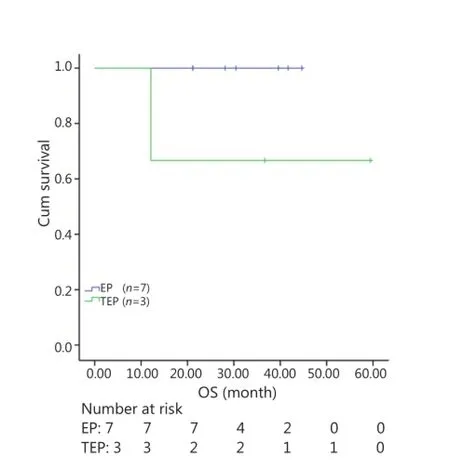
Figure 6 Kaplan-Meier curves for OS of 10 patients with stages I-II CSCLC. The median OS of 3-drug group was not significantly longer than that of 2-drug group (36.7 vs. 30.5 months, P=0.127). OS, overall survival; CSCLC, combined small-cell lung cancer.
The results showed that patients with KPS score ≤80, distant metastasis, lymph node metastasis, and tumors at stages III andIV displayed poor prognosis (P<0.001, P=0.005, 0.032, and 0.001, respectively). Furthermore, the absence of surgery and prophylactic cranial irradiation (PCI) were considered adverse prognostic factors (P=0.009, 0.042). However, age, gender, smoking index, tumor size, number of chemotherapy cycles, thoracic radiotherapy, second-/third-line therapy were not related to the prognosis (Table 3).
Multivariate analysis
A multivariate analysis of prognostic factors was performed using Cox’s regression model. The results showed that the KPS score, lymph node metastasis, distant metastasis, and PCI were independent predictors of prognosis (P=0.015, 0.007, 0.007, and 0.034, respectively) (Table 3).
Safety evaluation
The common chemotherapy-related adverse reactions included bone marrow suppression, gastrointestinal reaction, hepatic and renal function lesions, and skin rash. Most of these reactions were slight and reversible. The rates of grade IV bone marrow depression in the TEP/TCE group were significantly higher than those in the EP/CE group (26.3% vs. 7.0%, P=0.036, χ2=4.385). However, although the incidences of skin rash and diarrhea were higher in the TEP/TCE group than in the EP/CE group, both groups (10.5% vs. 0%, P=0.09, χ2=4.677) displayed no statistical difference. The statistical data showed that the replacement and incompletion of chemotherapy were more prevalent in the TEP/ TCE group than in the EP/CE group because of serious adverse reactions (31.6% vs. 4.7%, P=0.004, χ2=8.502), although the main reason for replacement and incompletion of chemotherapy in the EP/CE group was the disease progression (32.6% vs. 5.3%, P=0.021, χ2=5.353).
Discussion
CSCLC is currently defined by the WHO as a subset of SCLC, which not only exhibits the characteristics of small cell lung cancer, such as rapid growth and high malignant degree, but also displays the characteristics of NSCLC, such as chemotherapy resistance. Patients with CSCLC have demonstrated poor response to chemotherapy in previous studies13,14. Researchers attributed this finding to the combined NSCLC components. Thus, they attempted to explore ideal chemotherapy regimens. Luo et al.8compared the efficacy and safety of vinorelbine, ifosfamide, and cisplatin (NIP) with EP in treating advanced CSCLC, and they concluded that the ORR, PFS, and OS of patients in the NIP group were slightly inferior than those of patients under traditional EP regimen (83.8% vs. 90.6%, P=0.170; 6 vs. 6.5 months, P=0.163; 10.8 vs. 10.4 months, P=0.935, respectively). The TEP/TCE regimen has been widely used for CSCLC treatment at the Tianjin Medical University Cancer Institute and Hospital since 2005, with the aim of increasing the dose intensity and coverage rate of antitumor spectrum. The incidence of CSCLC in this institution was 22.78%, which was consistent with the findings of previous reports3,4. In our study, a large proportion of patients were male (79.03%), heavy smokers (61.29%), and beyond 60 years old, which also corresponded to the study conducted by Lu et al.15.
The ORRs of the TEP/TCE and EP/CE groups were 90% and 53%, respectively, which reached a significant difference (P=0.033, χ2=4.552). Furthermore, the DCRs of the two groups indicated no significant difference (100% vs. 86%, P=0.212, χ2=1.558). The PFS and OS of the patients in the TEP/TCE group were both slightly longer than those of the patients in the EP/CE group (11.86 vs. 12.14 months; 17.65 vs. 18.01 months, respectively). However, both groups failed to reach a statistical difference. Moreover, safety analysis showed that the incidence of grade IV bone marrow depression and grades III and IV diarrhea was significantly higher in the TEP/TCE group than in the EP/ CE group (P=0.004). Further analysis revealed that such adverse reactions in the TEP/TCE group were nearly consistent with the toxicity of paclitaxel. Statistical data revealed that the replacement and incompletion of chemotherapy were more prevalent in the TEP/TCE group than in the EP/CE group because ofserious adverse effects (31.6% vs. 4.7%, P=0.04, χ2=8.502). In summary, the administration of TEP/TCE regimen can provide beneficial short-term effects, but such effects cannot prolong the PFS and OS of patients with CSCLC. Multivariate analysis also confirmed that only the KPS score, lymph node metastasis, distant metastasis, and PCI were independent prognostic factors for patients with CSCLC. However, subgroup analysis of patients at stages III and IV revealed that the PFS and OS of patients treated with TEP/TCE regimen are slightly longer than those of patients treated with EP/CE regimen. Moreover, the differences nearly reached statistical significance (10.95 vs. 8.20 months, P=0.071; 10.2 vs. 17.62 months, P=0.089, respectively). This finding suggested that the TEP/TCE regimen may be beneficial to patients with advanced CSCLC and large tumor burden. The statistical data also showed that the main reason of replacement and completion of chemotherapy in the EP/CE group was disease progression (32.6% vs. 5.3%, P=0.021, χ2=5.353). Hence, broad-spectrum antitumor regimen may be superior to standard EP/CE regimen. However, making reasonable choices on the added drugs and reducing the incidence of side reactions areurgent problems that need to be solved. Ad is the most common combined component of CSCLC8,10,11and the effectiveness rate of paclitaxel combined with cisplatin in the treatment of NSCLC (including Ad) reached 22%-47%16-19. Although taxane is good for NSCLC, whether this drug induces the same effect on NSCLC components of CSCLC is unknown. Wagner et al.7determined that the NSCLC and SCLC components of CSCLC shared an identical immunophenotype with prevalent expression of synaptophysin and CD56 and loss of 22q13. Fukui et al.20also determined that patients with CSCLC with Ad shared an identical EGFR mutation in both SCLC and Ad components. Thus, the current findings suggested that NSCLC components of CSCLC may be close to SCLC in biology, and two kinds of components may exhibit the homology of gene sequence. We used next-generation sequencing method to compare and analyze whether the gene expression of Ad components in CSCLC is different from NSCLC. We believe that the results of our study will clarify the correlation of SCLC components with NSCLC components in CSCLC and contribute to the selection of the optimal treatment for CSCLC.
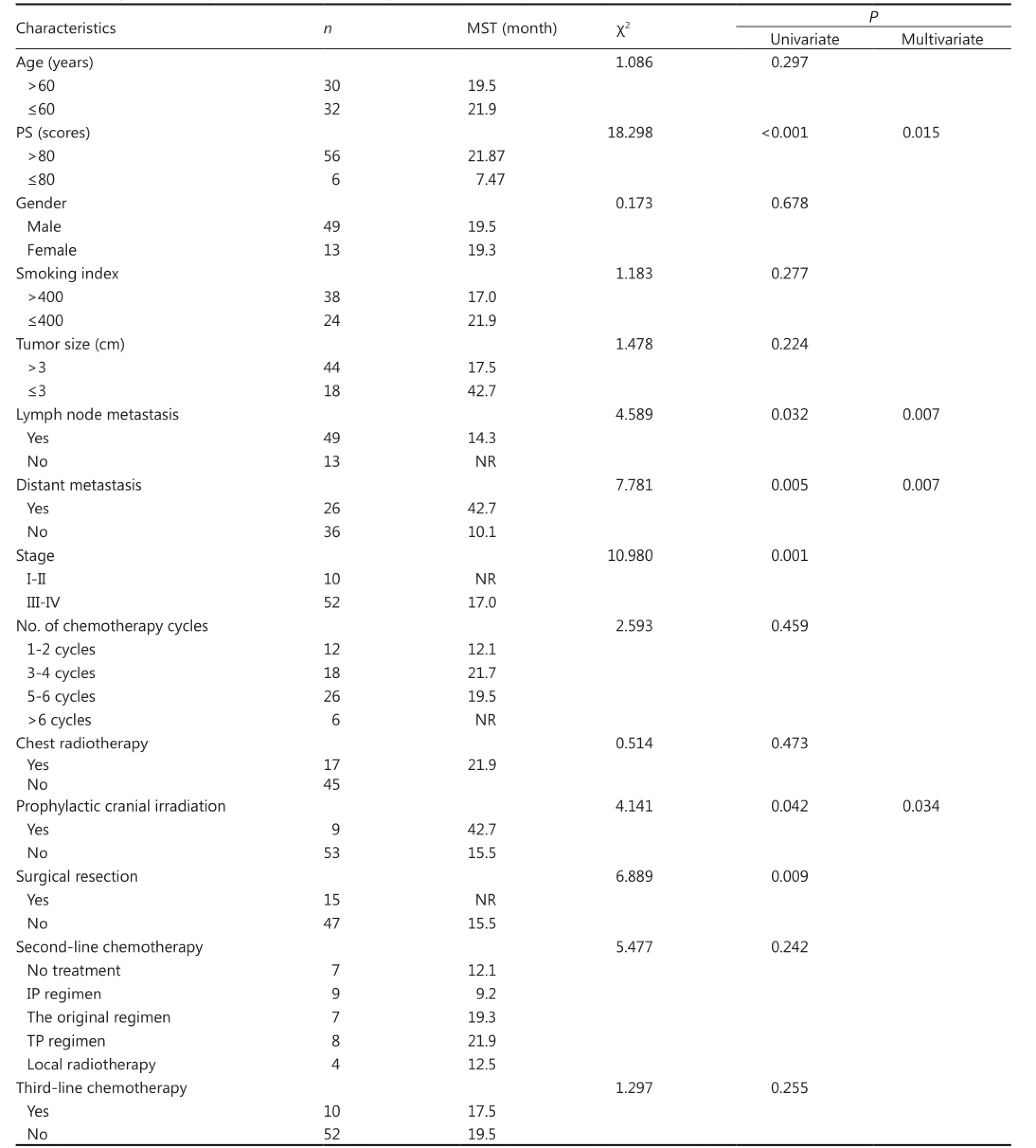
Table 3 Cox regression analysis of the factors affecting the survival of 62 patients
In conclusion, EP/CE regimen remains the standard regimen for majority of patients with CSCLC. However, three-drug regimen may increase the curative effect of patients in advanced stage. Nevertheless, this investigation is a retrospective, small, and nonrandomized study. Thus, the results need to be further confirmed by large prospective clinical trials.
Acknowledgements
This study was supported by grants from the Tianjin Municipal Science and Technology Project (Grant No. 11JCYBJC11300), National Natural Science Foundation of China (Grant No. 81372517), and National Science and Technology Major Project (Grant No. 09303001). This article was published originally in Chinese Journal of Clinical Oncology 2015;42(2): 91-95 (in Chinese).
Conflict of Interest Statement
No potential conflicts of interest are disclosed.
References
1. Stupp R, Monnerat C, Turrisi AT 3rd, Perry MC, Leyvraz S. Small cell lung cancer: state of the art and feature perspectives. Lung Cancer 2004;45:105-117.
2. Hermes A, Bergman B, Bremnes R, Ek L, Fluge S, Sederholm C, et al. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-Cell lung cancer: a randomized phase III trial. J Clin Oncol 2008;26:4261-4267.
3. Mangum MD, Greco FA, Hainsworth JD, Hande KR, Johnson DH. Combined small-cell and non-small-cell lung cancer. J Clin Oncol 1989;7:607-612.
4. Nicholson SA, Beasley MB, Brambilla E, Hasleton PS, Colby TV, Sheppard MN, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002;26:1184-1197.
5. Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC. World Health Organization Classification of Tumors. Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004:31-34.
6. Buys TP, Aviel-Ronen S, Waddell TK, Lam WL, Tsao MS. Defining genomic alteration boundaries for a combined small cell and nonsmall cell lung carcinoma. J Thorac Oncol 2009;4:227-239.
7. Wagner PL, Kitabayashi N, Chen YT, Saqi A. Combined small cell lung carcinomas: genotypic and immunophenotypic analysis of the separate morphologic Components. Am J Clin Pathol 2009;131:376-382.
8. Luo J, Wu FY, Li AW, Zheng D, Liu JM. Comparison of Vinorelbine, Ifosfamide and Cisplatin (NIP) and Etoposide and Cisplatin (EP) for Treatment of Advanced Combined Small Cell Lung Cancer (cSCLC) Patients: A Retrospective Study. Asian Pac J Cancer Prev 2012;13:4703-4706.
9. Hsiao HH, Tsai HJ, Liu YC, Tseng YT, Tseng SB, Chai CY, et al. A rare case of combined small cell lung cancer with unusual soft tissue metastasis. Kaohsiung J Med Sci 2006;22:352-356.
10. Luo J, Ni J, Zheng H, Li AW, Zhou CC. Clinical analysis of 88 cases with combined small cell carcinoma. Tumor 2009;29:156-159.
11. Liu SG, Liu JH, Li H. Diagnosis and Treatment for 31 Cases with Combined Small Cell Lung Cancer. Journal of Oncology 2013;19:900-902. (in Chinese).
12. Zhu YY, Ge W, Xu HL, Ming PP. The effect of Paclitaxe combined with Cisplatin and Etoposide comparing with Cisplatin and Etoposide on patients with small cell lung cancer: a systematic review. China Medical Herald 2014;11:62-66. (in Chinese).
13. Wang X, Jiang R, Li K. Prognostic significance of pretreatment laboratory parameters in combined small-cell lung cancer. Cell Biochem Biophys 2014;69:633-640.
14. Sehested M, Hirsch FR, Osterlind K, Olsen JE. Morphologic variations of small cell lung cancer: a histopathologic study of pretreatment and posttreatment specimens in 104 patients. Cancer 1986;57:804-807.
15. Lu HY, Mao WM, Cheng QY, Chen B, Cai JF, Wang XJ, et al. Mutation status of epidermal growth factor receptor and clinical features of patients with combined small cell lung cancer who received surgical treatment. Oncol Lett 2012;3:1288-1292.
16. Zhang S, Zhang QC, Jiang SJ. Effect of trichostatin A and paclitaxel on the proliferation and apoptosis of lung adenocarcinoma cells. Chin Med J (Engl) 2013;126:129-134.
17. Rigas JR. Taxane-platinum combinations in advanced non-small cell lung cancer: a review. Oncologist 2004;9 Suppl 2:16-23.
18. Jia JW, Wu YM, Guo SL, Li YL, Wu JX. Comparison of paclitaxel liposome and traditional taxol plus cisplatin in elderly patients with non -small cell lung cancer. Journal of the Fourth Military Medical University 2008;29:1604-1606. (in Chinese).
19. Scagliotti G. Optimizing chemotherapy for patients with advanced non- small cell lung cancer. J Thorac Oncol 2007;2 Suppl 2:S86-S91.
20. Fukui T, Tsuta K, Furuta K, Watanabe S, Asamura H, Ohe Y, et al. Epidermal growth factor receptor mutation status and clinicopathological features of combined small cell carcinoma with adenocarcinoma of the lung. Cancer Sci 2007;98:1714-1719.
Cite this article as: Li YY, Zhou C, Yang DX, Wang J, Liu ZJ, Wang XY, Li K. Paclitaxel-etoposide-carboplatin/cisplatin versus etoposide–carboplatin/ cisplatin as first-line treatment for combined small-cell lung cancer: a retrospective analysis of 62 cases. Cancer Biol Med 2015;12:117-125. doi: 10.7497/j.issn.2095-3941.2015.0012
Correspondence to: Kai Li
E-mail: likai5@medmail.com.cn
February 5, 2015; accepted May 18, 2015.
Available at www.cancerbiomed.org
Copyright ? 2015 by Cancer Biology & Medicine
 Cancer Biology & Medicine2015年2期
Cancer Biology & Medicine2015年2期
- Cancer Biology & Medicine的其它文章
- Predictive value of K-ras and PIK3CA in non-small cell lung cancer patients treated with EGFR-TKIs: a systemic review and meta-analysis
- Current approaches in treatment of triple-negative breast cancer
- Changes in tumor-antigen expression profile as human small-cell lung cancers progress
- Assays for predicting and monitoring responses to lung cancer immunotherapy
- Understanding the function and dysfunction of the immune system in lung cancer: the role of immune checkpoints
- Tumor immune microenvironment characterization and response to anti-PD-1 therapy
