Assays for predicting and monitoring responses to lung cancer immunotherapy
Cristina Teixidó, Niki Karachaliou, Maria González-Cao, Daniela Morales-Espinosa, Rafael Rosell,,3
1Pangaea Biotech, Quirón Dexeus University Hospital, Barcelona 08028, Spain;2Dr. Rosell Oncology Institute, Quirón Dexeus University Hospital, Barcelona 08028, Spain;3Cancer Biology and Precision Medicine Program, Catalan Institute of Oncology, Hospital Germans Trias i Pujol, Badalona 08916, Spain
REVIEW
Assays for predicting and monitoring responses to lung cancer immunotherapy
Cristina Teixidó1, Niki Karachaliou2, Maria González-Cao2, Daniela Morales-Espinosa2, Rafael Rosell1,2,3
1Pangaea Biotech, Quirón Dexeus University Hospital, Barcelona 08028, Spain;2Dr. Rosell Oncology Institute, Quirón Dexeus University Hospital, Barcelona 08028, Spain;3Cancer Biology and Precision Medicine Program, Catalan Institute of Oncology, Hospital Germans Trias i Pujol, Badalona 08916, Spain
Immunotherapy has become a key strategy for cancer treatment, and two immune checkpoints, namely, programmed cell death 1 (PD-1) and its ligand (PD-L1), have recently emerged as important targets. The interaction blockade of PD-1 and PD-L1 demonstrated promising activity and antitumor efficacy in early phase clinical trials for advanced solid tumors such as non-small cell lung cancer (NSCLC). Many cell types in multiple tissues express PD-L1 as well as several tumor types, thereby suggesting that the ligand may play important roles in inhibiting immune responses throughout the body. Therefore, PD-L1 is a critical immunomodulating component within the lung microenvironment, but the correlation between PD-L1 expression and prognosis is controversial. More evidence is required to support the use of PD-L1 as a potential predictive biomarker. Clinical trials have measured PD-L1 in tumor tissues by immunohistochemistry (IHC) with different antibodies, but the assessment of PD-L1 is not yet standardized. Some commercial antibodies lack specificity and their reproducibility has not been fully evaluated. Further studies are required to clarify the optimal IHC assay as well as to predict and monitor the immune responses of the PD-1/PD-L1 pathway.
Immunotherapy; lung cancer; programmed cell death 1 (PD-1); PD-1 ligand (PD-L1); antibody
Introduction
Lung cancer is among the most common cancers and the leading cause of cancer-related mortality worldwide1. Treatment options for lung cancer patients vary according to cell type, stage of disease, molecular profile, and functional status. Non-metastatic lung cancer is generally treated with curative intent using surgery, chemotherapy, targeted therapy, radiation therapy, or a combined modality approach2-8. However, the majority of the patients are diagnosed with extensive diseases and inoperable lesions9,10. Therefore, systemic therapy has become a mainstay for lung cancer management. Systemic treatments with chemotherapy have not improved patient prognosis in the last decade4,5,8,thereby emphasizing the need for new therapeutic strategies, such as immunotherapy, either as an adjunct to surgery and/or as a conventional form of cancer therapy11-13.
Lung cancer has long been considered poorly immunogenic because of the inactivity of different non-specific agents, such as Bacillus Calmette-Guerin14,15, interferon (IFN)-alpha16, and interleukin-217, as well as specific antibodies, such as trastuzumab18,19. However, emerging preclinical and clinical data suggest the opposite, and immunotherapy is currently widely investigated as a treatment for lung cancer20,21.
Immune checkpoints, which are inhibitory signaling pathways that can down-modulate the immune system responses of T cells, are pivotal in peripheral tissues and for maintaining immune self-tolerance. Among the many molecularly defined checkpoint proteins22,23, one of the most studied in lung cancer clinical trials is programmed cell death 1 (PD-1) receptor, also known as CD279 (cluster of differentiation 279), and its ligand (PD-L1), also known as B7-H1 or CD27413,24. We review the current literature on the PD-1 and PD-L1 pathways, withemphasis on PD-L1 as a potential predictive biomarker of response to anti-PD-L1 antibodies.
PD-1 and PD-L1 pathway
PD-1 is a type 1 transmembrane protein of the immunoglobulin superfamily25. In addition to its full length isoform, different splice variants of this protein (not all of which have been thoroughly studied) have been identified26. PD-1 plays an important role in limiting immune-mediated tissue destruction at sites with ongoing inflammation and/or infection. This immunoregulatory receptor is expressed on the surface of activated immune cell types, including T cells, B cells, natural killer (NK) cells, NKT cells, dendritic cells (DCs), and macrophages27, and is highly expressed on the surface of exhausted T cells. However, although nearly all exhausted cells express high levels of PD-1, not all cells expressing high levels of PD-1 are exhausted. Given that its blockade can restore the function of exhausted T cells28,29, PD-1 is considered a key immune checkpoint receptor that is expressed by activated T cells30.
PD-1 binds two B7 family ligands, namely, PD-L1 and PD-L2 (B7-DC or CD273)31,32. This interaction decreases the ability of activated T cells to produce an effective immune response and prevents the immune system from rejecting the tumor33. Among the ligands belonging to the B7 family, including PD-L1, PD-L2, B7-H3, and B7-H4, PD-L1 is the major membrane inhibitory ligand and the most studied in non-small cell lung cancer (NSCLC) clinical trials34. PD-L1 is expressed broadly in hematopoietic cells, including DCs, macrophages, mast cells, T cells, and B cells, and in non-hematopoietic cells, including endothelial, epithelial, and tumor cells35,36.
Cancer cells can activate PD-L1 expression through various oncogenic signaling pathways, such as phosphoinositide 3-kinase/ protein kinase B (PI3K/PKB)37, extracellular-signal-regulated kinases/mitogen-activated protein kinase (Erk/MAPK)38, anaplastic lymphoma kinase/signal transducers and activators of transcription 3 (ALK/STAT3)39, Janus kinase (JAK)/STAT40, and myeloid differentiation primary response gene 88/tumor necrosis factor receptor associated factor 6 (MYD88/TRAF6)41or in response to inflammatory cytokines that are produced by the infiltration of immune cells, such as IFNs42,43. Factors that influence PD-L1 expression may also depend on cell type. The receptor-ligand interaction PD-1/PD-L1 has been investigated as a target for cancer treatment in all of these situations.
Potential role of PD-L1 as a predictive biomarker for immunotherapy
Antibodies that target either PD-1 or PD-L1 are being developed to block ligand-receptor interaction and to improve antitumor immune response by allowing T cells to attack the tumor. To date, these antibodies have demonstrated exciting clinical responses against many cancer types.
PD-L1 is expressed in several tumor types, such as melanoma, glioblastoma, and cancers in lung, kidney, head and neck, stomach, colon, pancreas, breast, cervix, cervical, and ovarian cancer. This protein has also been observed in hematologic malignancies, such as multiple myeloma, lymphoma, and various leukemia types20,44-49.
PD-L1-positive cancers may indicate immune active tumors that could be sensitive to anti-PD-1 and/or PD-L1 therapies because of their correlation with poor prognosis in many of these malignancies, including lung adenocarcinoma50,51. However, the prognostic role of PD-L1 remains unclear. Other studies have found that the expression of PD-L1 is correlated both with better prognosis and no prognostic significance, making it difficult for researchers to make definitive conclusions43,52. Such discrepancies may be explained by the current use of non-standardized immunohistochemistry (IHC) techniques for measuring PD-L1 levels in tissue.
PD-L1 has dynamic expression, and its evaluation by IHC is not well standardized. Previous studies have used a range of various antibodies, treatments, tumor types, and criteria to determine the positivity of samples. Therefore, a coherent definition of PD-L1 positivity must be established to facilitate further study of PD-L1 as a potential biomarker for the PD-1/ PD-L1 pathway blockade. Given the intrinsic heterogeneity of PD-L1 expression in many tumors, the present results must be interpreted with caution. However, a biomarker of the response to a specific immunotherapy treatment is yet to be found.
In contrast, although testing the biopsied tumor tissue remains a recommended method for mutation analysis, challenges associated with serial tumor biopsy, particularly in NSCLC, have spurred the search for non-invasive blood-based assays that allow the frequent assessment of biomarkers as a part of routine clinical care53,54. Plasma and circulating tumor cells have also been proposed as alternative platforms for biomarker analysis55,56. A recent phase III clinical trial that compared high-dose chemotherapy with a rituximab regimen with standard rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in aggressive diffuse large B-cell lymphoma showed that the plasma PD-L1 protein in blood was associated with poorer prognosis for patients who were randomized within the R-CHOP arm57. Therefore, plasma PD-L1 protein could provide a promising alternative for monitoring PD-L1 levels with agents blocking PD-1/PD-L1 interaction, such as in advanced lung cancer.
PD-1 and PD-L1 in lung cancer
Immunotherapy has shown promising results in early NSCLC clinical trials involving PD-1 or PD-L1 antibodies58. These results have renewed the enthusiasm for immunotherapy as a treatment modality for lung cancer. Several drugs that target either the PD-L1 or PD-1 receptor are currently in preclinical and clinical development (Table 1). The first phase I trial with nivolumab [a human PD-1 blocking monoclonal antibody (mAb)] showed that PD-L1 expression in tumor cells could serve as a predictive biomarker to discriminate which patients would benefit from treatment. Only tumors expressing PD-L1 demonstrated an objective response rate (ORR). Reliable responses were observed in both non-squamous (ORR, 12%) and squamous histologies (ORR, 33%)30. Another phase I study with nivolumab (NCT00730639) showed that both PD-L1-positive and PD-L1-negative patients responded with an ORR of 44% and 17%, respectively, although numerically higher ORR, longer progression-free survival, and overall survival (OS) were observed in PD-L1 positive patients62. Nivolumab received U.S. Food and Drug Administration (FDA) approval in March 2015 and can be used for treating patients with advanced squamous NSCLC that progressed on or after platinum-based chemotherapy according to the CheckMate 017 phase III trial, which included squamous NSCLC patients regardless of their PD-L1 status. Median OS demonstrated superior performance for patients treated with nivolumab (9.2 months) compared with patients treated with docetaxel (6 months). Other studies involving nivolumab are ongoing, such as the phase III trial NCT01673867 comparing OS of nivolumab with docetaxel in subjects with non-squamous NSCLC after failure to prior platinum-based chemotherapy. Also ongoing are an open-labeled, randomized, phase III trials of nivolumab vs. investigator’s choice of chemotherapy (gemcitabine, cisplatin, carboplatin, paclitaxel, or pemetrexed) as first-line therapy for stage IV or recurrent PD-L1-positive NSCLC, and a phase I study of nivolumab in combination with gemcitabine/cisplatin, pemetrexed/cisplatin, carboplatin/paclitaxel, bevacizumab maintenance, erlotinib, ipilimumab, or as monotherapy in patients with stage IIIb/IV NSCLC.
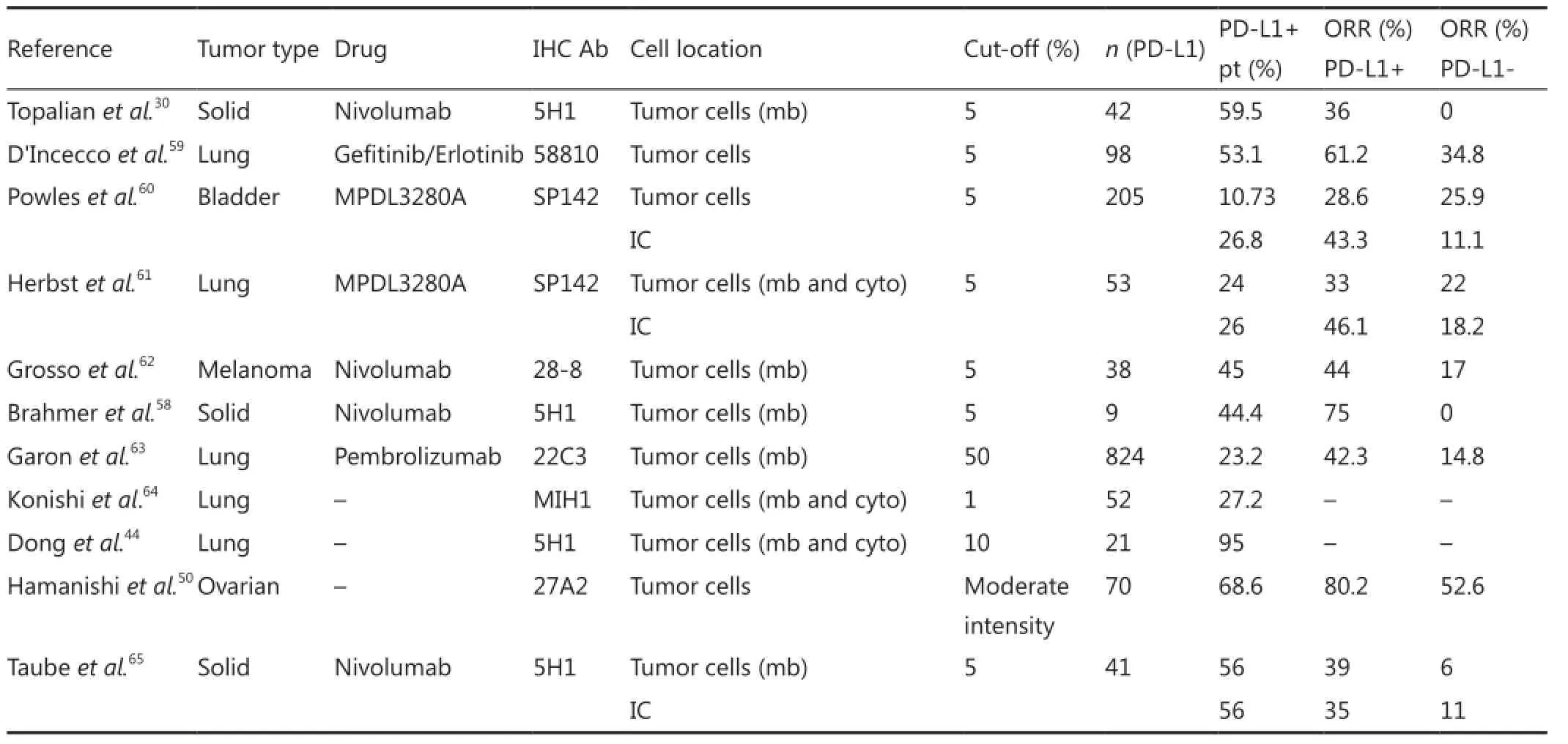
Table 1 PD-L1 expression by immunohistochemistry in different studies
BMS-936559 and MPDL3280A are anti-PD-L1 mAbs. BMS-936559 showed modest activity (ORR of 6%-17%) among patients with advanced cancers, including NSCLC, in a phase I multicenter trial (NCT00729664). Objective response (a complete or partial response) was observed in 5 of 49 evaluable NSCLC patients36. In a phase I study with anti-PD-L1 MPDL3280A, multiple tumor type responses (as evaluated by Response Evaluation Criteria in Solid Tumors, version 1.1) were observed among patients with tumors expressing high levels of PD-L1, especially when PD-L1 was expressed by tumor-infiltrating lymphocytes (TILs). A 46% ORR was reported in the cohort of patients with the highest PD-L1 positivity, 17% with moderate PD-L1 positivity, 21% with low intensity, and 13% with PD-L1-negative tumors61. Results of the phase II trials in the first and second lines and phase III trials of MPDL3280A were compared with those obtained when docetaxel was used for patients with locally advanced or metastatic NSCLC who failed platinum therapy (NCT01846416, NCT01903993, and NCT02008228, respectively). NCT02013219 is another interesting trial with MPDL3280A that combines phase Ib with tarceva for the treatment of EGFR- and NSCLC-positive patients.
PD-L1 is up-regulated in cancer and is expressed in tumor cells in 40%-50% of NSCLCs independent of tumor histology51,59. PD-1 is expressed on the majority of the TILs, and the presence of high levels of PD-1 on cytotoxic T lymphocytes suggests a reduced production of various cytokines and a proliferation of T cells64. A recent study suggested that PD-1 and PD-L1 checkpoint inhibitors could be more effective for NSCLC patients whose tumors showed somatic EGFR mutations. PD-L1 positivity was significantly associated with the presence of EGFR mutations, and PD-L1-positive patients had higher sensitivity to EGFR inhibitors, a longer time to progression from therapy, and better OS compared with PD-1-negative patients66,67.
Several new immune-based treatments for small cell lung cancer (SCLC) are currently in clinical development. These treatments include the mAb-targeting Delta-like ligand 4 (DLL4) demcizumab (NCT01859741) and nivolumab with or without ipilimumab (a mAb antibody against CTLA-4) (NCT01928394)68,69.
Available antibodies for IHC expression
Several companies have developed different primary antibodies for analyzing both PD-1 and PD-L1 proteins by IHC. Some studies suggest that tumor PD-L1 expression that is detected by IHC may predict clinical responses to anti-PD-1/PD-L1 therapy36,65. Therefore, PD-L1 expression has emerged as a potential predictive biomarker, but conflicting results have been obtained about the correlation between PD-L1 expression and effect on patient survival. Each company has developed PD-L1 detection techniques in isolation, thereby hampering the prospective validation of these tests and standardization for PD-L1 positive quantification. These contradicting results may be attributed to the lack of sensitivity and robustness of the antibodies that are used for detecting PD-L1 by IHC in clinical trials as well as the use of frozen versus formalin-fixed paraffinembedded (FFPE) specimens70.
The similarities among the PD-L1 antibodies that are used in trials, as well as the staining localization, threshold for signal detection, and test conditions, need to be investigated to obtain a robust protocol. Gadiot et al.71compared the performance of 15 anti-PD-L1 human antibodies that were used in IHC in FFPE melanoma cases. These antibodies included one mAb from eBioscience (San Diego, CA; MIH1), two mAbs from Otto Madjic (University of Vienna, Vienna, Austria; 5-496 and 2-272), one mAb from MBL International (Woburn, MA; 27A2), nine mAbs from Alan Korman (Medarex, Princeton, NJ; 16E11, 9A6, 16A4, 6H3, ETM-79, ETM-80, 1105, 25C8E8.F8 and 24B10.G6.D7), one mAb from L. Chen (Johns Hopkins University, Baltimore, MD; 5H1), and one polyclonal antibody from ProSci/Sigma (Poway, CA; 4059). The rabbit polyclonal antibody 4059 was the only one that did not result in background staining and blocked binding to PD-L1 by pre-incubation with a PD-L1 fusion protein and had the ability to stain FFPE.
The phase I study of Topalian et al.30in 2012 was performed among 296 patients (including 122 NSCLC patients) previously treated with nivolumab and showed that PD-L1 positive tumors by IHC (performed using the 5H1 clone) had an ORR of 36%, whereas PD-L1 negative tumors did not achieve any ORR. Clones 5H1 and 28-8 were also compared; the staining of membranous PD-L1 was tested in FFPE tissue samples comprising tumor cells and tumor infiltrating immune cells from NSCLC, melanoma, and renal cell carcinoma. Clone 28-8 demonstrated a better detection (higher histoscores) than 5H1, although binding abilities of these clones to membrane PD-L1 were similar72.
A recently published patent describes antibodies with specific sequences that bind to human PD-L1 as well as revealsthe benefit of detecting PD-L1 expression in FFPE human tissue samples by IHC (WO/2014/100079)73. The authors compared five commercially available PD-L1 human antibodies from eBioscience (San Diego, CA; MIH1), R&D Systems (Minneapolis, MN; AF-156), US biological (Salem, MA; 22 and 22E), and ProSci/ Sigma (4059) with two new mAbs from Merck (Whitehouse Station, NJ; 20C3 and 22C3) and found that none of these commercial antibodies had the required joint robustness, specificity, or sensitivity for using IHC in FFPE. However, the 22C3 and 20C3 antibodies were jointly robust, specific, and sensitive. The ability of the 22C3 antibody to detect a range of PD-L1 expression in different tumor types was also assessed by IHC in FFPE sections from different tumor types, including lung cancer74.
Roche/Genentech and Bristol–Myers Squibb have developed different companion assays for PD-L1 expression, each with its own experimental PD-L1 antibody. These antibodies include Spring Biosciences clone SP142 (MPDL3280A, Genentech, South San Francisco, CA), Spring Biosciences clone SP263 (MEDI4736, Astra Zeneca, London), and Dako clone 28-8 (nivolumab, Bristol-Myers Squibb, New York, NY). To date, the properties and concordance between these IHC antibodies have not been reported, and only clone SP142 is commercially available. The characteristics of all antibodies that are discussed in this section are listed in Table 2.
Staining pattern and threshold for signal detection of PD-L1 protein expression
For an antibody to be considered as a favorable diagnostic tool, it must show sensitivity, specificity, reproducibility, and robustness in detecting the target by IHC. Some of thenumerous available assays for detecting PD-L1 expression by IHC can only stain cancer cells, whereas the other assays can stain tumor-infiltrating immune cells. Therefore, patients with tumor cell staining and/or immune cell staining were examined in some studies, whereas the other studies only included patients with PD-L1 expression in tumor cells30,60,74. Given that these studies have not been compared, we could not ascertain whether the differences in these definitions can be attributed to antibody specificity, subjective interpretation, biological differences between the used immunotherapies, the nature of the analyzed patient tissues, or the technical issues that are related to tissue processing and storage. Taube et al.43studied the predictive function of PD-L1 expression in cancer and immune cells, as well as that of PD-1 expression on the immune infiltrate. They found that PD-L1 expression in cancer and immune cells was highly associated with PD-1 expression in TILs, thereby indicating that PD-L1 expression reflects an immune reactive microenvironment.
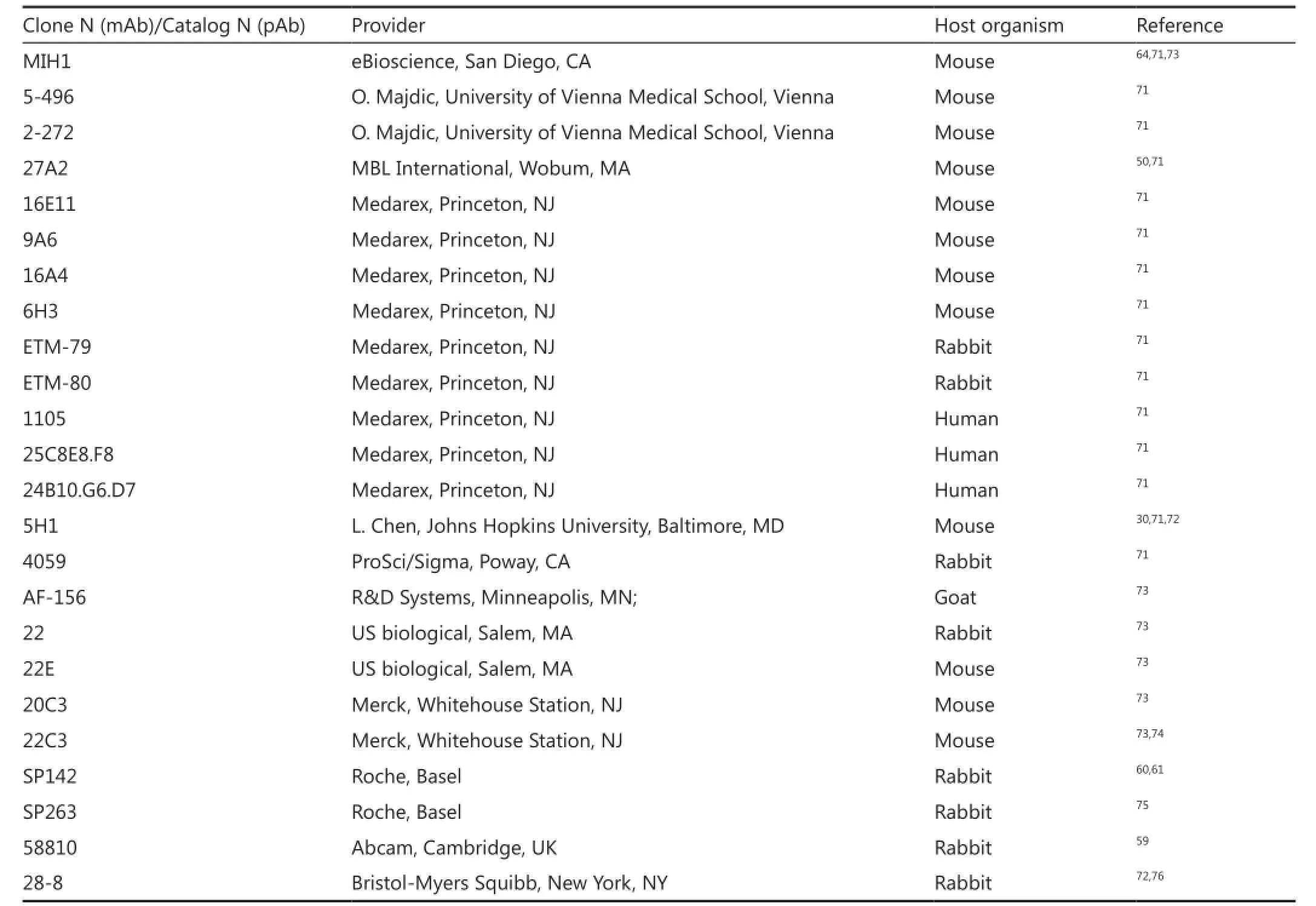
Table 2 anti-human-PD-L1 antibodies
Two patterns of cellular distribution of PD-L1, namely, membranous (cell surface) and cytoplasmic, have been described in tumor cells to indicate PD-L1 positivity. Membranous PD-L1 expression is present in tumors and inflammatory cells. PD-L1 staining pattern also differs between the assays. While some assays only evaluated membranous stain ing, others considered both membranous and cytoplasmic staining. PD-L1 is a type 1 transmembrane protein, and its cytoplasmic localization can represent intracellular stores of ligand that may relocate to the cell surface depending on cell stimulation. Interestingly, Brahmer et al.36found that the membrane expression of PD-L1 was the most relevant biomarker for predicting the clinical response to PD-1 pathway blockade65. Moreover, the various PD-L1 protein expression staining patterns that are obtained in immune and tumor cells demonstrate that the scoring system used in clinical trials and the required percentages of positive cells in a positive sample can also vary.
The literature provides four definitions of PD-L1 sample positivity that are independent of the sample location and the staining of cells, i.e., whether ≥1, ≥5, ≥10, or ≥50 of cells per area are stained positive for PD-L144,58,61,63. The specification of these parameters may explain why some patients who have been evaluated as PD-L1 positive respond to immunotherapy, whereas others do not respond. A standardized definition of PD-L1 positivity that links all the anti-PD-L1 antibodies by IHC must be provided to study the role of PD-L1 as a potential predictive biomarker for the therapeutic blockade of PD-1 and PD-L1. Without such definition, the comparison of clinical trial results using assays in different types of tumor will remain problematic.
Conclusion
Immunotherapy for lung cancer is a new and exciting therapeutic modality. Multiple mAb candidates that target the PD-1/PD-L1 immune checkpoint have demonstrated reliable responses in tumors, including lung cancer, whereas some mAb candidates have shown remarkable antitumor effects in different clinical trials. Unfortunately, the lack of a reliable biomarker of response obscures such a scenario. Data on the correlation between PD-L1 positivity and patient responses to the different PD-1/ PD-L1 blocking agents are also conflicting. PD-L1 is upregulated in many cells and cancer types and contributes to the malignancy of these cancers by interacting with PD-1 and inhibiting T cell activation, thereby limiting the detection and destruction of tumor cells by the immune system. This ligand may play important roles in the inhibition of immune responses in both lymphoid and non-lymphoid organs. If PD-L1 is upregulated in a tumor without an appropriate immune infiltrate, the blockade may have no effect because the tumor lacks the effector cells that fight the cancer.
Several immunohistochemical antibodies have been developed for detecting PD-L1 expression in FFPE tissue. Characterizing tumors and immune cells via PD-L1 protein expression by IHC may help identify those patients who can benefit from the mAb candidate anti-PD-1 and anti-PD-L1 agents. As a result, the ability of PD-L1 protein expression to become a favorable predictive marker of response has been measured in various ways in several clinical trials. The conflicting results of PD-L1 staining may be attributed to the small sample sizes that are tested in different assays and/or to the variability of the used antibodies. No precise cut-off has also been established for determining PD-L1 positivity by IHC. The limitations in the specificity and reproducibility of some antibodies may explain the contradictory relationships between assays.
Confirming PD-L1 as a predictive biomarker presents a promising new therapeutic opportunity to administer those agents that prevent PD-1/PD-L1 pathway interaction in advanced or metastatic lung cancers and other tumors. Further studies must be conducted to clarify the optimal IHC assay, validate and standardize the definition of PD-L1 positivity, and explore the relationship among various expression levels of the PD-L1 protein, as well as the effect of such levels on the prognosis of lung cancer patients with PD-1/PD-L1-directed therapies.
Conflict of Interest Statement
No potential conflicts of interest are disclosed.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29.
2. Hornback NB, Einhorn L, Shidnia H, Joe BT, Krause M, Furnas B. Oat cell carcinoma of the lung. Early treatment results of combination radiation therapy and chemotherapy. Cancer 1976;37:2658-2664.
3. Komaki R, Cox JD, Hartz AJ, Byhardt RW, Perez-Tamayo C, Clowry L, et al. Characteristics of long-term survivors after treatment for inoperable carcinoma of the lung. Am J Clin Oncol 1985;8:362-370.
4. Scagliotti GV, De Marinis F, Rinaldi M, Crinò L, Gridelli C, Ricci S, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol 2002;20:4285-4291.
5. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-smallcell lung cancer. N Engl J Med 2006;355:2542-2550.
6. Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzáles-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IBIIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-727.
7. Burdett S, Stewart LA, Rydzewska L. A systematic review and meta-analysis of the literature: chemotherapy and surgery versus surgery alone in non-small cell lung cancer. J Thorac Oncol 2006;1:611-621.
8. Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-3551.
9. Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 2005;40:90-97.
10. Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol 2013;31:992-1001.
11. McKneally MF, Maver C, Kausel HW. Regional immunotherapy of lung cancer with intrapleural B.C.G. Lancet 1976;1:377-379.
12. Roeslin N, Lang JM, Morand G, Wihlm JM, Witz JP. Regional immunotherapy in resectable squamous cell lung carcinoma. Analysis of a randomized study. Cancer Immunol Immunother 1982;13:174-175.
13. Sundar R, Soong R, Cho BC, Brahmer JR, Soo RA. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer 2014;85:101-109.
14. Maurer LH, Pajak T, Eaton W, Comis R, Chahinian P, Faulkner C, et al. Combined modality therapy with radiotherapy, chemotherapy, and immunotherapy in limited small-cell carcinoma of the lung: a Phase III cancer and Leukemia Group B Study. J Clin Oncol 1985;3:969-976.
15. Matthay RA, Mahler DA, Beck GJ, Loke J, Baue AE, Carter DC, et al. Intratumoral Bacillus Calmette-Guérin immunotherapy prior to surgery for carcinoma of the lung: results of a prospective randomized trial. Cancer Res 1986;46:5963-5968.
16. Jansen RL, Slingerland R, Goey SH, Franks CR, Bolhuis RL, Stoter G. Interleukin-2 and interferon-alpha in the treatment of patients with advanced non-small-cell lung cancer. J Immunother (1991) 1992;12:70-73.
17. Schiller JH, Morgan-Ihrig C, Levitt ML. Concomitant administration of interleukin-2 plus tumor necrosis factor in advanced non-small cell lung cancer. Am J Clin Oncol 1995;18:47-51.
18. Lara PN Jr, Laptalo L, Longmate J, Lau DH, Gandour-Edwards R, Gumerlock PH, et al. Trastuzumab plus docetaxel in HER2/ neu-positive non-small-cell lung cancer: a California Cancer Consortium screening and phase II trial. Clin Lung Cancer 2004;5:231-236.
19. Clamon G, Herndon J, Kern J, Govindan R, Garst J, Watson D, et al. Lack of trastuzumab activity in nonsmall cell lung carcinoma with overexpression of erb-B2: 39810: a phase II trial of Cancer and Leukemia Group B. Cancer. 2005;103:1670-1675.
20. Brahmer JR, Pardoll DM. Immune checkpoint inhibitors: making immunotherapy a reality for the treatment of lung cancer. Cancer Immunol Res. 2013;1:85-91.
21. Domingues D, Turner A, Silva MD, Marques DS, Mellidez JC, Wannesson L, et al. Immunotherapy and lung cancer: current developments and novel targeted therapies. Immunotherapy 2014;6:1221-1235.
22. Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008;8:467-477.
23. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-264.
24. Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol 2014;11:24-37.
25. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11:3887-3895.
26. Nielsen C, Ohm-Laursen L, Barington T, Husby S, Lillevang ST. Alternative splice variants of the human PD-1 gene. Cell Immunol 2005;235:109-116.
27. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704.
28. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682-687.
29. Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol 2007;81:2545-2553.
30. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-2454.
31. Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365-1369.
32. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261-268.
33. Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol 2013;94:25-39.
34. Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, et al. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer 2006;53:143-151.
35. Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007;8:239-245.
36. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-2465.
37. Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 2007;13:84-88.
38. Qin X, Liu C, Zhou Y, Wang G. Cisplatin induces programmed death-1-ligand 1(PD-L1) over-expression in hepatoma H22 cells via Erk /MAPK signaling pathway. Cell Mol Biol (Noisy-le-grand) 2010;56 Suppl:OL1366-OL1372.
39. Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci U S A 2008;105:20852-20857.
40. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219-242.
41. Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood 2007;110:296-304.
42. Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett 2006;580:755-762.
43. Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra137.
44. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800.
45. Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003;9:562-567.
46. Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006;108:19-24.
47. Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res 2007;13:709s-715s.
48. Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 2007;13:2151-2157.
49. Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 2008;14:3044-3051.
50. Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumorinfiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007;104:3360-3365.
51. Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011;28:682-688.
52. Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 2013;73:128-138.
53. Teixidó C, Karachaliou N, Peg V, Gimenez-Capitan A, Rosell R. Concordance of IHC, FISH and RT-PCR for EML4-ALK rearrangements. Transl Lung Cancer Res 2014;3:70-74.
54. Karachaliou N, Mayo-de-Las-Casas C, Molina-Vila MA, Rosell R. Real-time liquid biopsies become a reality in cancer treatment. Ann Transl Med 2015;3:36.
55. Thierry AR, Mouliere F, El Messaoudi S, Mollevi C, Lopez-Crapez E, Rolet F, et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat Med 2014;20:430-435.
56. Pailler E, Adam J, Barthélémy A, Oulhen M, Auger N, Valent A, et al. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-cell lung cancer. J Clin Oncol 2013;31:2273-2281.
57. Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia 2014;28:2367-2375.
58. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-3175.
59. D’Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 2015;112:95-102.
60. Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558-562.
61. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-567.
62. Grosso J, Horak CE, Inzunza D, Cardona DM, Simon JS, Gupta AK, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538). J Clin Oncol 2013;31:abstr 3016.
63. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N Engl J Med 2015;372:2018-2028.
64. Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 2004;10:5094-5100.
65. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014;20:5064-5074.
66. Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-1363.
67. Ji M, Liu Y, Li Q, Li XD, Zhao WQ, Zhang H, et al. PD-1/PD-L1 pathway in non-small-cell lung cancer and its relation with EGFR mutation. J Transl Med 2015;13:5.
68. Yang LL, Wu YL. Recent advances of immunotherapy in lung cancer: anti-programmed cell death-1/programmed death ligand-1 antibodies. Lung Cancer Management 2014;3:175-190.
69. Massarelli E, Papadimitrakopoulou V, Welsh J, Tang C, Tsao A. Immunotherapy in lung cancer. Transl Lung Cancer Res 2014;3:53-63.
70. Mullard A. New checkpoint inhibitors ride the immunotherapy tsunami. Nat Rev Drug Discov 2013;12:489-492.
71. Gadiot J, Hooijkaas AI, Kaiser AD, van Tinteren H, van Boven H, Blank C. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer 2011;117:2192-2201.
72. Cogswell JP, Goldberg SM, Gupta AK, Jure-Kunkel M, Wang XT, Wigginton JM. Cancer immunotherapy by disrupting pd-1/pd-l1 signaling. Google Patents; 2013: WO 2013173223 A1.
73. Pierce RH, Bourne P, Liang L, Bigler M. Antibodies that bind to human programmed death ligand 1 (pd-l1). Google Patents; 2014: WO 2014100079 A1.
74. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568-571.
75. Fury M, Ou SH, Balmanoukian A, Hansen A, Massarelli E, Blake-Haskins A, et al. Clinical Activity and Safety of MEDI4736, an Anti-PD-L1 Antibody, in Patients with Head and Neck Cancer. Ann Oncol 2014;25:iv340-iv356.
76. Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol 2013;31:4311-4318.
Cite this article as: Teixidó C, Karachaliou N, González-Cao M, Morales-Espinosa D, Rosell R. Assays for predicting and monitoring responses to lung cancer immunotherapy. Cancer Biol Med 2015;12:87-95. doi: 10.7497/ j.issn.2095-3941.2015.0019
Correspondence to: Cristina Teixidó
E-mail: cteixido@pangaeabiotech.com
March 19, 2015; accepted april 30, 2015.
available at www.cancerbiomed.org
Copyright ? 2015 by Cancer Biology & Medicine
As another anti-PD-1 mAb, pembrolizumab FDA approval in October 2014 and can be used for treating epidermal growth factor receptor (EGFR) mutation-negative and ALK rearrangement-negative NSCLC that has progressed on or after platinum-based chemotherapy. Approval was granted based on the results of a phase I trial by Garon et al.63, which showed that pembrolizumab had antitumor activity and a tolerable toxicity profile for patients with advanced NSCLC. Moreover, PD-L1 positivity in at least 50% of tumor cells was correlated with improved efficacy of pembrolizumab (response rate of 45.2%). Current or former smokers had a response rate of 22.5%, while non-smokers had a response rate of 10.3%. Pembrolizumabfor treating NSCLC is currently subjected to clinical trials, such as a phase I trial among advanced PD-L1-positive NSCLC patients (NCT02007070), a phase II/III study involving two doses of pembrolizumab vs. docetaxel for patients previously treated with PD-L1 positive NSCLC (NCT01905657), and combination studies with ipilimumab or chemotherapy for NSCLC patients (NCT02039674).
 Cancer Biology & Medicine2015年2期
Cancer Biology & Medicine2015年2期
- Cancer Biology & Medicine的其它文章
- Predictive value of K-ras and PIK3CA in non-small cell lung cancer patients treated with EGFR-TKIs: a systemic review and meta-analysis
- Paclitaxel-etoposide-carboplatin/cisplatin versus etoposidecarboplatin/cisplatin as first-line treatment for combined small-cell lung cancer: a retrospective analysis of 62 cases
- Current approaches in treatment of triple-negative breast cancer
- Changes in tumor-antigen expression profile as human small-cell lung cancers progress
- Understanding the function and dysfunction of the immune system in lung cancer: the role of immune checkpoints
- Tumor immune microenvironment characterization and response to anti-PD-1 therapy
