The in fl uence of amino acids on aztreonam spray-dried powders for inhalation
Xio-Fei Yng,Ying Xu*,D-Sheng Qu,Ho-Ying Li,**
aCollege of Chemistry,Chemical Engineering and Materials Science,Soochow University,Suzhou 215123,ChinabTesting and Analysis Centre,Soochow University,Suzhou 215123,China
cSuzhou Hui-Ren Biotech Co.Ltd,Suzhou 215513,China
Original Research Paper
The in fl uence of amino acids on aztreonam spray-dried powders for inhalation
Xiao-Fei Yanga,Ying Xub,*,Da-Sheng Quc,Hao-Ying Lic,**
aCollege of Chemistry,Chemical Engineering and Materials Science,Soochow University,Suzhou 215123,ChinabTesting and Analysis Centre,Soochow University,Suzhou 215123,China
cSuzhou Hui-Ren Biotech Co.Ltd,Suzhou 215513,China
ARTICLEINFO
Article history:
Received 31 May 2015
Received in revised form 6 August2015
Accepted 17 August 2015
Available online 24 August 2015
Aztreonam
The dry powder inhalation of antibiotics for the treatment of lung infections has attracted drastically increasing attention as it offers rapid local therapy at lower doses and minimal side effects. In this study, aztreonam (AZT) was used as the model antibiotic and spray-dried to prepare powders for inhalation. Amino acids of glycine (GLY), histidine (HIS) and leucine (LEU) were used as excipients to modify the spray-dried particles. It was demon-strated that the GLY-AZT spray-dried powders formed huge agglomerates with the size of 144.51 μm, which made it very difficult to be delivered to the lungs (FPF: 0.29% w/w only).In comparison with the AZT spray-dried powders, HIS-modified spray-dried powders showed increased compressibility, indicating larger distance and less cohesion between particles;while the LEU-modified spray-dried particles showed a hollow structure with significantly decreased densities.The fine particle fraction for HIS- and LEU-modified powders was 51.4% w/w and 61.7% w/w, respectively, and both were significantly increased (one-way ANOVA, Duncan’s test, P < 0.05) compared to that of AZT spray-dried powders (45.4% w/w), showing a great potential to be applied in clinic.
? 2015 The Authors. Production and hosting by Elsevier B.V. on behalf of Shenyang Phar-maceutical University. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Inhaled antibiotics attract increasing attention for the treatment of lung infections,as they are directly delivered to the local sites of infection,which therefore generate quick treatment at low dose and avoid systemic side effects[1].The delivery of antibiotics through inhalation has developed into a typical approach for the management of lung infections such as chronic Pseudomonas aeruginosa associated with cystic fibrosis and mycobacterial pulmonary infections[2].As a well recognized antibiotic for the remedy of respiratory infections,aztreonam(AZT)has been formulated as inhalation solution(Cayston?,75 mg/dose,approved by US FDA)and is nebulized to the lungs for the effective treatment of local infections that are associated with cystic fi brosis in most cases[3].However,the use of nebulization has several drawbacks associated with its usage including inconvenience of bulk device, low delivery ef fi ciency and the possibility of damage to the drug molecules by the high shear force[4,5].In contrast,dry powder inhalers(DPIs)are portable,easy to use and relatively costeffective.Additionally,rather than the use of propellent to produce aerosols as does the pressurized metered dose inhalers(pMDIs),DPIs utilize patients’inhalation to aerosolize drug particles and therefore avoid the coordination between the inhalation and actuation,which may be a problem associated with pMDIs[6].Furthermore,the biotherapeutics of proteins, peptides and genes may be more stable in dry state than in aqueous solutions.DPIs therefore have become an attractive approach for drug delivery to the lungs and have gained rapid development in the past decades.
Spray-drying has been con fi rmed to be an effectual approach to prepare dry powders with sizes within the inhalable range[7].More importantly,spray-drying technology favors manipulation of particles’physiochemical properties including particle size and size distribution,density,particle shape and morphology[8],which may therefore enhance the powder fl owability,dispersibility and deposition in the lower respiratory tract during inhalation.A few studies reported that excipient-free antibiotic(e.g.colistin)spray-dried powders have miraculous aerosolization performance[9],and the combination of different antibiotics(e.g.colistin and rifampicin) can create spray-dried powders with multifunctional characteristics of high delivery ef fi ciency and moisture protection[10].However,the excipients are usually used in spray-drying formulations to facilitate adjustment of the physiochemical properties of spray-dried powders in order to enhance their dispersibility and delivery ef fi ciency,where some of amino acids including arginine(ARG),phenylalanine(PHE) and leucine(LEU)have been investigated and veri fi ed as effective dispersibility enhancers.Based on their chemical structures,the mechanism for dispersibility enhancement is induced by the electrostatic repulsion(ARG,positively charged) and surface modi fi cation by hydrophobic molecules(PHE and LEU,low solubility)[11].However,the histidine(HIS),containing the imidazol functional group,and the hydrophilic glycine have not been investigated in terms of in fl uencing the physiochemical properties and subsequent aerosolization performance of spray-dried powders.Although LEU has been proven to be every effective in enhancing the dispersibility of spray-dried powders,there are no data to fully disclose the function of LEU on the AZT spray-dried powders for inhalation.
Therefore,in this study,AZT was used as a model drug and spray-drying was employed to prepare the dry powders for inhalation.GLY,HIS and LEU were used as the excipients and were dissolved in the spray-drying solution to modify the spray-driedAZT particles.The physiochemical properties,including shape and morphology,particle size distribution,density,moisture content,aerosolization performance and lung deposition,were fully investigated and compared,with the aim to generate dry powder formulations that have enhanced lung delivery ef fi ciency.This will help further construct the links between the structure of amino acids and the aerosolization performance of spray-dried powders.
2. Materials and methods
2.1. Materials
AZT was bought from Shanghai Hongrui Chemical Co.Ltd (Shanghai,China).The amino acids glycine(GLY),histidine(HIS) and leucine(LEU)were purchased from National Medicine Group Chemical Reagent Co.Ltd.(Shanghai,China).Potassium dihydrogen phosphate and ammonia solution were purchased from Sinopharm Chemical Reagent Co.Ltd(Shanghai,China).Methanol at HPLC grade was bought from Spectrum Chemicals&Laboratory Products(Cali,USA).The capsules(3 #Gelatine)were acquired from Suzhou Capsule Company (Suzhou,China).All other chemicals were of analytical grade and used as received.
2.2. Preparation of spray-dried powdersAZT spray-dried powders:two grams of AZT were dissolved in 40 mL of deionized distilled water(dd H2O)that was subsequently adjusted to pH 4.5 using 1%of ammonia solution to ensure complete drug dissolution.A laboratory scale spray dryer(Büchi Mini Spray Dryer B-290,Büchi Labortechnik AG, Switzerland)was used to prepare the spray-dried AZT powders in an open-cycle system with a pressure nozzle(co-current fl ow).The standard operation parameters were as follows:inlet temperature 125°C,aspiration rate 100%,spray fl ow rate 550 L/h and pump setting 8%(2.4 mL/min).These conditions resulted in an outlet temperature of 76-78°C.
2.2.1. Amino acid modi fi ed spray-dried powders
The amino acids of GLY,HIS and LEU were used respectively as the excipients to prepare spray-dried powders.For the preparation,a total mass of 2 g containing AZT and amino acid of GLY,HIS or LEU at the mass ratio of 40:60 was dissolved in dd H2O to prepare the spray-drying solutions that were subsequently spray-dried under the standard operation parameters to prepare dry powders.After spray drying,all dry powders were collected from the collection vessel,weighted,and stored in vacuum desiccators for further use.Each formulation was spraydried in triplicate,and the spray-dried yields were reported.
2.3. Powder characterization
The morphology of spray-dried powders was visualized using scanning electron microscope(SEM,S-5700,Hitachi Co.,Tokyo, Japan),operated at 15 kV under high vacuum.Samples of spraydried powders were sputter-coated with a thin layer(~50 nm) of gold under partial vacuum(HITACHI E-1010,Tokyo,Japan). Representative images of the spray-dried powders were taken under a magni fi cation of 3000-5000×.
Laser diffraction with a dry dispersion system(Mastersizer 2000E,Malvern Instrument,Malvern,UK)was utilized to determine the size distribution of dry powders.Approximately 200 mg of spray-dried powders was used to achieve the obligatory obscuration of 0.5-5%,and the size distribution was subsequently measured in triplicate for each sample.The average particle size was expressed as the volume-weighted mean.The particle size distribution is expressed in terms ofthe Span factor,which is calculated as(d[v,90]-d[v,10])/d[v, 50],where d[v,10],d[v,50],and d[v,90]are the sizes in diameter of a given percentage of particles smaller than the speci fi ed size.The smaller Span represents a narrower particle size distribution.
The weight loss of freshly prepared spray-dried powders as a function of temperature was measured by thermogravimetric analysis(TGA)using theThermal AdvantageTGA Q500(TA Instrument,USA)module.The samples(3-4 mg)were measured in platinum pans and heated at 25-150°C at a heating rate of 10°C/min under nitrogen purge.Measurements were made in triplicate.
The bulk density(ρb)and tap density(ρt)of spray-dried powders were determined by a veri fi ed method as previously reported with small modi fi cation[12].The ρbof dry powders was de fi ned as the ratio of the powder weight over the bulk volume,and was determined in a 1 mL of end-sealed syringe in which a pre-determined amount of dry powders was fi lled. The ρbwas subsequently calculated by dividing the powder weight by the observed bulk volume.Then,the syringe was capped with laboratory fi lm and tapped on a hard bench until no change in volume of the powder was observed.The ρtwas subsequently calculated from the powder weight divided by the tapped volume.The test was performed in triplicate for each powder formulation.Carr’s index was derived from(1-ρb/ ρt)×100%,and a value less than 25%indicates a fl uid powder.
2.4. Analysis of AZT
The content of AZT in the spray-dried powers was determined by high pressure liquid chromatography(HPLC). Approximately 5 mg of spray-dried powders was dissolved into 1 mL of 0.1%ammonia solution,and subsequently vortexed for 10 min to ensure the drug completely dissolved.A syringe fi lter with a pore size of 450 nm was utilized to purify the solution for the HPLC analysis of the drug contents.
The HPLC instrument,Agilent 1260 in fi nity,consisted of G1312B pump,G1329B auto sampler and G1314F UV detector. For the analysis of AZT,the drug samples(10 μL)were injected into the HPLC column(5 μm C18,250×4.6 mm,80 ?, ZORBAX Eclipse XDB,Agilent,USA)which was subsequently eluted at a fl ow rate of 0.6 mL/min using a mobile phase made up of phosphate buffer solution(pH 3 adjusted by phosphoric acid):methanol(70:30 v/v).AZT was detected at 263 nm. The retention time forAZT under these conditions was 6.5 min. The standard solutions at fi ve different concentrations were also used to construct the standard curve that was consequently used for the analysis of the drug samples[13].
2.5. In-vitro powder aerosolization
The aerosolization performance of the spray-dried powders from a dry powder inhaler(DPI)device was determined using a Next Generation Impactor(NGI,Copley Scienti fi c,Nottingham,UK)equipped with a USP throat and pre-separator.The NGI is an eight-stage inertial impactor that separates the aerosols into isolated size ranges based on aerodynamic diameter. For the test,the surface of NGI trays was coated with silicone oil to prevent particle bounce.The spray-dried powders(25 mg) were accurately weighed and loaded into size 3 gelatine capsules,and subsequently placed into an Aerolizer?(Novartis, Basel,Switzerland)DPI device,connected via a mouthpiece adapter to the USP throat.The powders were subsequently aerosolized(35%relative humidity,20°C)at 60 L/min for 5 seconds. Three capsules were used for each test,and the expected mass of AZT was equivalent to 75 mg and 30 mg for AZT-only and amino acid-modi fi ed spray-dried powders respectively.Each powder formulation was tested in triplicate.After administration,the capsules and inhaler device(DEV),NGI throat(THR), pre-separator(SEP),stages 1-7 and MOC were rinsed with water. These solutions were subsequently fi ltered through a 0.45 μm membrane,and 50 μL of the sample was employed for HPLC analysis.For each test,the total dose(TD)was de fi ned as the mass ofAZT loaded into the three capsules as mentioned above. The emitted dose(ED)was de fi ned as the mass of powder loaded into the capsule that was released during aerosolization,determined colorimetrically and expressed as a percentage of TD.The recovered dose(RD)was de fi ned as the total mass of AZT detected after each test(i.e.capsule,inhaler,throat,preseparator and stages 1-7 and MOC),expressed as a percentage of TD.The fi ne particle dose(FPD)was de fi ned as the mass of AZT detected on stage 2-MOC of the NGI(effective cut-off diameter<4.46 μm).The fi ne particle fraction(FPF)was calculated as the ratio of FPD to RD,expressed as a percentage.The mass median aerodynamic diameter(MMAD)of the powders was subsequently calculated,which referred to the particle size at the 50%mark of the cumulative fraction versus the effective cutoff diameter.
2.6. Statistical analysis
The data were statistically analyzed by the SPSS13.0 statistical software,and was presented using mean±SD.One-way analysis of variance(one-way ANOVA)with Duncan’s multiple comparison test was used and P<0.05 was accepted as signi fi cant.
3. Results and discussion
3.1. Preparation of spray-dried powders
AZT spray-dried powders,regarded as the control,showed a yield of 80.0%w/w(Table 1).The addition of different amino acids in the formulation generated an apparent dissimilar effect on the spray-dried yields.The addition of GLY signi fi cantly decreased(one-way ANOVA,Duncan’s test,P<0.05)the spraydried yield with only 29.3%w/w collected,as large amounts of GLY-AZT spray-dried powders were observed to be adsorbed on the inner surface of the spray-drying chamber and upper section of cyclone,indicating larger particle size and strong adhesion force for GLY-AZT spray-dried particles.The yield for HIS-AZT spray-dried powders was 74.8%w/w that was at the same level as that of the control.The LEU-modi fi ed spraydried powders showed a signi fi cantly increased(one-way ANOVA,Duncan’s test,P<0.05)yield of 85.2%w/w,which may be a sign of further improvement of powder fl owability,and the reason for that might be based on the alteration of physicochemical properties(e.g.particle size,density,surface morphology and porosity)of the spray-dried particles.
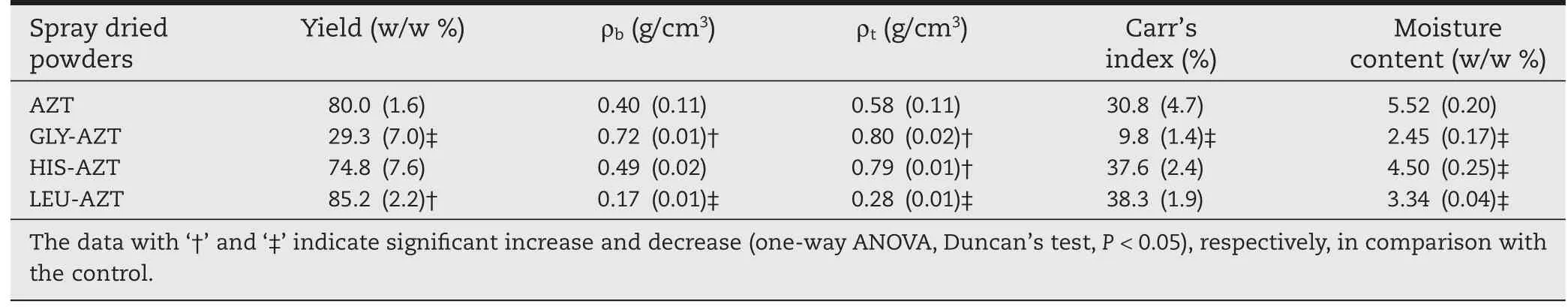
Table 1-Physiochemical properties of LEU-modi fi ed spray-dried AZT powders.The data were expressed as the mean±SD.
3.2. Characterization of physiochemical propertiesThe moisture content of spray-dried powders was determined by thermogravimetric analysis(TGA),and was estimated from the weight loss of dry powders at temperatures of up to 150°C(Table 1).In comparison with the moisture content (5.52%w/w)of AZT only spray-dried powders,the amino acidmodi fi ed spray-dried powders showed a signi fi cantly decreased (one-way ANOVA,Duncan’s test,P<0.05)moisture content in the range of 2.45-4.50%w/w,suggesting amino acid can diminish the water content in the spray-dried powders.The decrease of moisture content in the spray-dried powders is potentially supportive to improve the fl owability and further enhance the lung deposition.
It was demonstrated that the ρband ρtfor AZT spraydried powders were 0.40 and 0.58 g/cm3respectively(Table 1). The addition of GLY into the formulation signi fi cantly increased(One-way ANOVA,Duncan’s test,P<0.05)the ρb(0.72 g/ cm3)and ρt(0.80 g/cm3),suggesting that they were dense particles.In comparison with the control powders,the HISAZT spray-dried powders had a similar ρbof 0.49 g/cm3,and signi fi cantly higher(one-way ANOVA,Duncan’s test,P<0.05) ρtof 0.79 g/cm3,suggesting a better compressibility and a bigger distance between particles,further indicating less cohesion between particles and better dispersibility.Interestingly,the LEUAZT spray-dried powders demonstrated a very low ρband ρtof 0.17 g/cm3and 0.28 g/cm3respectively,signi fi cantly reduced (one-way ANOVA,Duncan’s test,P<0.05)than those of control powders.Carr’s Index value is an indication of free fl owing powders;a value less than 25%indicates a fl uid fl owing powder, whereas a value greater than 25%indicates cohesive powder characteristics.Carr’s index values for AZT,HIS-AZT and LEUAZT spray-dried powders were 30.8%,37.6%and 38.3% respectively,af fi rming poor fl owability for these dry powders. The GLY-AZT dry powders had a Carr’s index value of 9.8%, stating good fl owability.However,there was no relationship observed between particle size and the representative Carr’s index values.
The surface morphology of spray-dried powders was visualized by scanning electron microscopy(Fig.1).The surface morphology of AZT only spray-dried powders had a wrinkled surface with high roughness(Fig.1A).All amino acids,added into the spray-drying solution,can drastically change the particle morphology.The GLY-AZT spray-dried particles formed aggregates that were bigger than 10 μm,strongly suggesting a poor aerosolization performance and lower deposition in the lungs(Fig.1B).The HIS-AZT spray-dried particles showed a sphere-like shape with a relatively smooth and slightly wrinkled surface,comparable to those of AZT spray-dried particles.LEU, added into the formulation,can greatly change the surface morphology and structure of the spray-dried particles.The LEUAZT spray-dried particles showed broken surfaces with hollow structure inside,indicative of decreased density of dry powders and hence improved aerosolization performance.All spraydried particles of AZT,HIS-AZT and LEU-AZT showed the size less than 10 μm,which could potentially be inhaled into the lower respiratory tract.
Laser diffraction was utilized to determine the particle size distribution of spray-dried powders(Fig.2).The AZT only spraydried powders showed size distribution of less than 10 μm (Fig.2A).Although the GLY-AZT spray-dried powders showed a unimodal size distribution,the particle size was very large, up to~1000 μm(Fig.2B),while the HIS-and LEU-modi fi ed spraydried powders showed size distributions less than 10 μm(Fig.2C and D).All these data were in good agreement to the particle size observed under SEM.In order to further elucidate the particle size distribution,the mean diameter over the volume distribution D[4,3]and the Span were reported(Table 2).The AZT,HIS-AZT and LEU-AZT had a mean diameter of 2.15-2.31 μm,providing further evidence that they can be delivered to the lower respiratory tract.Additionally,the LEU-AZT had the smallest Span of 1.32,suggesting a narrowest size distribution and further indicating a superior uniform particle size, in comparison withAZT and HIS-AZT spray-dried powders.Conversely,the GLY-AZT spray-dried powders showed a larger mean diameter of 144.51 μm,with a Span of 2.07,which is expected to have a very poor drug deposition in the lower respiratory tract through inhalation.
Theprimaryaerodynamicdiameter(dae)wastheoreticallyestimated from the particle sizing(d)and tapped density by the formulaofdae=d(ρt/ρ1)1/2,whereρ1=1 g/cm3.Theexposedprimary particlediameterwas1.65 μmforAZTspray-driedpowders.The daewas signi fi cantly large(129.26 μm)for GLY-AZT spray-dried powders,which was far beyond the size range for inhalation. The HIS-and LEU-modi fi ed spray-dried powders had a daeof 1.91 μm and 1.22 μm respectively;the lower daeof the LEU-modi fi edpowderssuggeststhattheformulationcouldpotentially have higher dispersibility and could have an improved deposition in the lungs.For any single dry powder formulation,the daewas smaller than the particle size determined by laser diffraction(D[4,3]),suggesting the powders formed aggregates in the air fl ow during the laser diffraction test[14].
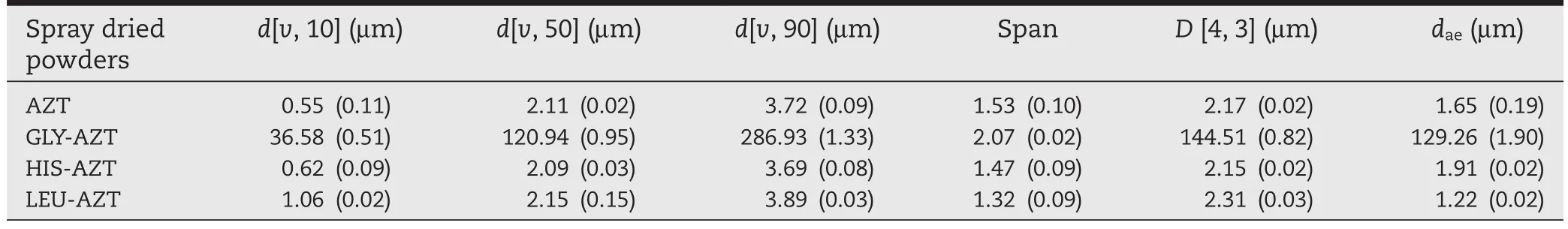
Table 2-Particle size distribution of spray-dried powders.The powders were tested in dry state,and the laser diffraction was utilized to determine the particle size distribution(data expressed as mean±SD).
3.3. In-vitro aerosolization and deposition
Next Generation Impactor(NGI)was employed to determine the in-vitro aerosolization and deposition of the spray-dried powders.For the test,Aerolizer?was utilized to aerosolize the dry powders where the amount of dry powders emitted from the capsules is expressed as a parameter of ED,which will re fl ect the in fl uence of amino acids on the fl owability of spraydried powders.ForAZT only spray-dried powders,approximately 85.6±1.6%was emitted from the capsules during the aerosolization.The ED for GLY-AZT spray-dried powders was 100±0.0%,suggesting all powders were emitted from the capsules,and further indicating GLY-AZT spray-dried powders had fl owability,which was in agreement with the data of Carr’s index value.The spray-dried powders modi fi ed by HIS and LEU had EDs of 92.8±0.7%and 98.0±0.7%respectively,suggesting LEU was more effective than HIS in improving the fl owability of spray-dried powders.Many reports have demonstrated that the use of LEU,either through coating on the particle surface, adding to the solution for spray-dried powders,or physically mixing with drug particles,can dramatically improve the fl owability of drug particles[12,15-17].Additionally,our previous paper has demonstrated that other amino acids such as arginine,phenylalanine and threonine can also improve the fl owability of spray-dried powders[11].In this paper,we further con fi rmed that use of amino acids did work well to improve aerosolization performance of spray-dried powders,and may further enhance the drug deposition in the lungs.
Undoubtedly,ED is a compulsory characteristic of feasible dry powder inhaler systems;however,the more crucial feature is the deposition site of drug particles in the respiratory tract once emitted from the inhaler.NGI has been well recognized as a device to test the in vitro lung deposition of inhaled particles,based on their aerodynamic size distribution[18],and it has been used as a standard method for the testing of inhaled products in the pharmacopeia of Europe,USA and other countries.The deposition pattern of spray-dried AZT alone demonstrated that a large amount of the powders remained in the device and capsules following aerosolization,and that a large proportion of the powder was deposited in the throat (Fig.3).A certain amount of drug particles can fl ow into lower stages and was mainly deposited in stages 2 and 3.All amino acid-modi fi ed spray-dried powders had a signi fi cantly decreased(one-way ANOVA,Duncan’s test,P<0.05)amount of drug that remained in the capsules and device,clearly exhibiting an improved aerosolization performance over the AZT powder.However,over 90%w/w of GLY-AZT spray-dried powders were deposited in the throat and pre-separator,with smaller proportion of the powders depositing on to the lower stages, suggesting that the inhalation of AZT powder would result in a signi fi cant deposition in the oropharyngeal region and the upper bronchi.Clearly,GLY-AZT spray-dried powders were unsuitable for the drug delivery to the lungs.On the other hand, the HIS-AZT spray-dried powders showed a signi fi cant increase of deposition in stage 2(cut-off diameter of 4.46 μm)over the control,indicating that the inhalation of HIS-AZT would generate an improved deposition in the lower respiratory tract. However,the LEU-modi fi ed spray-dried powders had a signi ficant reduction of deposition in the throat,indicating that the powder had an improved dispersibility.As expected,the LEU-AZT powder had an improved deposition in the lower stages.The drug deposition on stage 2 was signi fi cantly increased for LEU-AZT,with more than two times that of the control powder.Additionally,the total percentage of drug deposition on stage 5 to MOC(cut-off diameter<0.94 μm)was 26.2%for LEU-AZT powder,while only 13.7%was found for the control powder.Clearly,following inhalation,the LEU-modi fi ed powders would generate minimal deposition in the upper airways with a larger proportion of the dry powders fl owing into the lower respiratory tract,and the deposition in the alveolar regions would be highly enhanced.
For AZT spray-dried powders,the TD in each aerosolization test was 75 mg(25 mg powder per capsule,three capsules per test).The powder exhibited a FPD of 24.7 mg ofAZT(Table 3), and the FPF of spray-dried powder was 45.4%of the total recovered drug contents(54.4 mg).The reason could be attributed to the morphology of AZT microparticles that have decreased surface contact,therefore reducing the interaction forces between particles,and improving fl owability,dispersibility,and aerosolization performance[19].As expected,the GLY-AZT spray-dried powders,with huge particle size,had a minimal FPD of merely 0.04 mg,and a FPF of 0.29%of the RD(14 mg). These results,together with the data on the particle size and morphology,clearly demonstrated that the use of GLY in the formulation caused the drug particles to fuse together during the spray-drying process,damaging the micronized structure,and hence the delivery ability of spray-dried particles to the lungs.The HIS-AZT spray-dried powders demonstrated a FPF of 51.4%w/w(FPD of 13.0 mg divided by RD of 23.3 mg)that was signi fi cantly higher(one-way ANOVA,Duncan’s test, P<0.05)than the control powder,which was in good agreement with the particle sizing data that demonstrated a statistically smaller D[4,3]for HIS-AZT powder(2.15 μm)in comparison with that of AZT spray-dried powders(2.17 μm). These results clearly show that the addition of HIS in the formulation enhances the powder dispersibility.This could possible be due to the addition of HIS in the formulation that decreases the cohesion and adhesion of the spray-dried powders. This was also con fi rmed by the superior compressibleattribute of HIS-AZT dry powders(ρbwas only 0.49 g/cm3,while ρtwas highly increased to 0.79 g/cm3),suggesting a bigger distance between particles and subsequently having lower cohesive forces of HIS-modi fi ed spray-dried particles.On the other hand, the LEU-AZT spray-dried powders had FPD and RD of 12.8 mg and 20.8 mg respectively,resulting in a high FPF of 61.7%,which was signi fi cantly greater(one way ANOVA,Duncan’s test, P<0.05)than the control powders,suggesting that the majority of drug particles can be delivered to the peripheral regions of the lungs following inhalation.The signi fi cantly improved lung deposition of LEU-modi fi ed spray-dried powders may be related to their densities(ρband ρt)that were less than half of the control powders.The reduction in powder densities could play an important role to assist the powder aerosolization,and thereafter to improve the particle deposition in the lower stages.
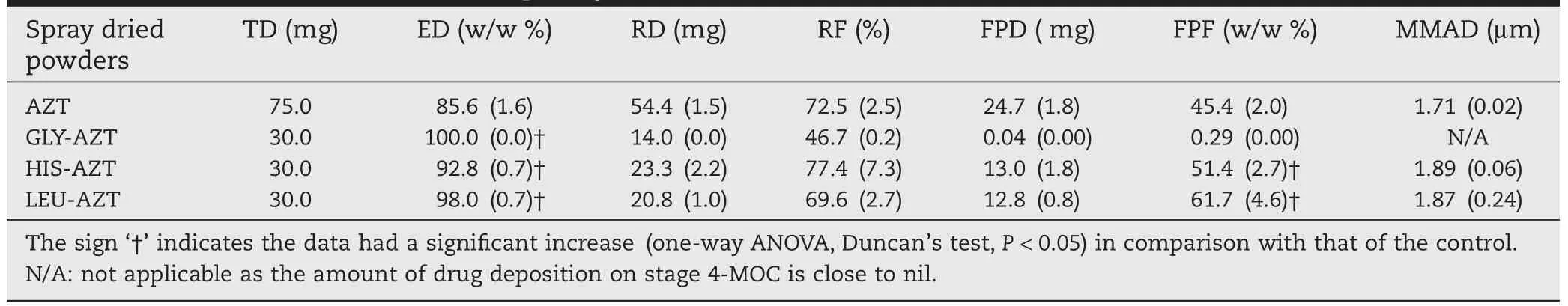
Table 3-In-vitro aerosolization of spray-dried powders.The in-vitro deposition of spray-dried AZT-LEU powders wasdetermined by NGI at the fl ow rate of 60 L/min for 5 s under the relative humidity of 35%.After deposition,the powders were collected from all parts including the capsules,device,throat,pre-separator and stage 1-MOC in use of 10 mL ofddH2O respectively.All solutions were fi ltered for HPLC test to determine the drug amount deposited in each part.The TD,ED,FPD,FPF,RD and RF were subsequently calculated as mentioned in the text.
The AZT,HIS-AZT and LEU-AZT spray-dried powders had MMAD in the range of 1.71-1.89 μm(Table 3),which was smaller than the physical diameter size of the particles,as determined by laser diffraction and con fi rmed by SEM visualization. This is the re fl ection that the furrowed surface morphology (AZT),decreased cohesion feature of particles(HIS-AZT,evidenced by high compressibility)and very low density(LEUAZT)resulted in improved dispersibility of particles.It has been proven that the increase in surface roughness can improve particle fl owability and dispersibility[20].However,in previous studies,the excipients were selected purposively to increase the surface roughness of spray-dried particles[12,21,22].In this study the active pharmaceutical ingredient(AZT)itself was able to generate the furrowed particles after spray-drying,which did provide AZT dry powders with good aerosolization ef ficiency.To the best of our knowledge,HIS as excipient to prepare spray-dried powders for inhalation has not been reported before. In this study,we have demonstrated that the addition of HIS to the spray-drying solution can generate powders with high condensability,through enhancing the distance between particles,leading to decreased cohesion between particles with subsequent increased dispersibility and improved deposition in the lungs.Furthermore,the use of LUE was shown to increase the fl owability,dispersibility and aerosolization performance of drug particles for inhalation,as reported in previous studies[8,12,15,23,24].The mechanism was probably related to its hydrophobic feature[25]and surfactant-like facet [26],which may facilitate the LEU molecules drift to the droplet surface during spray-drying and therefore modify surface characteristics,in particular the aerosolization characteristics.Moreover,this study also showed that the use of LEU at the concentration of 60%w/w in the formulation can generate hollow spray-dried particles with signi fi cant decreased powder densities,providing additional evidence in addition to the surface modi fi cation to enhance fl owability,dispersibility and lung deposition of LEU-modi fi ed spray-dried powders[27].
4. Conclusion
In this study,AZT spray-dried powders were prepared for inhalation and the in fl uence of different amino acids used as the excipients on the physiochemical properties and particularly in-vitro deposition was investigated.The use of GLY in the formulation resulted in the spray-dried powders fusing together agglomerates impacting on their aerosol performance,which was extremely poor.In comparison with AZT spray-dried powders,HIS-AZT dry powders were more compressible,indicating a bigger distance and lower cohesion between particles, while LEU-AZT spray-dried particles had hollow particle structures that drastically decreased the formulation densities.The changes in physiochemical properties resulted in these dry powders having enhanced dispersibility that resulted in an improved deposition to the lower respiratory tract,especially for LEU-modi fi ed spray-dried powders.
Acknowledgements
The authors are grateful for the fi nancial support from Natural Science Foundation of Jiangsu Province(BK2011295)andYouth Fund of Soochow University(SDY2011A21).
REFERENCES
[1]Zhou Q,Leung SSY,Tang P,et al.Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv Drug Deliv Rev 2015;85:83-99.
[2]Gibson RL,Retsch-Bogart GZ,Oermann C,et al.Microbiology, safety,and pharmacokinetics of aztreonam lysinate forinhalation in patients with cystic fi brosis.Pediatr Pulmonol 2006;41(7):656-665.
[3]Zeitler K,Salvas B,Stevens V,et al.Aztreonam lysine for inhalation:new formulation of an old antibiotic.Am J Health Syst Pharm 2012;69(2):107-115.
[4]Ibrahim BM,Tsifansky MD,Yang Y,et al.Challenges and advances in the development of inhalable drug formulations for cystic fi brosis lung disease.Expert Opin Drug Deliv 2011;8(4):451-466.
[5]Zhou QT,Tang P,Leung YSS,et al.Emerging inhalation aerosol devices and strategies:where are we headed?Adv Drug Deliv Rev 2014;75:3-17.
[6]Hoppentocht M,Hagedoorn P,Frijlink HW,et al. Technological and practical challenges of dry powder inhalers and formulations.Adv Drug Deliv Rev 2014;75:18-31.
[7]Nandiyanto ABD,Okuyama K.Progress in developing spraydrying methods for the production of controlled morphology particles:from the nanometer to submicrometer size ranges.Adv Powder Technol 2011;22(1):1-19.
[8]Seville PC,Li HY,Learoyd TP.Spray-dried powders for pulmonary drug delivery.Crit Rev Ther Drug Carrier Syst 2007;24(4):307-360.
[9]Zhou Q,Morton DAV,Yu HH,et al.Colistin powders with high aerosolisation ef fi ciency for respiratory infection: preparation and in vitro evaluation.J Pharm Sci 2013;102(10):3736-3747.
[10]Zhou Q,Gengenbach T,Denman J,et al.Synergistic antibiotic combination powders of colistin and rifampicin provide high aerosolization ef fi ciency and moisture protection.AAPS J 2014;16(1):37-47.
[11]Li HY,Seville PC,Williamson IJ,et al.The use of amino acids to enhance the aerosolisation of spray-dried powders for pulmonary gene delivery.J Gene Med 2005;7(3):343-353.
[12]Learoyd TP,Burrows JL,French E,et al.Sustained delivery by leucine-modi fi ed chitosan spray-dried respirable powders. Int J Pharm 2009;372(1-2):97-104.
[13]Zaja?c M,Jelin′ska A,Cielecka-Piontek J,et al.Stability of aztreonam in AZACTAM.Farmaco 2005;60(6-7):599-603.
[14]Adi H,Young PM,Chan HK,et al.Controlled release antibiotics for dry powder lung delivery.Drug Dev Ind Pharm 2010;36(1):119-126.
[15]Raula J,Lahde A,Kauppinen EI.Aerosolization behavior of carrier-free L-leucine coated salbutamol sulphate powders. Int J Pharm 2009;365(1-2):18-25.
[16]Prota L,Santoro A,Bifulco M,et al.Leucine enhances aerosol performance of naringin dry powder and its activity on cystic fi brosis airway epithelial cells.Int J Pharm 2011;412(1-2):8-19.
[17]Mangal S,Meiser F,Tan G,et al.Relationship between surface concentration of L-leucine and bulk powder properties in spray-dried formulations.Eur J Pharm Biopharm 2015;94:160-169.
[18]Taki M,Marriott C,Zeng XM,et al.Aerodynamic deposition of combination dry powder inhaler formulations in vitro:a comparison of three impactors.Int J Pharm 2010;388(1-2): 40-51.
[19]Chew NYK,Tang P,Chan H,et al.How much particle surface corrugation is suf fi cient to improve aerosol performance of powders?Pharm Res 2005;22(1):148-152.
[20]Adi S,Adi H,Tang P,et al.Micro-particle corrugation, adhesion and inhalation aerosol ef fi ciency.Eur J Pharm Sci 2008;35:12-18.
[21]Li HY,Seville PC.Novel pMDI formulations for pulmonary delivery of proteins.Int J Pharm 2010;385(1-2):73-78.
[22]Li HY,Birchall JC.Chitosan-modi fi ed dry powder formulations for pulmonary gene delivery.Pharm Res 2006;23(5):941-950.
[23]Minne A,Boireau H,Horta MJ,et al.Optimization of the aerosolization properties of an inhalation dry powder based on selection of excipients.Eur J Pharm Biopharm 2008;70(3):839-844.
[24]Stegemann S,Kopp S,Borchard G,et al.Developing and advancing dry powder inhalation towards enhanced therapeutics.Eur J Pharm Sci 2013;48(1-2):181-194.
[25]Black SD,Mould DR.Amino acid scale:hydrophobicity of physiological L-alpha amino acids.Anal Biochem 1991;193:72-82.
[26]Gliniski J,Chavepeyer G,Platten JK.Surface properties of aqueous solutions of L-leucine.Biophys Chem 2000;84(2): 99-103.
[27]Seville PC,Learoyd TP,Li HY,et al.Amino acid-modi fi ed spray-dried powders with enhanced aerosolisation properties for pulmonary drug delivery.Powder Technol 2007;178:40-50.
*Corresponding author.Testing and Analysis Centre,Soochow University,Suzhou 215123,China.Tel.:+86 512 65880383;fax:+86 512 65880383. E-mail address:xuying@suda.edu.cn(Y.Xu).
**Corresponding author.Suzhou Hui-Ren Biotech Co.Ltd,Suzhou 215513,China.Tel.:+86 512 51915785;fax:+86 512 51915785.
E-mail address:lihy98@hotmail.com(H.-Y.Li).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.08.002
1818-0876/?2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Spray-drying
Inhalation Amino acids
 Asian Journal of Pharmacentical Sciences2015年6期
Asian Journal of Pharmacentical Sciences2015年6期
- Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- Preface
- Novel potential for optimization of antitubercular therapy:Pulmonary delivery of rifampicin lipospheres
- Optimizing aerosolization of a high-dose L-arginine powder for pulmonary delivery
- Delivery of theophylline as dry powder for inhalation
- The effects of surface morphology on the aerosol performance of spray-dried particles within HFA 134a based metered dose formulations
