Delivery of theophylline as dry powder for inhalation
Original Research Paper
Delivery of theophylline as dry powder for inhalation
Bing Zhua,Mehra Haghia,b,Anphy Nguyena,Mary Goudc, Stewart Yeunga,Paul M.Younga,Daniela Trainia,*
aRespiratory Technology,The Woolcock Institute for Medical Research and Discipline of Pharmacology,Sydney Medical School,University of Sydney,NSW 2006,Australia
bFaculty of Pharmacy,University of Technology Sydney,NSW 2007,Australia
cAvans University of Applied Science,Breda,The Netherlands
ARTICLE INFO
Article history:
Received 3 July 2015
Received in revised form 18 August 2015
Accepted 18 August 2015
Available online 24 August 2015
Theophylline
Theophylline(TP)is a very well established orally or intravenously delivered antiasthma drug with many bene fi cial effects.This study aims to improve asthma treatment by creating a dry powder inhalable(DPI)formulation ofTP to be delivered directly to the lung,avoiding the side effects associated with conventional oral delivery.The DPI TP formulation was investigated for its physico-chemical characteristics using scanning electron microscopy,laser diffraction,thermal analysis and dynamic vapour sorption.Furthermore,aerosol performance was assessed using the Multi Stage Liquid Impinger(MSLI).In addition,a Calu-3 cell transport assay was conducted in vitro using a modi fi ed ACI to study the impact of the DPI formulation on lung epithelial cells.Results showed DPI TP to be physico-chemically stable and of an aerodynamic size suitable for lung delivery.The aerosolisation performance analysis showed the TP DPI formulation to have a fi ne particle fraction of 29.70±2.59%(P<0.05) for the TP formulation containing 1.0%(w/w)sodium stearate,the most ef fi cient for aerosolisation.Regarding the deposition of TP DPI on Calu-3 cells using the modi fi ed ACI, results demonstrated that 56.14±7.62%of the totalTP deposited(13.07±1.69 μg)was transported across the Calu-3 monolayer over 180 min following deposition,while 37.05±12.62% of the deposited TP was retained in the cells.This could be due to the presence of sodium stearate in the current formulation that increased its lipophilicity.A DPI formulation of TP was developed that was shown to be suitable for inhalation.
?2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Theophylline(TP)is a well established orally or intravenously delivered antiasthma drug[1-5].The main drawbacks of this active pharmaceutical ingredient(API)are related to its narrow therapeutic index and several side effects,i.e.nausea, headache,dizziness and vomiting[2,6].Increasing evidence shows that it has signi fi cant anti-in fl ammatory effects in chronic obstructive pulmonary disease at lower plasma concentrations[3].Already in the early 90s authors have demonstrated that delivery of sub-bronchodilator doses ofTP(serum concentration of about 8 μg/ml)signi fi cantly attenuated late asthmatic response,which is in fl ammatory in nature[7,8].The steady-state peak serumTP concentration is a function of the dose,dosing interval,and rate of TP absorption and clearance in the individual patient.Because of marked individual differences in the rate of TP clearance,the dose required to achieve a peak serum theophylline concentration in the 10-20 mcg/ml range varies fourfold among patients(e.g., 400-1600 mg/kg/d in adults<60 y old and 10-36 mg/kg/d in children)[9,10].The dose ofTP must then be individualised on the basis of peak serum concentration measurements in order to achieve a dose that will provide maximum potential bene fi t with minimal risk of adverse effects.
With the aim to reduce these side effects and exploit the anti-in fl ammatory bene fi ts of this old drug,there is the potential to reformulate this API for lung delivery as a dry powder inhaler(DPI).This should open up bene fi ts that would not be possible using the historical routes of delivery.The authors have already presented re-purposingTP as pressurised metered dose (pMDI)solution[11]with good in vitro aerosolisation and cell results,but the disadvantage of this delivery platform is that doses are limited to a few micrograms.
Dry powder inhalers have many bene fi ts over other lung drug delivery platforms,such as pMDIs or nebulisers.They can deliver high doses,have longer stability due to their solid state, absence of cold chain storage during transport,high patient compliance,shorter delivery times,lack of propellants and absence of the need of patient coordination when the API is delivered[12-14].
It is a common strategy to include lubricants or surfactants in an inhalable formulation to improve aerosol performance [15],reduce powder adhesion to inhaler device[16]and capsules or blisters[17,18].Common lubricants or surfactants usually include trileucine,leucine and cyclodextrin[15].Furthermore,lipophilic components,such as cholesterol and phospholipid[19],have also been utilised to achieve the abovementioned purposes.
Sodium stearate is the sodium salt of stearic acid with a hydrophobic chain of 18 carbons.Due to its lipophilic nature, it has been used as a lipophilic adjunct in the formulation of inhalable aerosols to promote microparticle aerosolisation and also formulation resistance to environmental humidity[19].For example,a previous study has demonstrated that the addition of a small amount of sodium stearate(1%,w/w)signi fi cantly improves the aerosol performance of tobramycin spray-dried powders[19].
A review of the literature suggests only few have engineered TP as a DPI for lung delivery.In 1994,Raeburn and Woodman [20]showed thatTP could be delivered intra-tracheally to guinea pigs via DPI,and investigated bronchospasm,in fl ammation and airway microvascular leakage.In 2011,Salem et al.[21]presented nanoparticles of TP for DPI delivery with a view to enhance the dissolution rate sinceTP is relatively insoluble in water.More recently,in 2013,Alhalaweh et al.[22]manufactured of a series ofTP co-crystals,with and without excipients or other APIs,using spray drying as a particle engineering approach.Their fi ndings produced SD-TP particles.Both the 2011 and 2013 manuscripts were focused more on the use of particle engineering(controlled agglomeration[21]or spray drying of co-crystals[22])for inhalation pharmaceutical applications and not on the actual re-formulation and aerosol characterisation of TP as a DPI,as studied here.Speci fi cally, the aim of this manuscript is to present a TP formulation and to study its optimisation,physico-chemical properties and interaction with lung epithelia using a Calu-3 epithelial cell model.
2. Materials and methods
Anhydrous theophylline(TP)and sodium stearate were supplied by MP Biomedicals,Australia.Water was puri fi ed by reverse osmosis(MilliQ,Millipore,France).All solvents used were of analytical grade and were supplied by Chemsupply(Victoria, Australia).All sterile culture plastic ware was supplied by Sarstedt(Adelaide,Australia).
2.1. Particle production
Solutions ofTP for spray drying were prepared by mixing equal volumes of individually prepared TP aqueous solution(30°C) and alcoholic solutions of sodium stearate at various concentrations(30°C).The total solid content was maintained at 20 mg/ml and the ratio of sodium stearate to TP was varied. Sodium stearate weight fractions of 0%,0.5%,1.0%and 1.5% w/w,respectively,based onTP mass were used for spray drying [19].Solutions were spray dried using a Büchi B-290 spray dryer (Büchi Mini Spray Dryer B-191,Flawil,Switzerland)under the following conditions:inlet temperature 100°C,measured outlet temperature 48°C,solution feed rate 40 ml/min,aspirator rate 100%and atomising air fl ow 700 l/h.
2.2. Scanning electron microscopy
The morphology of the spray driedTP microparticles containing various weight fractions of sodium stearate was studied using a scanning electron microscope(JEOL-6000,Tokyo,Japan) at 5 kV.TP raw material and spray-dried samples were deposited onto double-sided adhesive carbon tape,mounted to aluminium stubs and sputter-coated with gold at a coating thickness of 15 nm prior to imaging.
2.3. Thermal analysis of TP
The thermal response of TP raw material and spray dried formulations were analysed using differential scanning calorimetry(DSC,model 823e,Mettler Toledo International Inc.,Schwerzenbach,Switzerland)at a heating rate of 10°C/min between 25°C and 300°C.Samples(ca.5-8 mg)were loaded and crimp-sealed in 40 μl aluminium sample pans and the lids pierced to ensure constant pressure.Measurements were conducted under an inert gas stream(N2,25 cm3/min). Thermogravimetric analysis(TGA)was conducted using aTGA (Mettler-Toledo AG,Schwerzenbach,Switzerland).In brief,approximately 20 mg of TP raw material and each spray dried formulation were loaded and analysed at a heating rate of 10°C/min from 25°C to 200°C under an inert gas stream(N2,25 cm3/min)and the sample weight change(%)recorded.
2.4. Dynamic vapour sorption
The moisture sorption characteristic of spray-driedTP was assessed using dynamic vapour sorption(DVS Intrinsic,Surface Measurement Systems Limited,London,UK).Approximately 15 mg of spray-dried samples were loaded onto the stainless steel sample pan and dried at 0%relative humidity(RH)prior to analysis.The loaded samples were exposed to one 0~90% RH cycle at 25°C with 10%RH increment.Equilibrium of moisture sorption was determined by a change in mass-to-time ratio (dm/dt)of 0.0002%min-1.The data were presented as the sample mass change(%)over the investigated RH range.
2.5. In vitro aerosol performance analysis
The in vitro aerosol performance of theTP DPI formulations was determined using a multi-stage liquid impinger(MSLI)(Apparatus A,European Pharmacopoeia,Chapter 2.9.18)(Westech Scienti fi c Instruments,Bedfordshire,UK)using a RS01?DPI high-resistance device(Plastiape S.p.A,Osnago-Lecco,Italy).The fl ow rate through the MSLI was controlled using a rotary vein pump and solenoid valve timer(Westech Scienti fi c Instruments,Bedfordshire,UK)set to 60 l/min using a TSI fl owmeter(model 4040,TSI Instruments,MN,US).Deionised water (20 ml)was accurately added to each MSLI collection stage as particle recovery and rinsing solution.
A 5±0.2 mg aliquot of spray-driedTP formulations were preloaded into Size 3 hard gelatin capsules(Capsugel,Sydney, Australia)and placed in the chamber of the inhaler device.The device was fi tted into a silicon mouthpiece adapter,which was connected to the MSLI equipped with a US Pharmacopoeia throat.The formulation was actuated at the above mentioned fl ow rate over a four-second period.After actuation,the capsule,device and mouthpiece adaptor were thoroughly rinsed with 5 ml deionised water.All the stages were rinsed with deionised water into suitable volumetric fl asks for chemical quanti fi cation.Each formulation was tested in triplicate.
2.6. Calu-3 cell culture
Passages 37-41 of Calu-3 cells(HTB-55)(American Type Cell Culture Collection(ATTC),Rockville,USA)were grown in complete Dulbecco’s Modi fi ed Eagle’s medium:F-12(Sigma-Aldrich,Sydney,Australia)and propagated as described previously[23].In order to establish the air-liquid interface model,cells were seeded onto Snapwell?polyester inserts (0.4 μm pore size,1.12 cm2surface area)(Corning Costar,Lowell, MA,USA)at a density of 5×105cells/cm2and the monolayers were allowed to differentiate under air-interface for 14 d.This period of time in culture was selected based on the study by Haghi et al.[24]that determined the optimum time in culture for Calu-3 cells to form tight junctions and produce mucus in culture.
2.7. Cell integrated modi fi ed ACI assessment of TP drug deposition and transport
The novel integrated modi fi edAndersen cascade impactor(ACI) as described by Haghi et al.[24]was used to assess TP drug deposition and uptake across the Calu-3 epithelial cells.Fourteen days post seeding,the Snapwells,containing Calu-3 cells at the air-interface con fi guration,were placed on stage 4 of the ACI(aerodynamic cut-off diameter 2.1-3.3 μm).After particle deposition,the inserts were transferred to a 6 well plate containing 800 μl of Hank’s balanced salt solution(HBSS) (Invitrogen,Sydney,Australia)and returned to the incubator. Samples(100 μl)were taken at 30,60,120 and 180 min from the basal chamber and TP content was analysed using HPLC. After the fi nal sample was taken,the surface of the Calu-3 cells was washed with buffer and collected for analysis of residual apical drug.
To con fi rm barrier integrity of Calu-3 cells before and after TP deposition,the transepithelial electrical measurement(TEER) was performed using an EVOM Voltohmmeter(World Precision Instruments,FL,USA)with STX-2 chopstick electrodes. The measured TEER in control Snapwells and before TP deposition was 521±74 ohm·cm2.Following transport studies (180 min)the cell surface was washed with PBS buffer and the TEER measured at 458±39 ohm·cm2.
Finally,to quantify theTP retained in the cells,Calu-3 cells were harvested and lysed using CellLyticTMM Cell Lysis reagent (Sigma-Aldrich,Sydney,Australia)with 1%(v/v)protease inhibitor cocktail(Sigma-Aldrich,Sydney,Australia)according to the method described previously[23].This method allowed for the calculation of the total drug deposited by adding the amount of TP transported,retained in the cells and remaining on the surface of the epithelial cells.
2.8. High performance liquid chromatography
The quanti fi cation of drug from the MSLI and cell based studies was conducted by high performance liquid chromatography (HPLC).A Shimadzu HPLC system consisting of a LC20AT pump, a SIL20AHT autosampler and an SPD-20A UV-VIS detector (Shimadzu,Sydney,Australia)was used.Samples were injected into a reverse phase C18 column(4.6×150 mm and 5 μm, XBridge?Shield,Waters,USA)with 55%(v/v)methanol/ water mobile phase at a fl ow rate of 1 ml/min.The detection wavelength was 275 nm and injection volume was 100 μl.The standard solutions were prepared daily and the linearity of standard solutions in the concentration of 0.01~100 μg/ml was con fi rmed with an R2value≥0.999.
2.9. Statistical analysis
All results are expressed as mean±standard deviation(SD) of at least three separate determinants.The results were analysed using one-way ANOVA followed by post-hoc multiplecomparisons.Differences were deemed signi fi cant for P≤0.05. Unless otherwise stated,data are represented in terms of mean and standard deviation.
3. Results and discussion
In the current study,we developed and optimisedTP dry powder formulation by spray drying for pulmonary delivery using sodium stearate as lipophilic adjunct.Four batches ofTP powder with/without sodium stearate were produced.All batches showed a yield of approximately 60%~73%.The collected powders were evaluated in terms of surface morphology, physico-chemical properties,aerodynamic characteristics,and transport rate across Calu-3 epithelial cell monolayer.
3.1. Particle morphology
The morphology of spray dried TP particles by SEM is shown in Fig.1.As seen in Fig.1A,particles of TP raw material possessed elongated surface morphology.Spray-dried TP was spherical with coral-like surface morphology(Fig.1B).With the addition of 0.5%and 1.5%(w/w)sodium stearate,cospray dried particles lost the spherical morphology with the presence of crystal-like protrusions(Fig.1C)and clusters(Fig.1E). A previous study suggested that the fi nal distribution of each solute component in a binary aqueous system during the spray drying process was dependent on molecular structures of individual solutes and their relative molecular mass[19].Sodium stearate is used as a lipophilic adjunct in the current study, having a lower aqueous solubility than that ofTP[25,26].Subsequently,during the drying process,sodium stearate molecules may tend to move toward the air-liquid interface,depositing at the surface of a dried particle.Therefore,uneven solute transport rate from the inner core to 2the surface of a drying droplet may occur[27],resulting in different crystallisation rates of individual components under certain drying conditions[28].Furthermore,a suitable weight fraction ratio between the individual solute components in the binary system is also required to balance the crystallisation rates of each component in order to achieve the particle morphology suitable for inhalation delivery purpose[29].In general,1.0%w/w produced the most suitable(spherical and homogeneous) morphology(Fig.1D).
3.2. Physico-chemical characterisation
The thermal responses ofTP raw material and spray-dried formulations using DSC are shown in Fig.2.Sodium stearate produced no obvious effects on the thermal behaviours of the produced formulations,since weight fractions of sodium stearate in the dried particulate systems were considerably low.All samples subject to DSC analysis showed an endothermic peak at approximately 271°C,corresponding toTP melting event [30,31].No exothermic events were observed in the temperature region below 150°C,indicating all samples to be crystalline. Furthermore,there was no endothermic response noted at 70~90°C region forTP raw material and weight loss data from TGA were shown to be minimum(<0.01%,w/w;data not shown), con fi rming the anhydrous form.Weight loss analysis of all spray-dried formulations upon heating suggested prepared formulations to be anhydrate with an average weight loss of<0.1%(w/w).
To investigate the relative solid-state stability of the spray dried and co-spray dried powders,the in fl uence of humidity on moisture sorption was performed.Representative moisture sorption isotherms for the prepared formulations as a function of relative humidity(%)are shown in Fig.3.As seen, all formulations showed minimal weight gain of 0.4~0.6% between 0%and 90%RH.Theophylline is known to exist in three different solid-state forms,i.e.anhydrate,monohydrate and a metastable dehydrate[32].It has been reported that anhydrous TP converts to monohydrate when exposed to>80% relative humidity and the subsequent compound dehydrates at low RH(usually<20%)[33].The dehydration of TP monohydrate is considered as a two-step process consisting of dehydration and evaporation of crystal water[33].As shown in Fig.3A,theTP spray-dried formulation showed a small weight gain of 0.14%(w/w)after desorption.Previous research has demonstrated that TP monohydrate may transit to a metastable dehydrate during drying[34].Therefore,hysteresis in transition from metastable dehydrate to anhydrous form and incomplete evaporation of water molecules in the crystal lattice during the desorption cycle may contribute to the observed weight gain.Fig.4 shows the hysteresis of mass change between sorption and desorption cycles in the investigated RH range. As seen,the TP powder(i.e.0%sodium stearate)consistently showed positive hysteresis values between 10%and 80%RH with a decreasing trend when RH was below 50%,also suggesting the incomplete compound phase transition in the monohydrate dehydration process[34].
Fig.3B and D shows the representative moisture sorption isothermsofTPparticlescontaining0.5%and1.5%(w/w)sodium stearate,respectively.Asseen,TPmicroparticlescontaining0.5% and 1.5%(w/w)sodium stearate had increased desorption rates between 30%and 40%RH compared to 0%and 1.0%formulations.Previous study with NaSt in a range from 0 to 2%w/w [19]suggested micelle formation in the co-spray dried particleswhentheconcentrationofsodiumstearateinanatomised droplet was above its critical micelle concentration,between 4.0×10-4and 5.6×10-4M[35,36],with a parabolic dependence observedforNaStwithapeakaroundthe1%contentofsodium stearate.Positive hysteresis values for 0.5%and 1.5%formulationscanbefoundinFig.4inthe30%~40%RHrange.Therefore,it can be speculated that inclusion of sodium stearate in the particulate systems at low(0.5%,w/w)and high(1.5%,w/w) concentrations may have a great in fl uence on crystal lattice repacking during TP monohydrate-anhydrate transition,resultinginobservedabnormalitiesinthedryingcycle.TPparticles containing 1.0%(w/w)sodium stearate generally followed the sorption/desorption pro fi le of crystalline materials[37](Fig.3C) with minimal hysteresis in both cycles[19].
3.2.1. Impaction study and aerosolisation performance
Prepared DPI formulations were subjected to in vitro cascade impaction test via a multi-stage liquid impinger(MSLI)in order to evaluate their aerodynamic diameters and aerosol performances.Table 1 summarises the mass median aerodynamic diameters(MMAD)and geometric standard deviation(GSD)of tested formulations.The physical diameter of the resulting spray dried particles is governed by the solute concentration in a droplet once other parameters(e.g.atomised droplet diameter,drying temperature and solvent,etc.)are fi xed[38,39].This can potentially explain the similar MMADs and GSDs of prepared formulations in the current study,since overall solute concentration was maintained at the same level(20 mg/ml)and no other parameter varied[40,41].
Besides size,morphology is also one of the key factors governing the aerodynamic performance of a given particle[28].
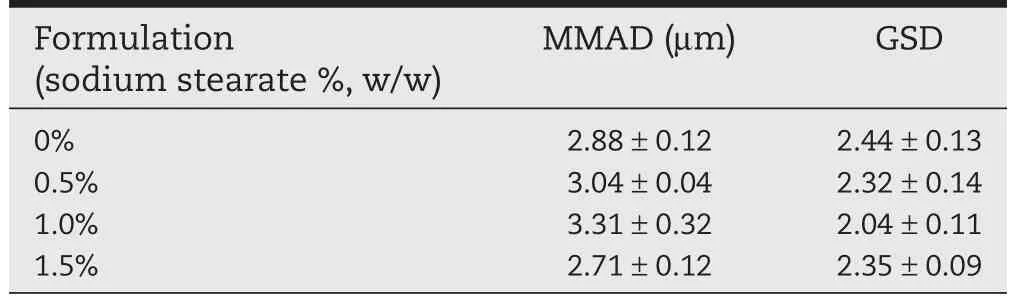
Table 1-Mass median aerodynamic diameter(MMAD) and geometric standard deviation(GSD)of theophylline particles containing different weight fractions of sodium stearate(n=3±SD).
Statistical analysis showed no signi fi cant difference in aerodynamic size among the formulations containing 0%,0.5%and 1.0%(w/w)sodium stearate,possibly due to their sphere-like morphological characteristics.However,signi fi cant statistical difference was observed between 1.0%and 1.5%(w/w)formulations.Since the MMAD of 1.5%(w/w)formulation was signi fi cantly lower than that of 1.0%(w/w)formulation,it can be speculated that the 1.5%(w/w)particle formulation may undergo fracture[42]during the aerosolisation process,since majority of them possessed needle-like surface morphology (Fig.1E).
Mass deposition of each formulation delivered via RS01 dry powder inhaler on each MSLI stage is expressed as the percentage of the total mass recovered(>95%of loaded dose)and shown in Fig.5.To further analyse the aerosol performance, the emptying ef fi ciency and aerosol performance parameters are shown in Fig.6.Fine particles were deemed as particles with an aerodynamic diameter≤6.8 μm(i.e.MSLI stage 3 to fi lter) and their fractions over loaded dose and emitted dose calculated as fi ne particle fractions of loaded and emitted doses(FPFLDand FPFED,respectively).
As seen in Fig.5,the 0%sodium stearate formulation had the highest powder retention in the capsule after dispersion under the pharmacopeia testing condition(i.e.60 l/min over a four-second period),with 41.16±0.21%of recovered mass retained within the capsule.Such rate was signi fi cantly lower for other formulations:4.99±1.61%,6.35±1.3%and 4.51±0.81% for 0.5%,1.0%and 1.5%formulation,respectively.As a result, emptying ef fi ciency ofTP spray-dried formulation was signi ficantly lower with approximately 42%mass emitted compared to~73%for co-spray dried formulations.The 0%formulation showed an induction port deposition of 13.38±0.12%,signi ficantly lower than the 20~30%obtained for the rest of prepared formulations(P<0.05).
In general,the majority of post-throat aerosols deposited in the aerodynamic size region≤6.8 μm.The addition of sodium stearate in the formulation in fl uenced the FPF of emitted aerosol cloud in a positive manner(Fig.6).For example,the FPFLDsigni fi cantly increased from 21.49±0.05%to 29.70±2.59%(P<0.05) when the weight fraction of sodium stearate was increased from 0%to 1.0%(w/w).This observation is possibly the result of surface distribution of sodium stearate molecules on the particles during the spray drying process,consequently reducing inter-particle cohesion[19].However,there was no clear linear correlation between the FPFLDand weight fraction of sodium stearate in the formulation as demonstrated by Parlati et al. [19].Peak FPFLDwas observed with 1.0%(w/w)sodium stearate added in the formulation and the difference of FPFLDvalues between 0.5%and 1.5%formulations was not signi fi cant (P>0.05,Fig.6).As mentioned previously,once the concentration of sodium stearate reaches the critical micelle concentration,micelle formation may occur,causing internalisation of a considerable amount of sodium stearate and thus insuf fi cient surface counts[19].The FPFEDof sodium stearate-containing formulations showed a similar trend of FPFLD(Fig.6).The FPFEDof 0%formulation was approximately 50%, possibly due to the porous nature of produced particles(Fig.1); however,the high capsule retention renders its aerosolisation ef fi ciency less desirable.
To summarise,all the prepared formulations had similar aerodynamic diameters and post-induction port distribution pro fi les.However,TP formulation containing 1.0%(w/w)sodium stearate showed the most suitable aerosolisation ef fi ciency.
3.3. In vitro TP transport using a modi fi ed ACI and Calu-3 model
Transport of TP microparticles containing 1%w/w magnesium stearate over 180 min is shown in Fig.7.Results demonstrated that 56.14±7.62%of the total TP deposited (13.07±1.69 μg)was transported across the Calu-3 monolayer over 180 min following deposition,while 37.05±12.62% of the depositedTP was retained in the cells.This could be due to the presence of sodium stearate in the current formulation that could have increased its lipophilicity.Further studies are currently under investigation to understand this phenomenon.In a previous study,where TP was formulated as a pressurised metered dose inhaler,it was observed that the amount of drug retained in the cells was negligible,while 97% of the depositedTP(1.25±0.40 μg)was transported over 180 min of study[11].In comparison,the current formulation shows a signi fi cantly faster transport rate over 30 min following drug deposition(~90 ngTP from the pressurised metered dose inhaler vs.~2.6 μg TP from the DPI formulation),which could indicate higher concentration of TP at the site of action(A3 Adenosine receptors located on the smooth muscle cells)30 min following deposition,potentially resulting in faster onset of action.
4. Conclusions
In the current investigation,a TP dry powder inhaler formulation containing 1.0%(w/w)sodium stearate as lipophilic adjunct was successfully developed.The formulation showed suitable physico-chemical and aerodynamic properties for lung delivery,as con fi rmed by the morphology and aerosol performance studies.Cell studies con fi rmed TP DPI delivered on Calu-3 cells to be able to transport across epithelial cells to reach its site of action.
The formulation shows potential for the repurposing of TP to treat asthma-related lung diseases via pulmonary route instead of the classical oral route.Future research interest will be investigating this formulation in vivo.
Acknowledgements
Professor Young is the recipient of an Australian Research Council Future Fellowship(project number FT110100996).Associate ProfessorTraini is the recipient of an Australian Research Council Future Fellowship(project number FT12010063).
REFERENCES
[1]Milgrom H,Bender B.Current issues in the use of theophylline.Am Rev Respir Dis 1993;147:S33-S39.
[2]Banner AS.Theophylline:should we discard an old friend? Lancet 1994;343:618.
[3]Barnes PJ.Theophylline:new perspectives for an old drug. Am J Respir Crit Care Med 2003;167:813-818.
[4]Barnes PJ.Theophylline for COPD.Thorax 2006;61:742-744.
[5]Barnes PJ.Theophylline.Am J Respir Crit Care Med 2013;188:901-906.
[6]Spector SL.Advantages and disadvantages of 24-hour theophylline.J Allergy Clin Immunol 1985;76:302-311.
[7]Ward AJ,McKenniff M,Evans JM,et al.Theophylline-an immunomodulatory role in asthma?Am Rev Respir Dis 1993;147:518-523.
[8]Sullivan P,Bekir S,Jaffar Z,et al.Anti-in fl ammatory effects of low-dose oral theophylline in atopic asthma.Lancet 1994;343:1006-1008.
[9]Wyatt R,Weinberger M,Hendeles L.Oral theophylline dosage for the management of chronic asthma.J Pediatr 1978;92:125-130.
[10]Hendeles L,Weinberger M,Wyatt R.Guide to oral theophylline therapy for the treatment of chronic asthma. Am J Dis Child 1978;132:876-880.
[11]Zhu B,Haghi M,Goud M,et al.The formulation of a pressurized metered dose inhaler containing theophylline for inhalation.Eur J Pharm Sci 2015;76:68-72.
[12]Crompton GK.Dry powder inhalers:advantages and limitations.J Aerosol Med 1991;4:151-156.
[13]Islam N,Gladki E.Dry powder inhalers(DPIs)-a review of device reliability and innovation.Int J Pharm 2008;360:1-11.
[14]Frijlink HW,De Boer AH.Dry powder inhalers for pulmonary drug delivery.Expert Opin Drug Deliv 2004;1:67-86.
[15]Pilcer G,Amighi K.Formulation strategy and use of excipients in pulmonary drug delivery.Int J Pharm 2010;392:1-19.
[16]Labiris NR,Dolovich MB.Pulmonary drug delivery.Part II:the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications.Br J Clin Pharmacol 2003;56:600-612.
[17]Price R,Young PM,Edge S,et al.The in fl uence of relative humidity on particulate interactions in carrier-based dry powder inhaler formulations.Int J Pharm 2002;246:47-59.
[18]Chew NY,Chan HK.Use of solid corrugated particles to enhance powder aerosol performance.Pharm Res 2001;18:1570-1577.
[19]Parlati C,Colombo P,Buttini F,et al.Pulmonary spray dried powders of tobramycin containing sodium stearate to improve aerosolization ef fi ciency.Pharm Res 2009;26:1084-1092.
[20]Raeburn D,Woodman VR.Effect of theophylline administered intratracheally as a dry powder formulation on bronchospasm and airway microvascular leakage in the anesthetized guinea-pig.Pulm Pharmacol 1994;7:243-249.
[21]Salem HF,Abdelrahim ME,Abo Eid K,et al.Nanosized rods agglomerates as a new approach for formulation of a dry powder inhaler.Int J Nanomedicine 2011;6:311-320.
[22]Alhalaweh A,Kaialy W,Buckton G,et al.Theophylline cocrystals prepared by spray drying:physicochemical properties and aerosolization performance.AAPS PharmSciTech 2013;14:265-276.
[23]Haghi M,Young PM,Traini D,et al.Time-and passage-dependent characteristics of a Calu-3 respiratory epithelial cell model.Drug Dev Ind Pharm 2010;36:1207-1214.
[24]Haghi M,Traini D,Young P.In vitro cell integrated impactor deposition methodology for the study of aerodynamically relevant size fractions from commercial pressurised metered dose inhalers.Pharm Res 2014;31(7):1779-1787.
[25]Zhu H,Yuen C,Grant DJ.In fl uence of water activity in organic solvent+water mixtures on the nature of the crystallizing drug phase.1.Theophylline.Int J Pharm 1996;135:151-160.
[26]British Pharmacopeia Commission.British pharmacopeia. London,England:2014.
[27]Chen XD,Sidhu H,Nelson M.Theoretical probing of the phenomenon of the formation of the outermost surface layer of a multi-component particle,and the surface chemical composition after the rapid removal of water in spray drying.Chem Eng Sci 2011;66:6375-6384.
[28]Vehring R.Pharmaceutical particle engineering via spray drying.Pharm Res 2008;25:999-1022.
[29]Sloth J,Jorgensen K,Bach P,et al.Spray drying of suspensions for pharma and bio products:drying kinetics and morphology.Ind Eng Chem Res 2009;48:3657-3664.
[30]Legendre B,Randzio SL.Transitiometric analysis of solid II/ solid I transition in anhydrous theophylline.Int J Pharm 2007;343:41-47.
[31]Colacio-Rodriguez E,Salas-Peregrin J.Thermal studies on purine complexes.V.Thermal behaviour of tetrachloropalladates of theophylline and theobromine and theophylline complexes of Cd(II)and Hg(II).Thermochim Acta 1984;74:45-54.
[32]Suzuki E,Shimomura K,Sekiguchi K.Thermochemical study of theophylline and its hydrate.Chem Pharm Bull 1989;37:493-497.
[33]Suihko E,Ketolainen J,Poso A,et al.Dehydration of theophylline monohydrate-a two step process.Int J Pharm 1997;158:47-55.
[34]Vora KL,Buckton G,Clapham D.The use of dynamic vapour sorption and near infra-red spectroscopy(DVS-NIR)to study the crystal transitions of theophylline and the report of a new solid-state transition.Eur J Pharm Sci 2004;22:97-105.
[35]Tabazadeh A.Organic aggregate formation in aerosols and its impact on the physicochemical properties of atmospheric particles.Atmos Environ 2005;39:5472-5480.
[36]Capelle HA,Britcher LG,Morris GE.Sodium stearate adsorption onto titania pigment.J Colloid Interface Sci 2003;268:293-300.
[37]Young PM,Salama R,Zhu B,et al.Multi-breath dry powder inhaler for delivery of cohesive powders in the treatment of bronchiectasis.Drug Dev Ind Pharm 2015;41(5):859-865.
[38]Vehring R,Foss WR,Lechuga-Ballesteros D.Particle formation in spray drying.J Aerosol Sci 2007;38:728-746.
[39]Salama R,Hoe S,Chan HK,et al.Preparation and characterisation of controlled release co-spray dried drug-polymer microparticles for inhalation 1:in fl uence of polymer concentration on physical and in vitro characteristics.Eur J Pharm Biopharm 2008;69:486-495.
[40]Elversson J,Millqvist-Fureby A,Alderborn G,et al.Droplet and particle size relationship and shell thickness of inhalable lactose particles during spray drying.J Pharm Sci 2003;92:900-910.
[41]Boraey MA,Hoe S,Sharif H,et al.Improvement of the dispersibility of spray-dried budesonide powders using leucine in an ethanol-water cosolvent system.Powder Technol 2013;236:171-178.
[42]Chew NY,Tang P,Chan HK,et al.How much particle surface corrugation is suf fi cient to improve aerosol performance of powders?Pharm Res 2005;22:148-152.
*Corresponding author.Respiratory Technology,The Woolcock Institute for Medical Research and Discipline of Pharmacology,Sydney Medical School,University of Sydney,NSW 2006,Australia.Tel.:0061-2-91140352.
E-mail address:daniela.traini@sydney.edu.au(D.Traini).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.08.005
1818-0876/?2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Powder formulation
Aerosol performance
Physiochemical characteristics
 Asian Journal of Pharmacentical Sciences2015年6期
Asian Journal of Pharmacentical Sciences2015年6期
- Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- Preface
- Novel potential for optimization of antitubercular therapy:Pulmonary delivery of rifampicin lipospheres
- The in fl uence of amino acids on aztreonam spray-dried powders for inhalation
- Optimizing aerosolization of a high-dose L-arginine powder for pulmonary delivery
- The effects of surface morphology on the aerosol performance of spray-dried particles within HFA 134a based metered dose formulations
