CuInS2/石墨烯混合物的合成及其在太陽能電池中的應(yīng)用
華 健,林運祥,周 雷,楊 曉,左學(xué)勤,李 廣,2*
(1.安徽大學(xué) 物理與材料科學(xué)學(xué)院,安徽 合肥 230601; 2.安徽省信息材料與器件重點實驗室,安徽 合肥 230601)
?
CuInS2/石墨烯混合物的合成及其在太陽能電池中的應(yīng)用
華健1,林運祥1,周雷1,楊曉1,左學(xué)勤1,李廣1,2*
(1.安徽大學(xué) 物理與材料科學(xué)學(xué)院,安徽 合肥230601; 2.安徽省信息材料與器件重點實驗室,安徽 合肥230601)
摘要:在不同的溶劑中通過溫和的溶劑熱法,成功地合成CuInS2納米晶體.這些合成好的CuInS2粉末被X光衍射表征后,又作為對電極被組裝成染料敏化太陽能電池.通過檢測可以發(fā)現(xiàn)乙二醇是合成CuInS2過程中最佳的溶液.這主要表現(xiàn)在用乙二醇合成的CuInS2作為電池對電極時的轉(zhuǎn)化率可以達到5.49%,這個值要比用其他溶液合成的CuInS2轉(zhuǎn)化率高.然后,將在乙二醇溶劑中合成的CuInS2粉末與石墨烯的氧化物混合形成CuInS2納米晶體/石墨烯納米復(fù)合材料,這種材料可以提高CuInS2在染料敏化太陽能電池方面的性能.通過透射電子顯微鏡法,可以證明CuInS2生長在石墨烯納米網(wǎng)中.與傳統(tǒng)的鉑對電極電池(6.90%)相比,這種納米復(fù)合材料具有相對較好的光電轉(zhuǎn)化率(6.28%).
關(guān)鍵詞:CuInS2;對電極;染料敏化太陽能電池(DSSC);石墨烯納米混合物;轉(zhuǎn)化率
0Introduction
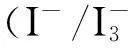
The most important part in CE is catalytic materials which are deposited on fluorine-doped tin oxide (FTO) glass substrate. Conventionally, it is platinum (Pt) that is used as this catalytic and electric material. A lot of experiments have shown that Pt is an excellent material for DSSC on account of outstanding electrochemical activity and stable property. However, as we know, Pt as relatively expensive and scare resources can not be used for quantity production. Furthermore, Pt is not suitable to be used in flexible plastics, because it needs higher temperature treatment while it is prepared as Pt CE[5]. Hence, many people try to use different materials to take the place of Pt as CE, such as carbon materials[6], conducting polymers materials[7],and inorganic materials (sulfides[4-5], nitrides[8], oxides[9]). Among these materials, more and more literatures study sulfides because it is abundant, cheap, and synthesized in mild conditions. In the family of sulfides, CuInS2(CIS) is becoming attractive in many aspects by the reason of its potential application[10].
CIS, as a ternary compound semiconductor, with the optical band gap of 1.5 eV[11]which matches well with solar spectrum, is an effective CE for light-absorbing. CIS has other excellent qualities such as good stability which can be irradiated for a long time, less toxicity compared with CdS and CuInSe2, and controllable morphology. Through numerical simulation, it can obtain 20.4% simulated efficiency[12]. Up to now, CIS can be synthesized through many ways, and to the best of our knowledge, it is wildly used in thin film solar cells in order to gain higher photo-conversion efficiency, for instance Wang and his co-workers have reached above 13%[13]. However, It does not show a satisfactory function if it is directly assembled on DSSC as CE, for instance it is reported that Yao and his co-workers have achieved 5.7%[14], which is also lower than Pt as CE.
In this literature, we report a new method to synthesize CE materials. We not only compound CIS nanocrystal but also synthesize CIS nanocrystal/reduced graphene oxide (CIS/RGO) nanocomposite by a solvothermal route to further enhance its performance. Graphene, with exceptionally high crystal, is a new two-dimensional (2D) material, which has one-auto-thick sheet and then form nano-networks. Graphene has many outstanding properties because of its particular structure. Graphene, as a conducting material, can work as electron reservoir[15-16]. In our work, CIS grown on graphene nano-networks exhibits remarkable photo-catalysis efficiency and electrical conductivity than bare CIS CE, which is close to Pt CE in the presence of visible light. So it can be anticipated that CIS/RGO must be one of the best materials to substitute Pt as CE and it may be widely used in DSSC in the future as well[16-19].
1Experimental Section
1.1 Materials
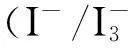
1.2 Synthesis of CuInS2 nanocrystals
In order to analyze the effect of solvent on synthesizing CIS nanocrystals, we choose different solvent but the other reactive conditions are the same. The reaction procedure was performed as follows. Absolute alcohol (30 mL), triethylene glycol (30 mL) and ethylene glycol (30 mL) were poured into different beakers (a1; a2; a3), respectively. InCl3·4H2O (1.0 mmol), CuCl (1.0 mmol) and thiocarbamide (2.5 mmol) were added into these three samples (a1; a2; a3). Then the chemicals were dispersed by the aid of magnetic stirring for a few minutes until they were completely dissolved. After that, the solutions were poured into a Teflon-lined stainless steel autoclave (50 mL). Then the sealed autoclaves were heated at 200 ℃ for 48 h. Afterward, the autoclaves were naturally cooled down to room temperature. The sediment filtered off from autoclave was washed off several times using distilled water and absolute ethanol. At last the sediment was dried in a vacuum at 60 ℃ for 12 h. As a result, we obtained the CIS powder.
1.3 Preparation of CuInS2/RGO nano-networks
In order to synthesize CuInS2/RGO nano-composites, in the first place we had to synthesis graphene oxide (GO) from natural graphite powder (KNGTM-150). In this work we chose Hummers and Offeman’s method[20-21]. The graphite powder was added into H2SO4/H3PO4(180∶20 mL) mixture solution. Then KMnO4and H2O2(30%) were added into the beakers until the mixture turned to bright yellow and using HCl solution filtered it. After that we washed it until the pH value reach 7. The next step, we should prepare CuInS2/RGO nano-composites. The method was close to the synthesis of CuInS2nanocrystals. However, the beaker was added one more GO (40 mg) and we used the ethylene glycol which is better than others as the solvent.
1.4 Fabrication of DSSC devices
DSSC was composed of three main parts. Among them, electrolyte and porous TiO2photoanode were bought from some companies. The most important thing we need to do was to fabricate CE. At first we washed the FTO glass using acetone and absolute alcohol. Besides, the FTO glass was masked by a 3 M Scotch tape (the thickness was about 5m) at two sides and the middle part was exposed about 0.5 cm×0.5 cm. Then dispersing CIS powder (0.1 g) or CIS/RGO was added into in agate mortar. Furthermore polyethylene glycol powder (0.025 g) and 3 mL absolute ethanol were also added in the agate mortar. The slurry was ground into a colloid. Afterwards, this colloid was coated over the exposing area of FTO coated glass plate using the doctor blade method. After the colloid dry naturally, the FTO was annealed at 450 ℃ for 30 min by the protection of argon atmosphere. So the CE was prepared.
The TiO2photo-anodes were immersed in 0.3 mM ethanolic solution of dye N-719 (Solaronix) for 12 h at room temperature in order to sufficiently absorb the dye. Then wash out excess adsorbed dye using absolute alcohol and dried it. Up to now, the DSSC was assembled by putting the CE and the TiO2photo-anode together, and it was with two clamps. After that, we injected electrolyte (0.05 M I2, 0.6 M 1-propyl-2,3-dimethylimidazolium iodide, 0.5 M LiI, and 0.5 M 4-tert-buylpyridine with acetonitrile as the solvent) into the middle of two FTO glasses.
1.5 Characterizations
The components and crystallographic structure of the as-prepared powdered products were characterized by X-ray diffraction(XRD) which uses CuKαradiation source (λ=1.540 6 ?) operated at 36 kV and 25 mA. The range of 2θis from 10° to 80°. The micro-morphology and the size of nanocrystal powder were measured by scanning electron microscopy (SEM, JSM-6700F) and high-resolution transmission electron microscopy (HRTEM; JEM-2100, Japan). We used UV-Vis spectrophotometers (JASCO V-550/V-570) to obtain UV-visable absorption spectra in the range from 250 nm to 850 nm.
2Results and discussion
Different CISs and CIS/RGO have been successfully synthesized. The structure and phase purity of these samples were measured by XRD. The XRD patterns of these three samples CIS powder (a1; a2; a3) in different solvents are shown in Fig.1 (CIS-a1-CIS-a3). From the major intensity of XRD diffraction peaks which appear at 2θ=27.1°, 32.0°, 46.1° and 54.7° approximately. These three samples can be indexed to chalcopyrite crystal structure (JCPDS No. 27-0159,a=0.552 3 nm,c=0.111 4 nm) and these 2θcan be attributed to (112), (204), (312) and (224) planes, respectively. The XRD diffraction peaks match with JCPDS card very well and no other peaks are detected, therefore we have obtained pure CIS powders which do not contain CuIn, CuS or In2S3and the CIS powder belong to tetragonal system. The crystallite size of these samples can be figured out on the basis of Debye-Scherer formula
(1)
whereKis a constant (0.94),λis the wavelength of X-ray source (0.154 056 nm),βdenotes the full width at half maximum of the diffraction strongest peak (112) andθis called the Bragg angle (13.937 5). The average size of a1-a3 samples calculated from three stronger peaks (112), (312), (224) using this equation is 44 nm, 37 nm and 35 nm. It indicates that the size is different just by altering solvents. Using ethylene glycol as the solvent, we can obtain the smallest CIS nanocrystal.
The CIS/RGO nano-composites (CIS is synthesized in ethylene glycol solution) is characterized by XRD as well (Fig.1 CIS/RGO). The positions of diffraction peaks are the same with CIS powders. However, the intensity of these peaks is much lower, which may be attributed to the RGO sheet restrain the intensity of CIS’s peaks.
The optical absorption property of this CIS powder is investigated by UV-Vis spectra (Fig.2). It owns higher absorption coefficient and broad absorption shoulder band.
Fig.2 shows the strongest absorption peak at 377.5 nm approximately. It is less than the value of bulk CIS (810 nm)[22], which demonstrates that the blue-shift of this CIS powder. It is mainly because of surface effect and quantum dimension effect by the decrease in average diameter of CIS particles. In order to calculate the band gap energy (Eg), we draw (αhν)2versus hν curve inserted in Fig.2. It can be confirmed through the equation
(2)
CIS is a direct band gap semiconductor, so it appears (αhν)2. Where α is the absorption coefficient,Ais a constant. Afterwards, we construct the tangent line along the straight-line portion, where the line intersects thehνaxis. The point of the intersection isEg. It is obvious that towards the CIS nanocrystals the value ofEgis 1.58 eV. This value is similar with Qi et al[23]and is close to the result of theoretical calculation value (Eg=1.5 eV)[10]. The band gap of this CIS nano-crystal is very suitable for absorbing solar spectrum. Therefore, CIS nano-crystal is one of ideal materials as CE for DSSC.
The surface morphology of the as-prepared CIS and CIS/RGO powder shown in Fig.3(a,b,d) and (c,e,f) respectively are examined by the SEM and TEM images.
The solvent possessing particular properties can affect the morphology of CIS nanocrystals. Through observing different CIS synthesized by different solvents using SEM images, it is found that the ethylene glycol is the optimal solvent to compound the regular CIS nano-particle. We conclude that the viscosity of solvent has an effect on the surface force of these CIS nanocrystals. Fig.3a and 3b show the pure CIS structure in ethylene glycol solution. The CIS porous microsphere with the diameter of 4.6m approximately is shown in Fig.3a. Fig.3b reveals the magnified image of Fig.3a. By detailed observation, we can find that the porous microsphere is composed of many irregular nanoparticles and among the different nanoparticles a lot of small holes and openings appear. The diameter of these small nanoparticles rangs from 50 nm to 100 nm. Higher resolution transmission electron microscopy (HRTEM) image taken from CIS is exhibited in Fig.3c. In this image, the lattice fringes can be seen clearly. In addition, the distance between the nearest fringes is about 0.32 nm, which agrees with the tetragonal phase CIS which is already measured by XRD patterns. This result is the same with other people’s study[23].
Fig.3(d-f) indicates the SEM and TEM images of CIS/RGO hybrids. Through the Fig.3d and 3f, the white graphene sheets with wrinkled shape are clearly visible, which prove that the graphene is prepared successfully. There are many CIS nano-crystals rather than microsphere attached on graphene sheets. This is because the graphene sheets provide the supporting place for the growth of CIS nano-crystals, rather than the CIS nano-crystals aggregate into microsphere (Fig.3a). By a further observation (Fig.3f), it is obvious that the diameter of these attached nano-crystals is about 100-150 nm which is much smaller than microsphere in Fig.3a (4.6m).
For purpose of researching the photovoltaic properties of CIS and CIS/RGO CEs, the DSSCs assembled by the diverse CEs (CIS-a1, CIS-a2, CIS-a3,CIS/RGO,Pt) were under 100 mW·cm-2illuminating condition. Fig.4a describes the J-V cures and the P-V (power density and voltage) cures are illustrated in Fig.4b. Tab.1 reveals accordingly the detailed photovoltaic parameters. At first, we analyze the photovoltaic performance of CIS synthesized in various solutions (CIS-a1, CIS-a2, CIS-a3). CIS-a3 exhibits a relatively preferable property with the power conversion efficiency of 5.49%, a short-circuit current density (Jsc) of 11.56 mA·cm-2, an open-circuit voltage (Voc) of 745.7 mV, and a fill factor (FF) of 63.10%. CIS-a1 and CIS-a2 have lower power conversion efficiency than CIS-a3, which is 4.55% and 5.03% respectively, and lower Voc and FF. We speculate the diameter and shape of the particles are the main reason for this result. By the management above, the smaller diameter of CIS-a3 with regular shape has been proved. So, it has larger superficial area to absorb sunlight.
With the purpose of explaining the reason why CIS/RGO nano-composites CE exhibited superior electrochemical activity,these as-prepared CEs were measured by EIS measurements using sandwich-like cell (CE/electrolyte/CE). The Nyquist plots of different CEs were shown in Fig.5 and the corresponding data were listed in Tab.1. The first point of intersection between semicircle and real axis denote Ohmic serial resistance (Rs) which means the outside circuit resistance. The diameter of semicircle in left can be assigned to charge transfer resistance (Rct) corresponding to the ability to transfer electron at the electrode and electrolyte interface[14,25]. It is obvious that original CIS has larger Rs (9.08 Ω) and Rct (4.84 Ω) relatively, which demonstrates that pure CIS has lower electrocatalytic activity. On the contrary, CIS/RGO nano-composites with lower Rs (5.04 Ω) and Rct (1.95 Ω) illustrate CIS grown on Graphene nano-networks promote catalytic performance of DSSC. Furthermore, the Rs and Rct of CIS/RGO nanocomposites are even close proximity to the values of Pt CE (4.04 Ω and 1.73 Ω). So, CIS nanocrystals grown on graphene nano-networks can enhance the properties of DSSC as CE.
3Conclusions
In summary, CuInS2nanocrystals have been successfully synthesized through a facile solvothermal method in different solvent systems. Ethylene glycol is an appropriate solvent towards the size and shape of CIS nanocrystals. Then this CIS nanocrystal was grown along graphene nano-networks successfully. Compared with pristine CIS (5.49%), CIS/RGO substantially enhances the efficiency of DSSC (6.31%), which is much more close to Pt CE. Therefore, CIS/RGO is a potential material for substituting Pt as CE. So, our work provides a meaningful prospect for the application of DSSC.
References:
[1]Hagfeldt A, Boschloo G, Sun L, et al. Dye-sensitized solar cells[J]. Chem Rev,2010,110(11):6595-6663.
[4]Guo J, Wang X, Zhou W H, et al. Efficiency enhancement of dye-sensitized solar cells(DSSCs) using ligand exchanged CuInS2NCs as counter electrode materials3[J]. RSC Advances,2013,3(34):14731-14736.
[5]Sun H C, Qin D, Huang S Q, et al. Dye-sensitized solar cells with NiS counter electrodes electro deposited by a potential reversal technique[J]. Energy Environ Sci,2011,4(8):2630-2637.
[6]Wu M X, Wang Y D, Lin X, et al. Economical and effective sulfide catalysts for dye-sensitized solar cells as counter electrodes[J]. Physical Chemistry Chemical Physics,2011,13(43):19298-19301.
[8]Jiang Q W, Li G R, Liu S, et al. Surface-nitrided nickel with bifunctional structure as low-cost counter electrode for dye-sensitized solar cells[J]. The Journal of Physical Chemistry C,2010,114(31):13397-13401.
[9]Wu M X, Lin X, Hagfeldt A, et al. A novel catalyst of WO2nanorod for the counter electrode of dye-sensitized solar cells[J]. Chemical Communications,2011,47(15):4535-4537.
[10]Konovalov I. Material requirements for CIS solar cells[J]. Thin Solid Films,2004,451/452:413-419.
[11]Xiao J P, Xie Y, Tang R, et al. Synthesis and characterization of ternary CuInS2nanorods via a hydrothermal route[J]. Journal of Solid State Chemistry,2001,161(2):179-183.
[12]Shang X Z, Wang Z Q, Li M K, et al. A numerical simulation study of CuInS2solar cells[J].Thin Solid Films,2014,550:649-653.
[13]Klenk R, Klaer J, Koble C, et al. Development of CuInS2-based solar cells and modules[J]. Solar Energy Materials and Solar Cells,2011,95(6):1441-1445.
[14]Yao R Y, Zhou Z J, Hou Z L, et al. Surfactant-free cuins2nanocrystals: an alternative counter-electrode material for dye-sensitized solar cells[J]. ACS Applied Materials & Interfaces,2013,5(8):3143-3148.
[15]Chen Z, Liu S Q, Yang M Q, et al. Synthesis of uniform CdS nanospheres/graphene hybrid nanocomposites and their application as visible light photocatalyst for selective reduction of nitro organics in water[J]. ACS Applied Materials & Interfaces,2013,5(10):4309-4319.
[16]Das S, Sudhagar P, Kang Y S, et al. Graphene synthesis and application for solar cells[J]. Journal of Materials Research,2014,29(3):299-319.
[17]Ahn H J, Kim I H, Yoon J C, et al. p-Doped three-dimensional graphene nano-networks superior to platinum as a counter electrode for dye-sensitized solar cells[J]. Chem Commun,2014,50(19):2412-2415.
[18]Singh P K, Singh U, Bhattacharya B, et al. Electrochemical synthesis of graphene oxide and its application as counter electrode in dye sensitized solar cell[J]. Journal of Renewable and Sustainable Energy,2014,6(1):013125.
[19]Wang R, Wu Q D, Lu Y, et al. Preparation of nitrogen-doped TiO2/graphene nanohybrids and application as counter electrode for dye-sensitized solar cells[J]. ACS Applied Mater & Interfaces,2014,6(3):2118-2124.
[20]Kovtyukhova N I, Ollivier P J, Martin B R, et al. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations[J]. Chem Mater,1999,11(3):771-778.
[21]Sen T, Patra A. Recent advances in energy transfer processes in gold-nanoparticle-based assemblies[J]. J Phys Chem C,2012,116(33):17307-17317.
[22]Asgary S, Mirabbaszadeh K, Nayebi P, et al. Synthesis and investigation of optical properties of TOPO-capped CuInS2semiconductor nanocrystal in the presence of different solvent[J]. Materials Research Bulletin,2014,51:411-417.
[23]Qi Y X, Tang K B, Zeng S Y, et al. Template-free one-step fabrication of porous CuInS2hollow microspheres[J]. Microporous and Mesoporous Materials,2008,114(1/2/3):395-400.
[24]Kavan L, Yum J H, Nazeeruddin M K, et al. Graphene nanoplatelet cathode for Co (III)/(II) mediated dye-sensitized solar cells[J]. ACS Nano,2011,5(11):165-172.
[25]Roy-Mayhew J D, Bozym D J, Punckt G, et al. Functionalized graphene as a catalytic counter electrode in dye-sensitized solar cells[J]. ACS Nano,2010,4(10):6203-6211.
(責(zé)任編輯于敏)
doi:10.3969/j.issn.1000-2162.2015.01.014

