pH-responsive mesoporous silica nanoparticles employed in controlled drug delivery systems for cancer treatment
Ke-Ni Yang, Chun-Qiu Zhang, Wei Wang, Paul C.Wang, Jian-Ping Zhou, Xing-Jie Liang
1State Key Laboratory of Natural Medicines, Department of Pharmaceutics, China Pharmaceutical University, Nanjing 210009,China; 2CAS Key Laboratory for Biological Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology, Chinese Academy of Sciences, Beijing 100190, China; 3Laboratory of Molecular Imaging, Department of Radiology,Howard University, Washington DC 20060, USA
Introduction
Cancer is the leading cause of death worldwide.Conventional chemotherapy is often characterized by clinical inefficiency and serious side-effects, mainly because of the leaking out of drugs during blood circulation and nonspecific cell/tissue biodistribution.The development of nanotechnology and nanomedicine in the past decades has facilitated the development of various nanovehicles for experimental and clinical application as drug delivery systems to solve these problems1,2.Nanovehicles benefit from surface properties and nanoscales and can thus accumulate in tumor tissue effectively with grafted multiple targeting ligands for ‘a(chǎn)ctive targeting’, while exhibiting enhanced permeability and retention effect (EPR) for ‘passive targeting’,which mainly improves local drug concentration and reduces nonspecific tissue biodistribution3-5.Nanovehicles can carry a large payload of cargoes and be conveniently modified to perform desirable functions, such as controlling drug release6,improving blood circulation half-life7, increasing bioavailability,and bypassing multidrug resistance mechanism8,9.
The most commonly used nanovehicles include liposomes10,micelles11, dendrimers12, nanoparticles13, and inorganic materials14.However, several barriers block clinical translocation of these nanovehicles to a certain extent because of the premature release and early extraction before reaching the target, uncontrollable rate of release to obtain low local concentration, and inefficient cellular uptake and endosomal escape15-17.Thus, controlled drug delivery systems should be designed.In such systems, controlled drug release at special time and space on demand can be achieved with a ‘zero release’ effect in blood circulation to protect healthy tissues from toxic drugs and to prevent drug decomposition.Several controlled drug delivery nanovehicles based on organic platforms have been fabricated18-20.Discoveries based on inorganic materials have recently opened up new and exciting possibilities in designing controlled drug delivery systems.These materials include gold nanoparticles14, magnetic nanoparticles21, and silica nanoparticles22.
Among these inorganic materials, mesoporous silica nanoparticles(MSNs) have aroused significant interest and rapidly developed into an important candidate for nanomedical applications since a MCM-41-type mesoporous silica material was first reported as a drug delivery system in 200123.As shown in Figure 1, the simple, scalable,and cost-effective fabrication, as well as non-toxic nature, large surface area and pore volume, and high density silanol-containing surface are apparent advantages of MSNs.On one hand, the textural characteristics of MSNs increase the loading amount of anti-cancer drugs that are encapsulated in pore tunnels.On the other hand,the silanol-containing surface can be easily modified with various molecules, resulting in an enhanced pro file for the pharmacokinetics and pharmacodynamics of therapeutic agents22.Moreover, the nanotunnels that encapsulate cargoes can be sealed with various gatekeepers, and such cargoes will not be released until triggered by stimuli, which offers an opportunity to design stimuli-responsive drug delivery systems for controlled release.
The stimulus can be divided into endogenous stimulus and exogenous stimulus17.Endogenous stimuli arise from the microenvironment differences between normal tissues and tumor, such as reduced intercellular/intracellular pH, higher redox potential, and increased level of certain enzymes24,25.However, exogenous stimuli are based on extracorporeal physical alterations, including temperature changes, magnetic fields,ultrasounds, as well as light and electric fields17.Among these stimuli, low pH is easy to achieve and has become the focus of numerous investigations in oncology because the extracellular pH of normal tissues and blood is approximately 7.4, whereas that in a tumor microenvironment is between 6.0 and 7.0,which is mainly caused by high glycolysis rate and high level of CO226.The pH value will drop further from the extracellular microenvironment of a tumor to intracellular organelles, such as endosomes (pH=5.5) and lysosomes (pH<5.5).Therefore,the abnormal pH gradients combined with the advantages of MSNs provide opportunities to realize pH-responsive MSNs as controlled drug delivery systems for cancer treatment.
Many groups have reported on pH-responsive MSNs modi fied with various gatekeepers.The triggered release of anti-cancer drugs from nanotunnels of mesoporous materials has mainly been achieved by using polyelectrolytes, supramolecularnanovalves,pH-sensitive linkers, and acid-decomposable inorganic materials27.
In this paper, we review the recent advances in drug delivery of pH-responsive MSNs with four categories of gatekeepers for cancer treatment based on tumor microenvironment.
pH-responsive MSNs with polyelectrolytes gatekeepers
Polyelectrolytes, which are polymers with repeating units that bear electrolyte groups, are either absorbed or covalently bonded to the surface of MSNs to serve as a mechanized stimulus-responsive release system by transformation under different pH values28.Under neutral or weakly basic conditions, the polyelectrolytes tightly wrap around the particle surface and block multiple openings.With decreasing pH value, the polyelectrolytes are triggered to go through swelling or coiling so that the cargoes are released from the unblocked pores29.
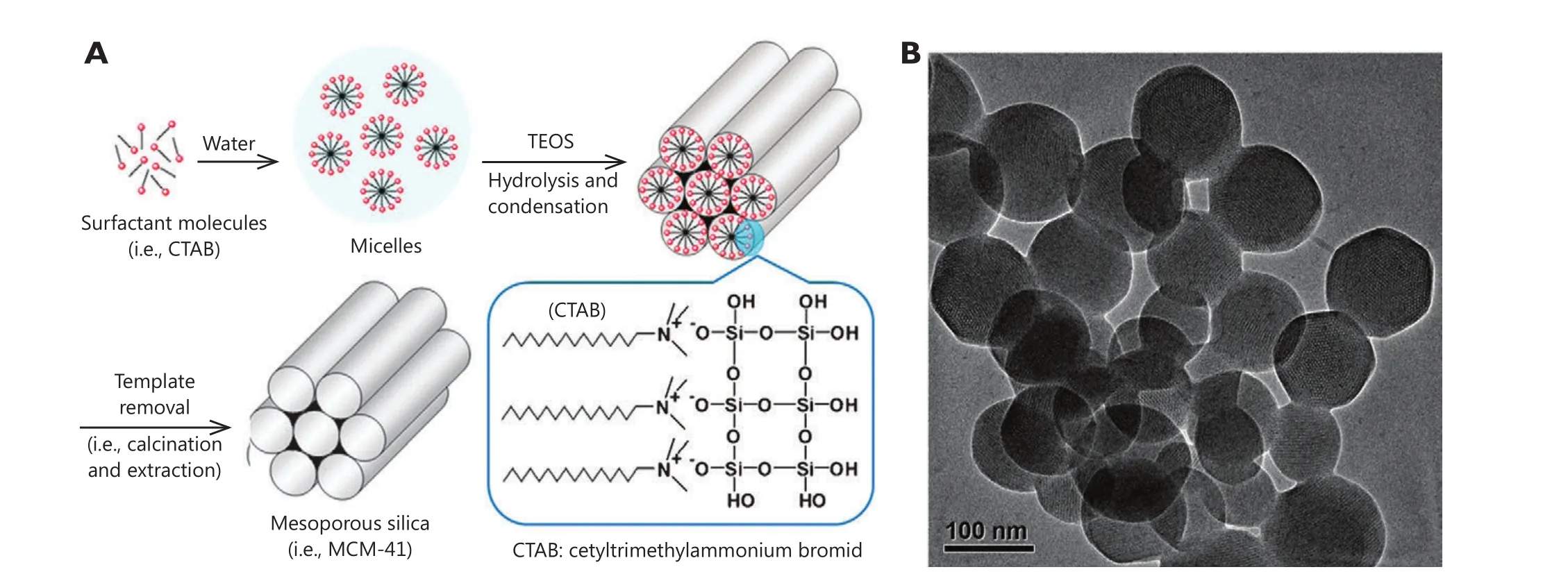
Figure 1 Synthesis scheme for the preparation of MSNs (A) and transmission electron microscopy (TEM) images of MCM-41 (B).(Figure 1A is adapted from Ref.22 with permission of The Royal Society of Chemistry).
Feng et al.30synthesized a type of pH-responsive MSNs with polyelectrolyte multilayers (PEM) composed of poly (allylamine hydrochloride) (PAH) and sodium poly(styrene sulfonata) (PSS)using a layer-by-layer technique.A schematic illustration of the construction and release mechanism of PEM-MSNs is shown in Figure 2A and 2B.The PEM-MSNs with eight polymer layers possess maximum encapsulation efficiency, and the release of DOX is accelerated under acidic conditions with incompact PAH/PSS multilayers (Figure 2C).In HeLa cells, DOX-loaded PEM-MSNs are almost distributed in the cytoplasm within 6 h,and some DOX is released from carriers into the nucleus for 12 h.Meanwhile, free DOX rapidly enter cancer cells and accumulate in the nucleus within 0.5 h.Thus, DOX-loaded PEM-MSNs are internalized into endosomes/lysosomes, and then pH-triggered DOX release occurs from nanotunnels because of the low pH(~5.0) in the endosomes/lysosomes followed by the delivery of released DOX from cytoplasm to nucleus.This process prolongs the accumulation of DOX in the nucleus to enhance the anticancer efficiency.Moreover, the blood profiles of DOX after intravenous injection of free DOX and DOX-loaded MSNs show different patterns (Figure 2D).The majority of free DOX have a rapid clearance within 2 h of administration, followed by a slow clearance phase.By contrast, DOX-loaded PEM-MSNs show low and sustained drug concentration in rat plasma up to 24 h post-injection, possibly because of the relatively high pH value of blood and the close state of PEM-MSNs.Furthermore,the determination of DOX levels in major organs, as well as the histological examination, indicates that DOX-loaded PEM-MSNs have lower systemic toxicity than free DOX (Figure 2E,F).
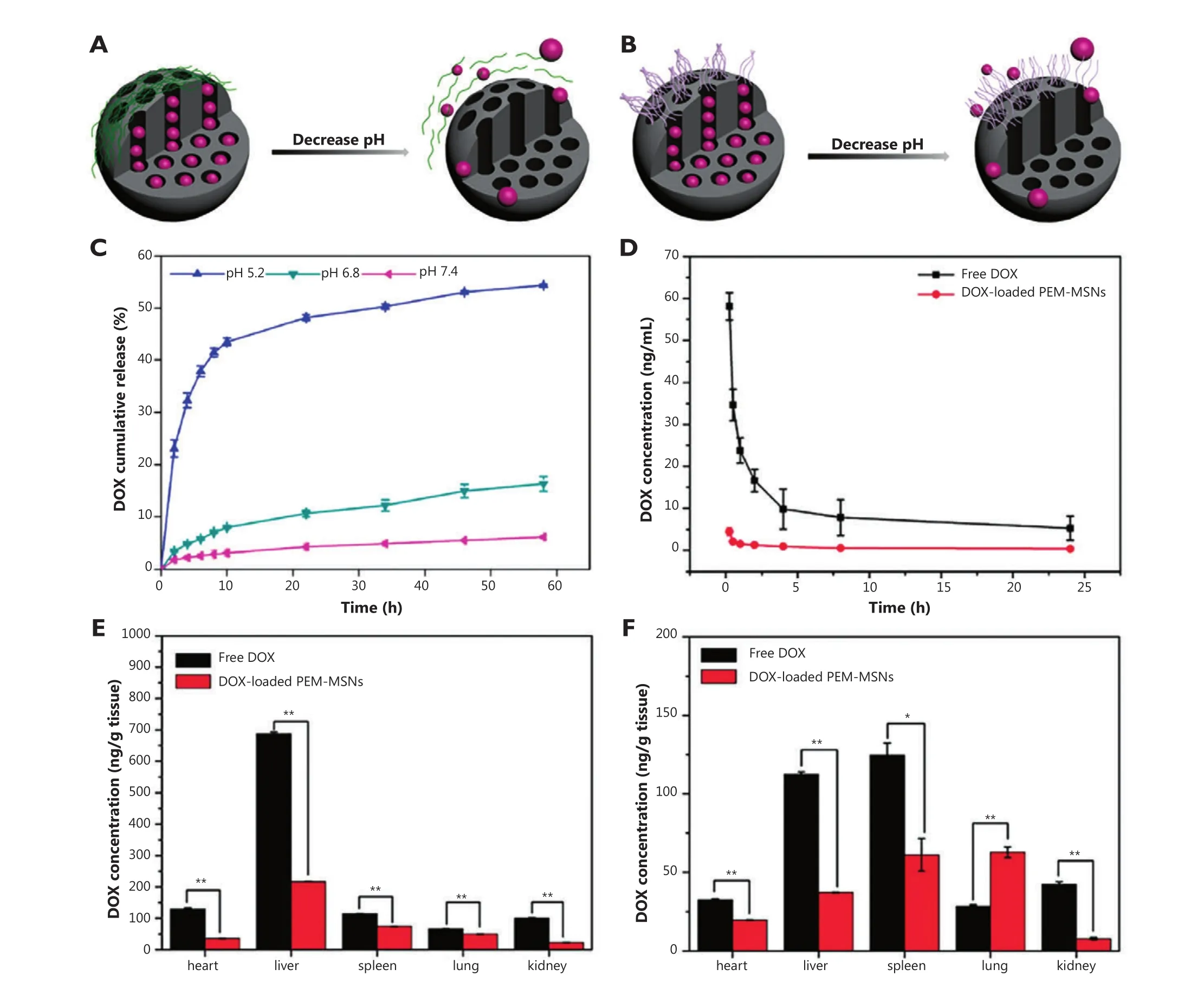
Figure 2 Graphical representation of pH-responsive MSNs with polyelectrolyte multilayers (A) and polyelectrolyte brushes (B).Release pro files of DOX from PEM-MENs (eight layers) in different pH media (C).DOX concentrations in plasma after DOX and DOX-loaded PEM-MSNs were injected intravenously through the vein for incremental time (D).Biodistribution of DOX in healthy SD rats at 2 h (E) and 24 h (F) after DOX and DOX-loaded PEM-MSNs at 2 mg/kg DOX equivalent were injected intravenously through the vein.*P<0.05 and **P<0.01 compared with free DOX group.(Figure 2C,D,E,F are adapted from Ref.30 with permission from The Royal Society of Chemistry).
Sun et al.31selected poly[2-(diethylamino)ethyl methacrylate](PDEAEMA) to functionalize the MSNs through the surfaceinitiated atom transfer radical polymerization of DEAEMA.Under neutral and alkaline conditions, PDMAEMA chains are prone to aggregate together with polymer chain-chain interactions to seal the nanotunnels of MSNs.However, under acid conditions,the tertiary amine in PDEAEMA can easily obtain a proton to form quaternary ammonium.This process is followed by polymer chain stretching with the electrostatic repulsions and strong chain-solvent interaction (Figure 2B), which facilitates cargo release.Yang et al.32-36also studied the ways by which to employ polyelectrolytes as pH-responsive gatekeepers.For instance, in their pH-sensitive system of poly (glutamic acid) grafted MSNs(MSN-PLGA), the drug loading experiment was performed at pH 8.0 because of the electrostatic attraction between DOX and the nanoparticles.The drug release behavior of MSN-PLGA loaded DOX was then studied at different pH values (5.5, 6.8, and 7.4).The results indicated that MSN-PLGA had high drug loading efficiency and exhibited a significantly pH-dependent drug release behavior.This finding can be attributed to the fact that the protonation of poly (glutamic acid) with decreasing pH results in the dissociation of the electrostatic interaction between PLGA and DOX and consequently facilitates DOX release.Many other polyelectrolytes were developed as gatekeepers for designing pH-responsive MSNs, such as poly(4-vinyl pyridine)37, chitosan38,39and poly(acrylic acid)40.Thus, the weak acid tumor tissues make pH-responsive release systems suitable for the controlled release of anti-cancer drugs.
pH-responsive MSNs with supramolecularnanovalves
The development of supramolecular chemistry enabled supramolecular assembly to be made into a ‘nanovalve’ machine responding to various stimuli, such as chemical, light, and electrical stimuli41.The supramolecularnanovalve, as a gatekeeper for controlling cargo release, includes an immobilized stalk molecule covalently attached to silica surface and a mobile cyclic molecule encircling the stalk via non-covalent interactions29.Under certain conditions, the binding constant between cyclic caps and stalks weakens, thus resulting in large-amplitude sliding motions of the caps and the unblocking of nanotunnels.Therefore, supramolecularnanovalves provide opportunities for MSNs to construct a pH-responsive drug delivery system for responding to weak acidic tumor tissues (Figure 3A).
Meng et al.42reported a novel MSNs delivery system based on the function of β-cyclodextrin (β-CD) nanovalves that were responsive to the acidic conditions of endosomes in cancer cells.In this system, N-methylbenzimidazole is chosen to serve as stalks for the optimized pKa(5.67) (Figure 3B), which binds to the β-CD rings strongly at pH 7.4 with trapping cargoes in nanotunnels while causing dissociation with the β-CD caps at pH <6 in the acidifying endosomal compartment.The profiles of drug release accompanied by β-CD detachment from MSNs before and after acid stimuli are presented in Figure 3C, which shows typical pH-responsive release characteristics.To improve the rate and quantity of DOX release, the interior of the silica surface is modified with 7.5% ammonium (Figure 3D).In squamous carcinoma (KB-31) cells, DOX-loaded nanoparticles are taken up into the perinuclear regions efficiently within 3 h, where drug release to the nucleus is observed.The release is followed by apoptosis at 60 h, nuclear fragmentation after 80 h, and finally cell death (Figure 3E, left panel).However,the release profile of DOX dramatically changes after NH4Cl treatment of KB-31 cells, in which most drugs are retained inside nanotunnels and little evidence of nuclear staining and cell death is observed (Figure 3E, right panel).In addition, quantitative analysis of the nuclear DOX fluorescence signal and MTS assays further confirm that the cargo release caused by lysosomal acidification is made feasible by the pH-sensitive nanovalves during in vitro operation.
Similarly, Du et al.43successfully synthesized a biocompatible pH-responsive nanovalve based on MSNs comprising α-cyclodextrin (α-CD) rings and p-anisidino linkers modi fied on the silica surface.Luminescence spectroscopy demonstrates that the pH-responsive system exhibits good bio-stability and no drug leakage at pH ~7.4, as well as excellent drug release performance not only in H2O but also in cell culture medium at pH ~5.5 upon the protonation of p-anisidino nitrogen atoms (part of the linker).Therefore, Du et al.explored the applications of the α-CD nanovalves based on MSNs by testing their delivery capability in different types of human cancer cells at lysosomal pH levels.
In addition, cucurbit[n]uril, the structure of which is similar to cyclodextrin, is capable of blocking the pores of MSNs as nanovalves and of preventing the cargoes from leaking out until they are detached from the stalks or positioned far away from the pore entrances by sliding under acidic stimuli41.In a typical design,Angelos et al.44developed supramolecularnanovalves composed of cucurbit(6)uril [CB(6)]/trisammoniumpseudorotaxanes that are attached to MSNs surfaces and encapsulate cargo molecules at neutral pH and then release the cargoes under mildly acidic conditions.Owing to the difference in the binding affinity of CB(6) with(CH2)6and(CH2)4, the CB(6)ring shuttles to the distal hexamethylenediammonium recognition unit once the anilinium nitrogen atom protonated, which then unblocks the pore orifice and facilitates cargo release.More importantly, the pH at which the MSNs system responds can be tuned through rational chemical modi fication of the stalk.

Figure 3 Graphical representation of the pH-responsive MSNs with supramolecularnanovalves (A).Synthesis of the stalk on the surface of MSNs for further β-CD capping on the pore (B).Fluorescence intensity plots for the release of Hoechst dye, doxorubicin, and the pyrene-loaded cyclodextrin cap from MSNs (C) and release pro files of doxorubicin from ammonium-modi fied (7.5%, w/w) nanoparticles showing a faster and larger response compared with that of unmodi fied MSNs (D).Confocal images of KB-31 cells incubated with MSNs containing doxorubicin for the indicated times: KB-31 cancer cells effectively endocytosed the doxorubicin-loaded FITC-MSNs at 3 h.This action is followed by nuclear fragmentation after 80 h.However, with NH4Cl treatment, most of the doxorubicin was con fined to nanoparticles, such that no observable cell death occurred (E).(Figure 3B,C,D,E are adapted with permission from Ref.42.Copyright 2010, American Chemical Society).
pH-responsive MSNs with pH-sensitive linkers
The pH-sensitive linkers, such as acetal bond, hydrazine bond,and ester bond can be cleaved with decreasing pH value, thus providing opportunities for designing pH-responsive MSNs.On one hand, the pH-sensitive linkers modified over the pore entrances of MSNs can induce bulky groups as nanogates to block the pores and control drug release (Figure 4).On the other hand, the pH-sensitive linkers can also be modi fied in the nanotunnels to bond with drugs covalently.These drugs will then be released with the cleavage effects of linkers between drugs and MSNs under acidic conditions.
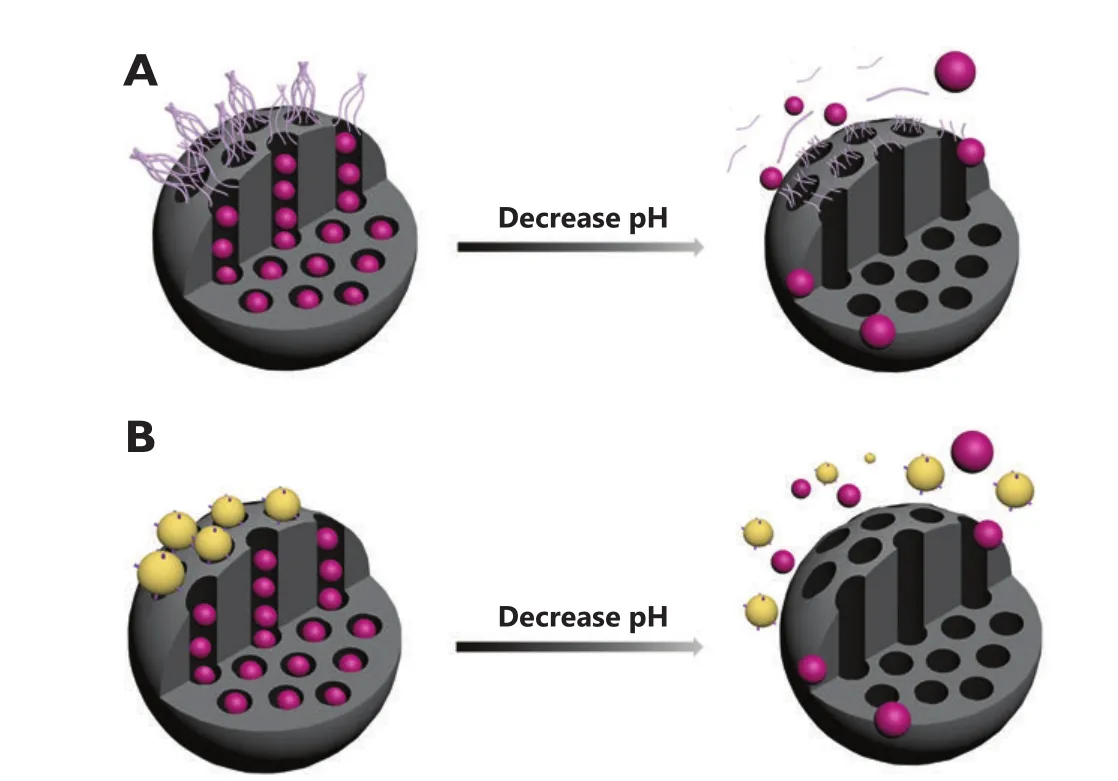
Figure 4 Graphical representation of the pH-responsive MSNs capped with polymers (A) and nanoparticles (B) that linked to the surface of MSNs via pH-sensitive linkers.
Gao et al.45employed functionalized MSNs as drug reservoirs and then blocked the mesopores with polypseudorotaxanes through pH-sensitive benzoic-imine bonds hydrolyzed under very weak acidic conditions but stable at neutral basic pH because of the proper π-π conjugation extent.The polypseudorotaxanes consist of poly (ethylene glycol) (PEG) and α-CD, with PEG serving as the guest polymer for CD hosts and imparting in vivo longevity to MSNs by preventing nonspecific protein adsorptions during the circulation.Under weak acidic tumor extracellular pH (~6.8), the benzoic-imine linkages begin partially hydrolyzing to accelerate DOX release and meanwhile generate positive amino groups to facilitate internalization of particles.Subsequently, in the more acidic endosomal pH (~5.0),the increasing hydrolysis of the benzoic-imine bond would intensify the removal of the polypseudorotaxane caps and thus accelerate the release of DOX into the cytoplasm.In HepG2 cells, DOX-loaded MSNs are initially located within endosomal intracellular compartments and release drugs in the cytosol region in a sustained manner.Moreover, the different results of confocal fluorescence microscopy and cytotoxicity assay between cells exposed to DOX-loaded MSNs at pH =6.8 and pH =7.4 again prove that enhanced tumor-specific uptake and intracellular delivery can be achieved through the inclusion of the benzoic-imine linkage.
Analogously, Liu et al.46reported a new pH-responsive nanogated construction by capping gold nanoparticles onto mesoporous silica through acid-labile acetal linkers (Figure 4B).At neutral pH, the linker remains intact, and pores are blocked by gold nanoparticles to inhibit cargo diffusion.However, at acidic pH, the hydrolysis of the acetal group removes the caps and allows release of cargoes.Aside from bulky groups capped on the outlets via pH-sensitive linkers, Lee et al.47conjugated DOX to the inner wall of MSNs nanochannels via liable hydrazone bonds.Through EPR effects, the Atto-647-MSN-hydrazone-DOX inherently accumulates in solid tumors of the liver.Nanoparticles then highly concentrate within endosomes and lysosomes of cancer cells.Sustained release of drug payload is observed because of the leakage of hydrazone bonds at endosomal and lysosomal pH.Moreover, apart from DOX, the pH-sensitive drug release mechanism can be applied to other anti-cancer drugs that possess functional ketones or aldehydes.
pH-responsive MSNs with aciddecomposable inorganic gatekeepers
The common strategies used for surface functionalization include grafting organic species.However, such strategies have been limited by tedious and intricate organic synthesis steps and the lack of a clear de finition of the body toxicity of dismantled pore-blocking agents.Some acidic-decomposable inorganic materials have recently been reported as gatekeepers to control drug release, offering opportunities to design promising specific carriers for therapeutic agents (Figure 5A).
Rim et al.48introduced inorganic calcium phosphate (CaP) as a novel pore blocker through the enzyme-mediated mineralization on the MSN surface, which can be dissolved in intracellular endosomes as nontoxic ions to initiate drug release.The construction of the nanoparticle involves urease functionalization of MSN surfaces and subsequent enzyme-mediated surface CaP mineralization in the presence of urea under mild conditions within a short time.For pH-controlled DOX release from mineralized MSNs, pH variation between physiological pH (pH 7.4) and low pH (pH 4.5)is employed.The results show that a large amount of DOX was released after 24 h under low pH conditions (Figure 5B).Furthermore, the pH-dependent dissolution kinetics of Hap-like coating support the DOX release pro files from CaP capped MSNs(Figure 5C), which confirms that the dissolution of pore blocks results in the opening of the pore and then triggers DOX release.In breast cancer MCF-7 cells, DOX-loaded mineralized MSNs(DOX-Si-MP-CaP) carry DOX in nanopores effectively before endocytosis, and DOX release can be facilitated in lysosomes by the dissolution of mineral coatings, followed by the DOX release and accumulation in the nucleus (Figure 5D).Moreover, the evaluation of the in vivo efficacy of DOX-Si-MP-CaP using xenograft models of MCF-7 human breast cancer shows that a single intratumoral administration of DOX-Si-MP-CaP is significantly more effective in tumor reduction than control groups including free DOX and DOX-Si-MP-CaP (Figure 5E,F).
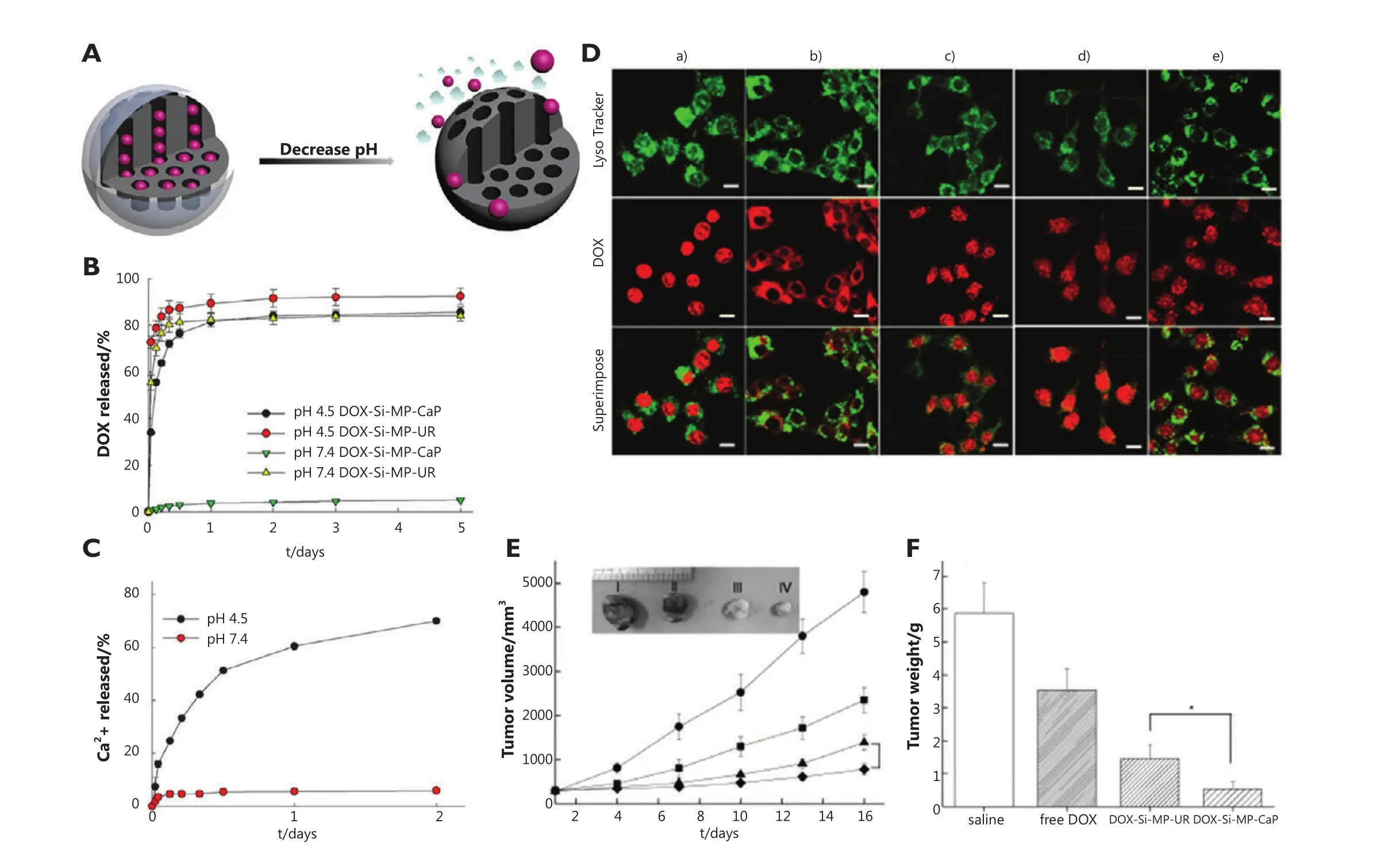
Figure 5 (A) Graphical representation of pH-responsive MSNs with acid-decomposable inorganic gatekeepers.(B) DOX release pro files from DOX-Si-MP-UR and DOX-Si-MP-CaP under pH control.(C) Kinetics of calcium dissolution from DOX-Si-MP-CaP under pH control.(D) CLSM images of live MCF-7 cells treated with Lyso Tracker (50 nm), free DOX (5 μg/mL), and DOX-Si-MP-CaP (DOX =5 μg/mL), thereinto, (a) free DOX for 1 h exposure; (b) DOX-Si-MP-CaP for 1 h exposure; (c) DOX-Si-MP-CaP for 5 h exposure; (d) DOX-Si-MP-UR for 1 h exposure; and (e) DOXSi-MP-UR for 5 h exposure.(Green fluorescence is associated with Lyso Tracker; the red fluorescence is expressed by free DOX, released DOX,and DOX retained within MSNs).Scale bar: 20 μm.(E) In vivo therapeutic efficacy after a single intratumoral injection of saline (●), free DOX (█),DOX-Si-MP-UR (▲), and DOX-Si-MP-CaP(◆) at a DOX-equivalent dose of 10 mg/kg.Inset: images of excised tumors at 16 days after treatment.I:saline, II: free DOX, III: DOX-Si-MP-UR, IV: DOX-Si-MP-CaP.(F) Tumor weights at 16 days after treatment.The results represent the means ± SDs(n=4); *P<0.05.(Figure 4B,C,D,E,F are adapted from Ref.48 with permission of John Wiley and Sons).
Muhammad et al.49employed acid-decomposable luminescent ZnO quantum dots (QDs) to seal the nanopores of MSNs in order to inhibit premature drug (DOX) release.After internalization into HeLa cells, the ZnO QD lids are dissolved rapidly in the acidic intracellular compartments, followed by loaded drug release from MSNs into cytosol.In this pH-responsive drug delivery system,ZnO QDs behave as a dual-purpose entity that not only serves as a lid but also imposes a synergistic anti-tumor effect on cancer cells.Zheng et al.50reported a pH-responsive controlled release system via using acid-decomposable layered double hydroxides (LDHs)as inorganic nanovalves, by virtue of the electrostatic interaction of LDH nanosheets on the surface of MSNs.The preparation procedure of the pH-responsive MSNs is free from complicated organic synthesis.Guest molecules are loaded and capsulated in neutral and released in acidic pH depending on the dissolution of LDHs.Thus, acid-decomposable inorganic materials are promising candidates for designing pH-responsive MSNs.
Conclusion and outlook
In this review, we highlighted the exciting research advances on pH-responsive drug delivery systems based on MSNs.Various materials can be used as gatekeepers to control drug release under acidic conditions.These materials have great potential for application in tumor therapy and for improving anti-cancer drug efficiency and decreasing side effects.However, most work is focused on in vitro studies51.Thus, several challenges still need to be overcomed for the further advancement of the biological and biomedical applications of pH-responsive MSNs.First, the differences in the pH values between tumor microenvironment and normal tissues are minimal, making the manipulation of the stimuli-responsive drug delivery system in vivo via pH difficult.Second, the targeting effects of pH-responsive MSNs depending on EPR effects are low, thus causing nanoparticles accumulation in some organs, such as the heart, liver, and spleen.Upon accumulation in normal tissues, MSNs can be internalized into cells via endocytosis to trigger drug release, which may result in side effects.Third, the biodistribution, acute and chronic toxicities, changes in molecule level, long-term stability, and circulation properties of stimulus-responsive drug delivery systems need to be further investigated before implementation in clinical practice.Therefore, future work in designing stimulusresponsive MSNs will most likely be directed toward the integration of multiple stimuli strategies that can respond to two or more stimuli simultaneously and can bear targeting molecules for efficiently directing the nanoparticles to tumor tissues, with low toxicity and good pharmacokinetic pro file.
Acknowledgements
This work was supported by the Chinese Natural Science Foundation Project (Grant No.30970784 and 81171455),a National Distinguished Young Scholars Grant (Grant No.31225009) from the National Natural Science Foundation of China, the National Key Basic Research Program of China(Grant No.2009CB930200), the Chinese Academy of Sciences(CAS) ‘Hundred Talents Program’ (Grant No.07165111ZX),the CAS Knowledge Innovation Program, and the State High-Tech Development Plan (Grant No.2012AA020804).The authors also appreciate the support by the ‘Strategic Priority Research Program’ of the Chinese Academy of Sciences(Grant No.XDA09030301).This work was also supported in part by NIH/NIMHD 8 G12 MD007597 and USAMRMC W81XWH-10-1-0767 grants.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Nazir S, Hussain T, Ayub A, Rashid U, MacRobert AJ.Nanomaterials in combating cancer: therapeutic applications and developments.Nanomedicine 2014;10:19-34.
2.Farokhzad OC, Langer R.Impact of nanotechnology on drug delivery.ACS Nano 2009;3:16-20.
3.Maeda H.The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting.Adv Enzyme Regul 2001;41:189-207.
4.Cho K, Wang X, Nie S, Chen ZG, Shin DM.Therapeutic nanoparticles for drug delivery in cancer.Clin Cancer Res 2008;14:1310-1316.
5.Davis ME, Chen ZG, Shin DM.Nanoparticle therapeutics: an emerging treatment modality for cancer.Nat Rev Drug Discov 2008;7:771-782.
6.Mu L, Feng SS.A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS.J Control Release 2003;86:33-48.
7.Prencipe G, Tabakman SM, Welsher K, Liu Z, Goodwin AP,Zhang L, et al.PEG branched polymer for functionalization of nanomaterials with ultralong blood circulation.J Am Chem Soc 2009;131:4783-4787.
8.Brigger I, Dubernet C, Couvreur P.Nanoparticles in cancer therapy and diagnosis.Adv Drug Deliv Rev 2002;54:631-651.
9.Cuvier C, Roblot-Treupel L, Millot JM, Lizard G, Chevillard S,Manfait M, et al.Doxorubicin-loaded nanospheres bypass tumor cell multidrug resistance.Biochem Pharmacol 1992;44:509-517.
10.Malam Y, Loizidou M, Seifalian AM.Liposomes and nanoparticles:nanosized vehicles for drug delivery in cancer.Trends Pharmacol Sci 2009;30:592-599.
11.Blanco E, Kessinger CW, Sumer BD, Gao J.Multifunctional micellar nanomedicine for cancer therapy.Exp Biol Med(Maywood) 2009;234:123-131.
12.Nanjwade BK, Bechra HM, Derkar GK, Manvi FV, Nanjwade VK.Dendrimers: emerging polymers for drug-delivery systems.Eur J Pharm Sci 2009;38:185-196.
13.Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z.Polysaccharides-based nanoparticles as drug delivery systems.Adv Drug Deliv Rev 2008;60:1650-1662.
14.Ghosh P, Han G, De M, Kim CK, Rotello VM.Gold nanoparticles in delivery applications.Adv Drug Deliv Rev 2008;60:1307-1315.
15.Talelli M, Iman M, Varkouhi AK, Rijcken CJ, Schiffelers RM, Etrych T, et al.Core-crosslinked polymeric micelles with controlled release of covalently entrapped doxorubicin.Biomaterials 2010;31:7797-7804.
16.Andresen TL, Jensen SS, J?rgensen K.Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release.Prog Lipid Res 2005;44:68-97.
17.Mura S, Nicolas J, Couvreur P.Stimuli-responsive nanocarriers for drug delivery.Nat Mater 2013;12:991-1003.
18.Sawant RR, Torchilin VP, Liposomes as ‘smart’ pharmaceutical nanocarriers.Soft Matter 2010;6:4026-4044.
19.Zhang Q, Ko NR, Oh JK.Recent advances in stimuliresponsive degradable block copolymer micelles: synthesis and controlled drug delivery applications.Chem Commun (Camb)2012;48:7542-7552.
20.Kojima C.Design of stimuli-responsive dendrimers.Expert Opin Drug Deliv 2010;7:307-319.
21.Yuan Q, Venkatasubramanian R, Hein S, Misra RD.A stimulusresponsive magnetic nanoparticle drug carrier: magnetite encapsulated by chitosan-grafted-copolymer.Acta Biomater 2008;4:1024-1037.
22.Yang P, Gai S, Lin J.Functionalized mesoporous silica materials for controlled drug delivery.Chem Soc Rev 2012;41:3679-3698.
23.Chen T, Fu J.pH-responsive nanovalves based on hollow mesoporous silica spheres for controlled release of corrosion inhibitor.Nanotechnology 2012;23:235605.
24.Torchilin V.Multifunctional and stimuli-sensitive pharmaceutical nanocarriers.Eur J Pharm Biopharm 2009;71:431-444.
25.Zhu L, Torchilin VP.Stimulus-responsive nanopreparations for tumor targeting.Integr Biol (Camb) 2013;5:96-107.
26.Danhier F, Feron O, Préat V.To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery.J Control Release 2010;148:135-146.
27.Xing L, Zheng H, Cao Y, Che S.Coordination polymer coated mesoporous silica nanoparticles for pH-responsive drug release.Adv Mater 2012;24:6433-6437.
28.Fu Q, Rao GR, Ista LK, Wu Y, Andrzejewski BP, Sklar LA, et al.Control of molecular transport through stimuli-responsive ordered mesoporous materials.Adv Mater 2003;15:1262-1266.
29.Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI.Mesoporous silica nanoparticles in biomedical applications.Chem Soc Rev 2012;41:2590-2605.
30.Feng W, Zhou X, He C, Qiu K, Nie W, Chen L, et al.Polyelectrolyte multilayer functionalized mesoporous silica nanoparticles for pH-responsive drug delivery: layer thicknessdependent release pro files and biocompatibility.J Mater Chem B 2013;1:5886-5898.
31.Sun JT, Hong CY, Pan CY.Fabrication of PDEAEMA-coated mesoporous silica nanoparticles and pH-responsive controlled release.J Phys Chem C 2010;114:12481-12486.
32.Chang B, Sha X, Guo J, Jiao Y, Wang C, Yang W.Thermo and pH dual responsive, polymer shell coated, magnetic mesoporous silica nanoparticles for controlled drug release.J Mater Chem 2011;21:9239-9247.
33.Chen Y, Yang W, Chang B, Hu H, Fang X, Sha X.In vivo distribution and antitumor activity of doxorubicin-loaded N-isopropylacrylamide-co-methacrylic acid coated mesoporous silica nanoparticles and safety evaluation.Eur J Pharm Biopharm 2013;85:406-412.
34.Tang H, Guo J, Sun Y, Chang B, Ren Q, Yang W.Facile synthesis of pH sensitive polymer-coated mesoporous silica nanoparticles and their application in drug delivery.Int J Pharm 2011;421:388-396.
35.Zheng J, Tian X, Sun Y, Lu D, Yang W.pH-sensitive poly(glutamic acid) grafted mesoporous silica nanoparticles for drug delivery.Int J Pharm 2013;450:296-303.
36.Wen H, Guo J, Chang B, Yang W.pH-responsive composite microspheres based on magnetic mesoporous silica nanoparticle for drug delivery.Eur J Pharm Biopharm 2013;84:91-98.
37.Liu R, Liao P, Liu J, Feng P.Responsive Polymer-Coated Mesoporous Silica as a pH-Sensitive Nanocarrier for Controlled Release.Langmuir 2011;27:3095-3099.
38.Popat A, Liu J, Lu GQ, Qiao SZ.A pH-responsive drug delivery system based on chitosan coated mesoporous silica nanoparticles.J Mater Chem 2012;22:11173-11178.
39.Hu X, Wang Y, Peng B.Chitosan-capped mesoporous silica nanoparticles as pH-responsive nanocarriers for controlled drug release.Chem Asian J 2014;9:319-327.
40.Yuan L, Tang Q, Yang D, Zhang JZ, Zhang F, Hu J.Preparation of pH-responsive mesoporous silica nanoparticles and their application in controlled drug delivery.J Phys Chem C 2011;115:9926-9932.
41.Yang YW.Towards biocompatible nanovalves based on mesoporous silica nanoparticles.Med Chem Comm 2011;2:1033-1049.
42.Meng H, Xue M, Xia T, Zhao YL, Tamanoi F, Stoddart JF, et al.Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves.J Am Chem Soc 2010;132:12690-12697.
43.Du L, Song H, Liao S.A biocompatible drug delivery nanovalve system on the surface of mesoporous nanoparticles.Micropor Mesopor Mat 2012;147:200-204.
44.Angelos S, Khashab NM, Yang YW, Trabolsi A, Khatib HA,Stoddart JF, et al.pH clock-operated mechanized nanoparticles.J Am Chem Soc 2009;131:12912-12914.
45.Gao Y, Yang C, Liu X, Ma R, Kong D, Shi L.A multifunctional nanocarrier based on nanogated mesoporous silica for enhanced tumor-specific uptake and intracellular delivery.Macromol Biosci 2012;12:251-259.
46.Liu R, Zhang Y, Zhao X, Agarwal A, Mueller LJ, Feng P.pH-responsive nanogated ensemble based on gold-capped mesoporous silica through an acid-labile acetal linker.J Am Chem Soc 2010;132:1500-1501.
47.Lee CH, Cheng SH, Huang IP, Souris JS, Yang CS, Mou CY, et al.Intracellular pH-responsive mesoporous silica nanoparticles for the controlled release of anticancer chemotherapeutics.Angew Chem Int Ed Engl 2010;49:8214-8219.
48.Rim HP, Min KH, Lee HJ, Jeong SY, Lee SC.pH-Tunable calcium phosphate covered mesoporous silica nanocontainers for intracellular controlled release of guest drugs.Angew Chem Int Ed Engl 2011;50:8853-8857.
49.Muhammad F, Guo M, Qi W, Sun F, Wang A, Guo Y, et al.pH-Triggered controlled drug release from mesoporous silica nanoparticles via intracelluar dissolution of ZnO nanolids.J Am Chem Soc 2011;133:8778-8781.
50.Zheng Q, Hao Y, Ye P, Guo L, Wu H, Guo Q, et al.A pH-responsive controlled release system using layered double hydroxide (LDH)-capped mesoporous silica nanoparticles.J Mater Chem B 2013;1:1644-1648.
51.Tang F, Li L, Chen D.Mesoporous silica nanoparticles: synthesis,biocompatibility and drug delivery.Adv Mater 2012;24:1504-1534.
 Cancer Biology & Medicine2014年1期
Cancer Biology & Medicine2014年1期
- Cancer Biology & Medicine的其它文章
- Inhalation treatment of lung cancer: the influence of composition, size and shape of nanocarriers on their lung accumulation and retention
- Interferon-alpha-2b induces autophagy in hepatocellular carcinoma cells through Beclin1 pathway
- Uptake of prostate cancer screening and associated factors among Chinese men aged 50 or more: a population-based survey
- Mechanistic considerations for the use of monoclonal antibodies for cancer therapy
- Cancer metabolic reprogramming: importance, main features,and potentials for precise targeted anti-cancer therapies
- Instructions for Authors
